282036f43f08d0b5b88ea68fdcafd075.ppt
- Количество слайдов: 24
 Zen & the Art of Guideline Development Duncan Service Senior Information Officer
Zen & the Art of Guideline Development Duncan Service Senior Information Officer
 OUTLINE • • • What are guidelines? Methods of guideline development Evidence based guidelines: – What are they? – Why are they useful? – What are their limitations? Guidelines and the information professional High quality guideline development -the AGREE instrument SIGN in 2012
OUTLINE • • • What are guidelines? Methods of guideline development Evidence based guidelines: – What are they? – Why are they useful? – What are their limitations? Guidelines and the information professional High quality guideline development -the AGREE instrument SIGN in 2012
 METHODS OF GUIDELINE DEVELOPMENT I • Expert opinion – guideline reflects the views of opinion leaders / specialist societies – inexpensive – high potential for bias – potential for hidden conflicts of interest
METHODS OF GUIDELINE DEVELOPMENT I • Expert opinion – guideline reflects the views of opinion leaders / specialist societies – inexpensive – high potential for bias – potential for hidden conflicts of interest
 METHODS OF GUIDELINE DEVELOPMENT II • Formal consensus – A number of methods exist, including Delphi, nominal group technique and consensus conferences – Results may be affected by way in which questions are posed, selection of participants and methods used – Some potential for bias
METHODS OF GUIDELINE DEVELOPMENT II • Formal consensus – A number of methods exist, including Delphi, nominal group technique and consensus conferences – Results may be affected by way in which questions are posed, selection of participants and methods used – Some potential for bias
 METHODS OF GUIDELINE DEVELOPMENT III • Evidence based clinical guidelines – systematically developed statements to help professionals assimilate and evaluate the ever-increasing amount of information on best practice in the management of conditions – less susceptible to bias in their conclusions and recommendations than those based on consensus or a non-systematic review of the evidence • Evidence based guidelines: – provide an easily accessible summary of current evidence and recommended practice based upon that evidence – allow clinicians from different specialties easy access to best practice in other areas – provide a good source of information for others (including patients, carers, politicians etc)
METHODS OF GUIDELINE DEVELOPMENT III • Evidence based clinical guidelines – systematically developed statements to help professionals assimilate and evaluate the ever-increasing amount of information on best practice in the management of conditions – less susceptible to bias in their conclusions and recommendations than those based on consensus or a non-systematic review of the evidence • Evidence based guidelines: – provide an easily accessible summary of current evidence and recommended practice based upon that evidence – allow clinicians from different specialties easy access to best practice in other areas – provide a good source of information for others (including patients, carers, politicians etc)
 A NOTE OF CAUTION. . • Guidelines are only useful if they are: – developed using a robust methodology – relevant to clinicians – up to date – realistic • Guidelines therefore need to be produced or adapted by the people who are going to use them • They need to be produced by credible agencies
A NOTE OF CAUTION. . • Guidelines are only useful if they are: – developed using a robust methodology – relevant to clinicians – up to date – realistic • Guidelines therefore need to be produced or adapted by the people who are going to use them • They need to be produced by credible agencies
 GUIDELINES & INFORMATION PROFESSIONALS • Information professionals are crucial to the dissemination and production of clinical guidelines • Involvement can be at 3 levels: – Identifying good quality guidelines for local dissemination & implementation – Providing literature searching for guideline development – Critical appraisal in guideline development
GUIDELINES & INFORMATION PROFESSIONALS • Information professionals are crucial to the dissemination and production of clinical guidelines • Involvement can be at 3 levels: – Identifying good quality guidelines for local dissemination & implementation – Providing literature searching for guideline development – Critical appraisal in guideline development
 GUIDELINES & INFORMATION PROFESSIONALS I IDENTIFYING GOOD QUALITY GUIDELINES • Many thousands of guidelines exist • Important task is to identify high quality, up top date guidelines for local implementation • Main tool to use is the AGREE instrument • Alternatively, in the UK accreditation by NHS Evidence (www. evidence. nhs. uk ) is an indicator of high quality guideline development
GUIDELINES & INFORMATION PROFESSIONALS I IDENTIFYING GOOD QUALITY GUIDELINES • Many thousands of guidelines exist • Important task is to identify high quality, up top date guidelines for local implementation • Main tool to use is the AGREE instrument • Alternatively, in the UK accreditation by NHS Evidence (www. evidence. nhs. uk ) is an indicator of high quality guideline development
 THE AGREE INSTRUMENT • Quality of the guideline development process can be analysed using the AGREE instrument - a real world example of international collaboration • The AGREE Instrument has been translated into 7 European languages • The WHO has endorsed the AGREE Instrument and it is used as the basis of HS Evidence Accreditation • AGREE II has recently been launched and is being translated in to many languages BUT • Despite AGREE there are still issues in guideline development….
