ЯМР спектроскопия 5 13С 2D.pptx
- Количество слайдов: 33
 ЯМР спектроскопия для химиков-органиков Научная школа-семинар
ЯМР спектроскопия для химиков-органиков Научная школа-семинар
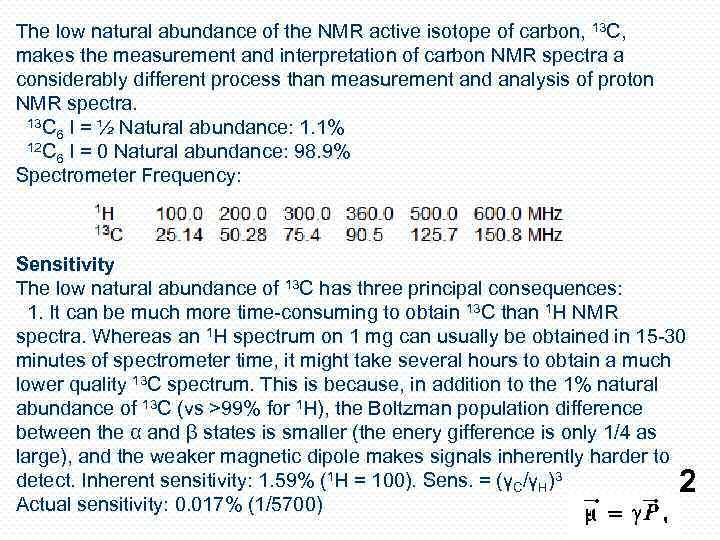 The low natural abundance of the NMR active isotope of carbon, 13 C, makes the measurement and interpretation of carbon NMR spectra a considerably different process than measurement and analysis of proton NMR spectra. 13 C 6 I = ½ Natural abundance: 1. 1% 12 C 6 I = 0 Natural abundance: 98. 9% Spectrometer Frequency: Sensitivity The low natural abundance of 13 C has three principal consequences: 1. It can be much more time-consuming to obtain 13 C than 1 H NMR spectra. Whereas an 1 H spectrum on 1 mg can usually be obtained in 15 -30 minutes of spectrometer time, it might take several hours to obtain a much lower quality 13 C spectrum. This is because, in addition to the 1% natural abundance of 13 C (vs >99% for 1 H), the Boltzman population difference between the α and β states is smaller (the enery gifference is only 1/4 as large), and the weaker magnetic dipole makes signals inherently harder to detect. Inherent sensitivity: 1. 59% (1 H = 100). Sens. = (γC/γH)3 Actual sensitivity: 0. 017% (1/5700) 2
The low natural abundance of the NMR active isotope of carbon, 13 C, makes the measurement and interpretation of carbon NMR spectra a considerably different process than measurement and analysis of proton NMR spectra. 13 C 6 I = ½ Natural abundance: 1. 1% 12 C 6 I = 0 Natural abundance: 98. 9% Spectrometer Frequency: Sensitivity The low natural abundance of 13 C has three principal consequences: 1. It can be much more time-consuming to obtain 13 C than 1 H NMR spectra. Whereas an 1 H spectrum on 1 mg can usually be obtained in 15 -30 minutes of spectrometer time, it might take several hours to obtain a much lower quality 13 C spectrum. This is because, in addition to the 1% natural abundance of 13 C (vs >99% for 1 H), the Boltzman population difference between the α and β states is smaller (the enery gifference is only 1/4 as large), and the weaker magnetic dipole makes signals inherently harder to detect. Inherent sensitivity: 1. 59% (1 H = 100). Sens. = (γC/γH)3 Actual sensitivity: 0. 017% (1/5700) 2