THE AGREE INSTRUMENT • Quality of the guideline development process can be analysed using the AGREE instrument - a real world example of international collaboration • The AGREE Instrument has been translated into 7 European languages • The WHO has endorsed the AGREE Instrument and it is used as the basis of HS Evidence Accreditation • AGREE II has recently been launched and is being translated in to many languages BUT • Despite AGREE there are still issues in guideline development….
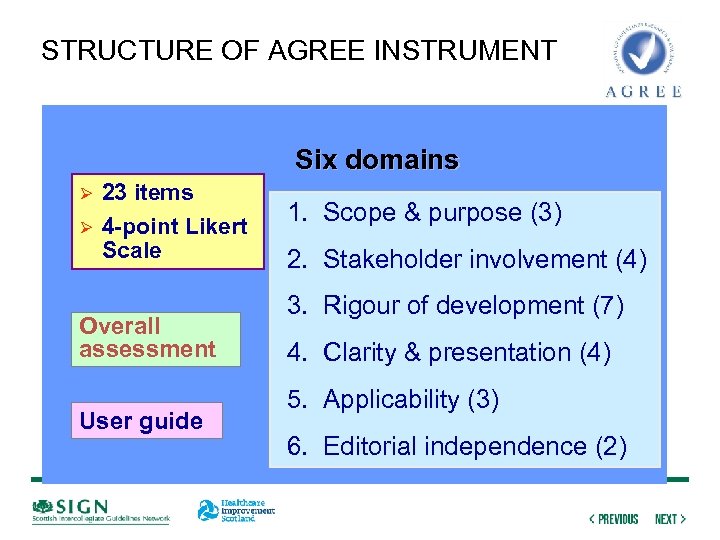 STRUCTURE OF AGREE INSTRUMENT Six domains Ø Ø 23 items 4 -point Likert Scale Overall assessment User guide 1. Scope & purpose (3) 2. Stakeholder involvement (4) 3. Rigour of development (7) 4. Clarity & presentation (4) 5. Applicability (3) 6. Editorial independence (2)
STRUCTURE OF AGREE INSTRUMENT Six domains Ø Ø 23 items 4 -point Likert Scale Overall assessment User guide 1. Scope & purpose (3) 2. Stakeholder involvement (4) 3. Rigour of development (7) 4. Clarity & presentation (4) 5. Applicability (3) 6. Editorial independence (2)
 ISSUES IN THE QUALITY OF CLINICAL GUIDELINES • • Quality of development process Conflicts of interest Multidisciplinary involvement Grading systems Resources required Adaptation / accreditation of others’ work Updating Etc…
ISSUES IN THE QUALITY OF CLINICAL GUIDELINES • • Quality of development process Conflicts of interest Multidisciplinary involvement Grading systems Resources required Adaptation / accreditation of others’ work Updating Etc…
 CONFLICTS OF INTEREST • AGREE criteria - conflicts of interest of guideline development members should be recorded. • It is not possible to completely eradicate competing interests • They need to be managed not demonised • Most important issue is making them explicit at all stages of the guideline development process • May be particular issues from patient interest groups
CONFLICTS OF INTEREST • AGREE criteria - conflicts of interest of guideline development members should be recorded. • It is not possible to completely eradicate competing interests • They need to be managed not demonised • Most important issue is making them explicit at all stages of the guideline development process • May be particular issues from patient interest groups
 MULTIDISCIPLINARY AND PATIENT INVOLVEMENT • AGREE criteria - the guideline development group should include individuals from all the relevant professional groups. AND - the patients' views and preferences should be sought • Multidisciplinary involvement promotes a sense of ownership and facilitates implementation • Many guideline developers have made this a centrepiece of their process, but not all • Scepticism about contributions from non specialists is still common • Even those that do may not do this that well…
MULTIDISCIPLINARY AND PATIENT INVOLVEMENT • AGREE criteria - the guideline development group should include individuals from all the relevant professional groups. AND - the patients' views and preferences should be sought • Multidisciplinary involvement promotes a sense of ownership and facilitates implementation • Many guideline developers have made this a centrepiece of their process, but not all • Scepticism about contributions from non specialists is still common • Even those that do may not do this that well…
 GRADING SYSTEMS • AGREE criteria - there should be an explicit link between the recommendations and the supporting evidence. And, the health benefits, side effects and risks should be considered in formulating the recommendations. • There a number of different grading systems available – all have advantages & disadvantages- and the choice of system remains controversial for many guideline developers
GRADING SYSTEMS • AGREE criteria - there should be an explicit link between the recommendations and the supporting evidence. And, the health benefits, side effects and risks should be considered in formulating the recommendations. • There a number of different grading systems available – all have advantages & disadvantages- and the choice of system remains controversial for many guideline developers
 LIMITED RESOURCES • Guideline development is expensive and time consuming • For example, the average time to develop a full clinical guideline in SIGN is around 29 months and can involve around 20 guideline development group members and the majority of SIGN staff at some stage • Likewise, keeping extant guidelines up to date is an increasing problem • As a result, guideline developers are looking for ways to reduce costs and duplication of effort • Adaptation of others’ guidelines offers one possible solution: – Reduce duplication of effort, especially for the systematic review portion of guideline – Potentially less time and fewer resources required than for de novo development
LIMITED RESOURCES • Guideline development is expensive and time consuming • For example, the average time to develop a full clinical guideline in SIGN is around 29 months and can involve around 20 guideline development group members and the majority of SIGN staff at some stage • Likewise, keeping extant guidelines up to date is an increasing problem • As a result, guideline developers are looking for ways to reduce costs and duplication of effort • Adaptation of others’ guidelines offers one possible solution: – Reduce duplication of effort, especially for the systematic review portion of guideline – Potentially less time and fewer resources required than for de novo development
 GUIDELINES & INFORMATION PROFESSIONALS II LITERATURE SEARCHING • Many guideline development agencies (including SIGN) use information professionals to identify the literature to be used in clinical guidelines • Role of the information professional is: – To work with healthcare professionals to produce PICO format questions to be addressed by the guideline – To produce explicit search strategies to identify relevant studies – To work with healthcare professionals to agree explicit inclusion/exclusion criteria to select studies for review – To run the searches, undertake sifting according to the inclusion / exclusion criteria – To manage all information used in the guideline development process
GUIDELINES & INFORMATION PROFESSIONALS II LITERATURE SEARCHING • Many guideline development agencies (including SIGN) use information professionals to identify the literature to be used in clinical guidelines • Role of the information professional is: – To work with healthcare professionals to produce PICO format questions to be addressed by the guideline – To produce explicit search strategies to identify relevant studies – To work with healthcare professionals to agree explicit inclusion/exclusion criteria to select studies for review – To run the searches, undertake sifting according to the inclusion / exclusion criteria – To manage all information used in the guideline development process
 GUIDELINES & INFORMATION PROFESSIONALS III PROVIDING CRITICAL APPRAISAL OF • At SIGN information professionals extraction from PUBLISHED PAPERS also undertake dataprofessionals, assess papers identified and, in collaboration with healthcare th quality of the papers reviewed • This information is presented in evidence tables which summarise all the evidence used to produce the recommendations • The increased role of information professionals has we believe had several important effects: – Reduced the burden on front line staff in guideline development and the time taken to produce guidelines – Streamlined the evidence management process – Improved the quality of the evidence review process
GUIDELINES & INFORMATION PROFESSIONALS III PROVIDING CRITICAL APPRAISAL OF • At SIGN information professionals extraction from PUBLISHED PAPERS also undertake dataprofessionals, assess papers identified and, in collaboration with healthcare th quality of the papers reviewed • This information is presented in evidence tables which summarise all the evidence used to produce the recommendations • The increased role of information professionals has we believe had several important effects: – Reduced the burden on front line staff in guideline development and the time taken to produce guidelines – Streamlined the evidence management process – Improved the quality of the evidence review process
 SIGN IN 2012 • Part of Healthcare Improvement Scotland’s Evidence & Technologies function, allowing better co-ordination of this work nationally • Emphasis is on revising existing guidelines rather than new topics • New process being introduced for ‘refreshing’ guidelines with a shorter timescales • To reduce clinical time requirement to develop guidelines, more work is done in house with data extrapolation and initial critical appraisal undertaken by SIGN staff • Guidelines are now produced in a variety of formats which support the interaction between patient and healthcare professional, including – Algorithms – Patient pathways – Smart phone applications
SIGN IN 2012 • Part of Healthcare Improvement Scotland’s Evidence & Technologies function, allowing better co-ordination of this work nationally • Emphasis is on revising existing guidelines rather than new topics • New process being introduced for ‘refreshing’ guidelines with a shorter timescales • To reduce clinical time requirement to develop guidelines, more work is done in house with data extrapolation and initial critical appraisal undertaken by SIGN staff • Guidelines are now produced in a variety of formats which support the interaction between patient and healthcare professional, including – Algorithms – Patient pathways – Smart phone applications