 2. The effects of 13 C nuclei on spectra of other nuclei (e. g. , 1 H, 19 F, 31 P) are very minor. Each proton signal is surrounded by 13 C satellites separated by 1 JC-H (typically 120 -150 Hz), each with an intensity of 0. 5% of the central peak. The central peak arises from the 98. 9% of 12 C which is NMR transparent. The 13 C satellites can be readily detected for sharp peaks. In addition to being useful for measuring proton-carbon couplings, these satellites can sometimes be used for measurement of JHH between equivalent protons. 3. Coupling between carbons (JCC) is not usually observed, because two adjacent 13 C nuclei occur in only 1. 1% of the carbons. There are thus 13 C satellites on the carbon peaks (each about 0. 5% of the intensity of the main peak), in the same way that there are 13 C satellites on proton spectra. The couplings can be measured directly with some difficulty by accumulating many scans on a very concentrated sample, but a better way is to use one of the multi-pulse 2 D experiments (e. g. , INADEQUATE) which nulls the central peaks due to adjacent 12 C atoms. Even so, large samples and long acquisition times are required . 3
2. The effects of 13 C nuclei on spectra of other nuclei (e. g. , 1 H, 19 F, 31 P) are very minor. Each proton signal is surrounded by 13 C satellites separated by 1 JC-H (typically 120 -150 Hz), each with an intensity of 0. 5% of the central peak. The central peak arises from the 98. 9% of 12 C which is NMR transparent. The 13 C satellites can be readily detected for sharp peaks. In addition to being useful for measuring proton-carbon couplings, these satellites can sometimes be used for measurement of JHH between equivalent protons. 3. Coupling between carbons (JCC) is not usually observed, because two adjacent 13 C nuclei occur in only 1. 1% of the carbons. There are thus 13 C satellites on the carbon peaks (each about 0. 5% of the intensity of the main peak), in the same way that there are 13 C satellites on proton spectra. The couplings can be measured directly with some difficulty by accumulating many scans on a very concentrated sample, but a better way is to use one of the multi-pulse 2 D experiments (e. g. , INADEQUATE) which nulls the central peaks due to adjacent 12 C atoms. Even so, large samples and long acquisition times are required . 3
 Decoupling Most 13 C NMR spectra are very complex. The methyl carbon of an ethoxy group will appear as a large quartet, with each line further split into triplets. Even in fairly simple molecules each carbon may be coupled to a number of different protons. In complicated molecules, these multiplets overlap badly, and may be impossible to analyze. 