 OUTPUTS • SIGN no longer published hard copies of full guidelines • In addition to guidelines, the website includes: ü Links to SIGN apps ü SIGN Rockets – summaries of recommendations in one click ü Patient pathways ü Audit tools ü Editable specialty specific QRGs for antibiotic prophylaxis ü Facebook page ü Twitter coming soon. . • The website receives around 20 million hits every year
OUTPUTS • SIGN no longer published hard copies of full guidelines • In addition to guidelines, the website includes: ü Links to SIGN apps ü SIGN Rockets – summaries of recommendations in one click ü Patient pathways ü Audit tools ü Editable specialty specific QRGs for antibiotic prophylaxis ü Facebook page ü Twitter coming soon. . • The website receives around 20 million hits every year
 WHERE SIGN FITS IN I Quality Strategy Effective Ambition • The most appropriate treatments, interventions, support and services will be provided at the right time to everyone who will benefit, and wasteful or harmful variation will be eradicated • SIGN produces recommendations on the most appropriate treatments and interventions for people with clinical conditions • SIGN supports implementation by ensuring each guideline is accompanied by appropriate implementation support tools
WHERE SIGN FITS IN I Quality Strategy Effective Ambition • The most appropriate treatments, interventions, support and services will be provided at the right time to everyone who will benefit, and wasteful or harmful variation will be eradicated • SIGN produces recommendations on the most appropriate treatments and interventions for people with clinical conditions • SIGN supports implementation by ensuring each guideline is accompanied by appropriate implementation support tools
 WHERE SIGN FITS IN II Quality Strategy Patient Centred Ambition • There will be mutually beneficial partnerships between patients, their families and those delivering healthcare services which respect individual needs and values and which demonstrate compassion, continuity, clear communication and shared decision-making • SIGN works with patients and carers to ensure that SIGN guidelines meet their needs and contribute to shared decision making • SIGN produces materials for patients and carers in plain English to enhance communication and support shared decision making
WHERE SIGN FITS IN II Quality Strategy Patient Centred Ambition • There will be mutually beneficial partnerships between patients, their families and those delivering healthcare services which respect individual needs and values and which demonstrate compassion, continuity, clear communication and shared decision-making • SIGN works with patients and carers to ensure that SIGN guidelines meet their needs and contribute to shared decision making • SIGN produces materials for patients and carers in plain English to enhance communication and support shared decision making
 WHERE SIGN FITS IN III NHSScotland Efficiency & Productivity Framework • Seven Cost Reduction work streams, underpinned by the support and enablers, identify where there are further savings and quality improvements to be made: • • evidence based care preventative and early intervention outpatients, community and primary care acute flow and capacity management workforce productivity prescribing, procurement, support/shared services, and service redesign, innovation and transformation SIGN produces recommendations for evidence based care in primary, secondary and tertiary care including areas for disinvestment
WHERE SIGN FITS IN III NHSScotland Efficiency & Productivity Framework • Seven Cost Reduction work streams, underpinned by the support and enablers, identify where there are further savings and quality improvements to be made: • • evidence based care preventative and early intervention outpatients, community and primary care acute flow and capacity management workforce productivity prescribing, procurement, support/shared services, and service redesign, innovation and transformation SIGN produces recommendations for evidence based care in primary, secondary and tertiary care including areas for disinvestment
 THANK YOU Contact us at www. sign. ac. uk duncan. service@nhs. net sara. twaddle@nhs. net
THANK YOU Contact us at www. sign. ac. uk duncan. service@nhs. net sara. twaddle@nhs. net