1 JCH = 100 -250 Hz 2, 3 JCH = 2 -10 Hz To simplify 13 C spectra, we usually use some form of broadband decoupling (noise decoupling) to remove the effect of proton couplings. This also dramatically increases signal intensity, since now all carbons appear as singlets (assuming absence of other spin 1/2 nuclei like 31 P or 19 F). The increase is actually greater by a factor of 2 -3 than would be predicted on the basis of simply combining the 13 C multiplet intensities because the Nuclear Overhauser Effect causes additional increases in signal intensity. 4
Decoupling Most 13 C NMR spectra are very complex. The methyl carbon of an ethoxy group will appear as a large quartet, with each line further split into triplets. Even in fairly simple molecules each carbon may be coupled to a number of different protons. In complicated molecules, these multiplets overlap badly, and may be impossible to analyze. 1 JCH = 100 -250 Hz 2, 3 JCH = 2 -10 Hz To simplify 13 C spectra, we usually use some form of broadband decoupling (noise decoupling) to remove the effect of proton couplings. This also dramatically increases signal intensity, since now all carbons appear as singlets (assuming absence of other spin 1/2 nuclei like 31 P or 19 F). The increase is actually greater by a factor of 2 -3 than would be predicted on the basis of simply combining the 13 C multiplet intensities because the Nuclear Overhauser Effect causes additional increases in signal intensity. 4
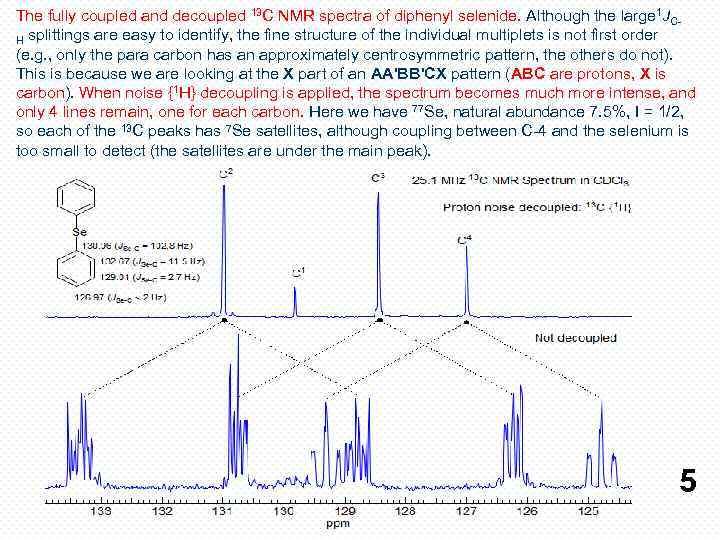 The fully coupled and decoupled 13 C NMR spectra of diphenyl selenide. Although the large 1 JCH splittings are easy to identify, the fine structure of the individual multiplets is not first order (e. g. , only the para carbon has an approximately centrosymmetric pattern, the others do not). This is because we are looking at the X part of an AA'BB'CX pattern (АВС are protons, X is carbon). When noise {1 H} decoupling is applied, the spectrum becomes much more intense, and only 4 lines remain, one for each carbon. Here we have 77 Se, natural abundance 7. 5%, I = 1/2, so each of the 13 C peaks has 7 Se satellites, although coupling between C-4 and the selenium is too small to detect (the satellites are under the main peak). 5
The fully coupled and decoupled 13 C NMR spectra of diphenyl selenide. Although the large 1 JCH splittings are easy to identify, the fine structure of the individual multiplets is not first order (e. g. , only the para carbon has an approximately centrosymmetric pattern, the others do not). This is because we are looking at the X part of an AA'BB'CX pattern (АВС are protons, X is carbon). When noise {1 H} decoupling is applied, the spectrum becomes much more intense, and only 4 lines remain, one for each carbon. Here we have 77 Se, natural abundance 7. 5%, I = 1/2, so each of the 13 C peaks has 7 Se satellites, although coupling between C-4 and the selenium is too small to detect (the satellites are under the main peak). 5
 DEPT (Distortionless Enhancement of Polarization Transfer): The DEPT technique has proven superior to others in providing information on attached protons reliably, efficiently and with high selectivity. It is a proton-carbon polarization transfer method, so DEPT spectra are actually more sensitive than normal acquisitions. A set of spectra with pulse delays adjusted for π/2 (DEPT-90) and 3π/4 (DEPT-135) are taken. The DEPT-90 spectrum shows only CH carbons, the DEPT-135 shows positive CH 3 and CH, and negative CH 2 signals. It is important to understand that the appearance of positive and negative signals can be reversed by phasing, so it is necessary to have some way of determining whether the spectrum has been phased for CH 2 positive or negative. Quaternary carbons are invisible 6
DEPT (Distortionless Enhancement of Polarization Transfer): The DEPT technique has proven superior to others in providing information on attached protons reliably, efficiently and with high selectivity. It is a proton-carbon polarization transfer method, so DEPT spectra are actually more sensitive than normal acquisitions. A set of spectra with pulse delays adjusted for π/2 (DEPT-90) and 3π/4 (DEPT-135) are taken. The DEPT-90 spectrum shows only CH carbons, the DEPT-135 shows positive CH 3 and CH, and negative CH 2 signals. It is important to understand that the appearance of positive and negative signals can be reversed by phasing, so it is necessary to have some way of determining whether the spectrum has been phased for CH 2 positive or negative. Quaternary carbons are invisible 6
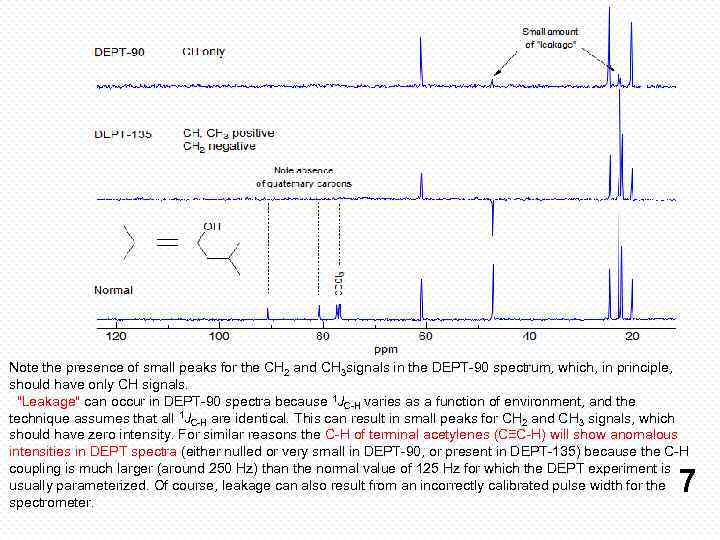 Note the presence of small peaks for the CH 2 and CH 3 signals in the DEPT-90 spectrum, which, in principle, should have only CH signals. "Leakage" can occur in DEPT-90 spectra because 1 JC-H varies as a function of environment, and the technique assumes that all 1 JC-H are identical. This can result in small peaks for CH 2 and CH 3 signals, which should have zero intensity. For similar reasons the C-H of terminal acetylenes (C≡C-H) will show anomalous intensities in DEPT spectra (either nulled or very small in DEPT-90, or present in DEPT-135) because the C-H coupling is much larger (around 250 Hz) than the normal value of 125 Hz for which the DEPT experiment is usually parameterized. Of course, leakage can also result from an incorrectly calibrated pulse width for the spectrometer. 7
Note the presence of small peaks for the CH 2 and CH 3 signals in the DEPT-90 spectrum, which, in principle, should have only CH signals. "Leakage" can occur in DEPT-90 spectra because 1 JC-H varies as a function of environment, and the technique assumes that all 1 JC-H are identical. This can result in small peaks for CH 2 and CH 3 signals, which should have zero intensity. For similar reasons the C-H of terminal acetylenes (C≡C-H) will show anomalous intensities in DEPT spectra (either nulled or very small in DEPT-90, or present in DEPT-135) because the C-H coupling is much larger (around 250 Hz) than the normal value of 125 Hz for which the DEPT experiment is usually parameterized. Of course, leakage can also result from an incorrectly calibrated pulse width for the spectrometer. 7
 8
8
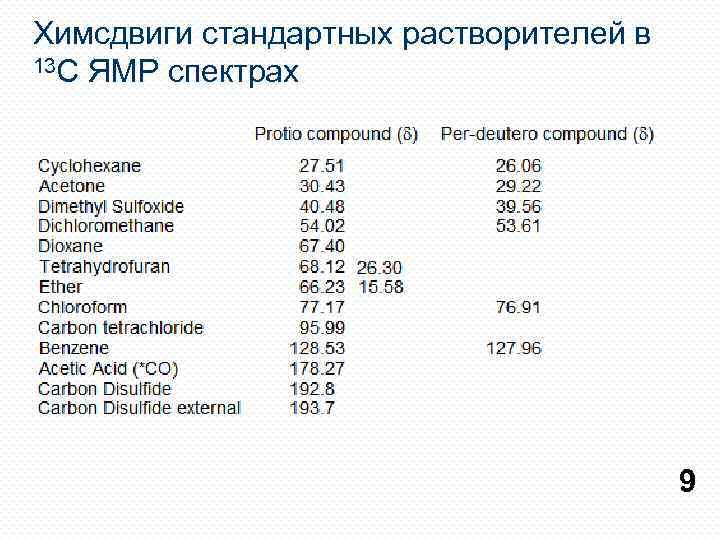 Химсдвиги стандартных растворителей в 13 С ЯМР спектрах 9
Химсдвиги стандартных растворителей в 13 С ЯМР спектрах 9
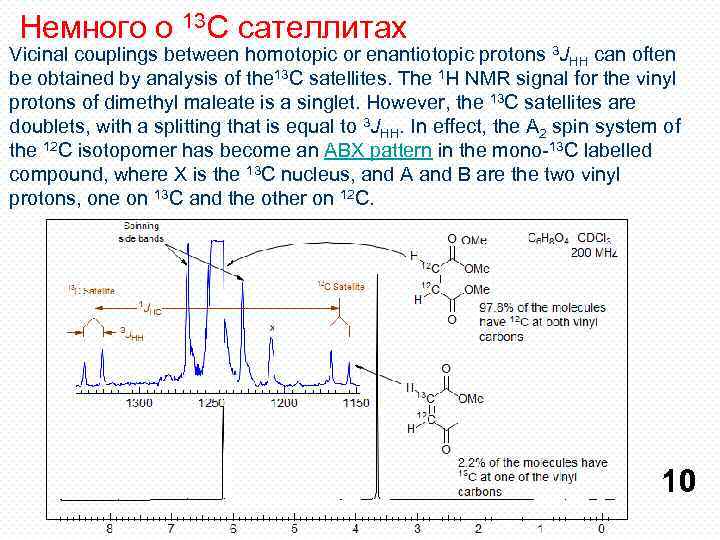 Немного о 13 С сателлитах Vicinal couplings between homotopic or enantiotopic protons 3 JHH can often be obtained by analysis of the 13 C satellites. The 1 H NMR signal for the vinyl protons of dimethyl maleate is a singlet. However, the 13 C satellites are doublets, with a splitting that is equal to 3 JHH. In effect, the A 2 spin system of the 12 C isotopomer has become an ABX pattern in the mono-13 C labelled compound, where X is the 13 C nucleus, and A and B are the two vinyl protons, one on 13 C and the other on 12 C. 10
Немного о 13 С сателлитах Vicinal couplings between homotopic or enantiotopic protons 3 JHH can often be obtained by analysis of the 13 C satellites. The 1 H NMR signal for the vinyl protons of dimethyl maleate is a singlet. However, the 13 C satellites are doublets, with a splitting that is equal to 3 JHH. In effect, the A 2 spin system of the 12 C isotopomer has become an ABX pattern in the mono-13 C labelled compound, where X is the 13 C nucleus, and A and B are the two vinyl protons, one on 13 C and the other on 12 C. 10
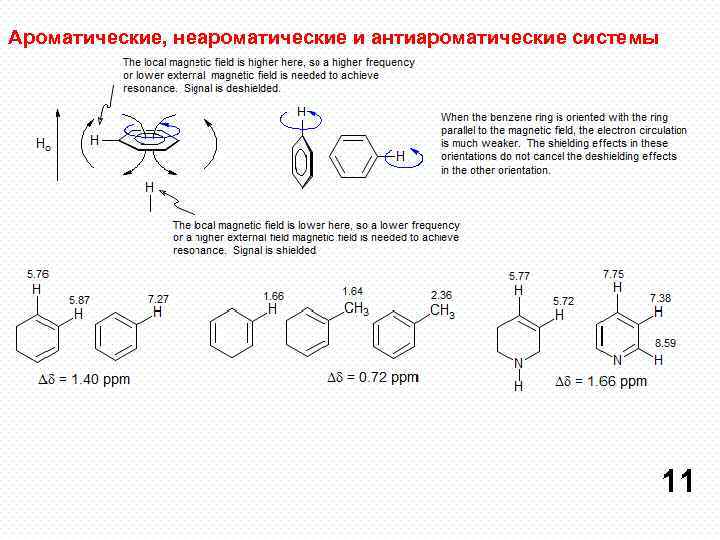 Ароматические, неароматические и антиароматические системы 11
Ароматические, неароматические и антиароматические системы 11
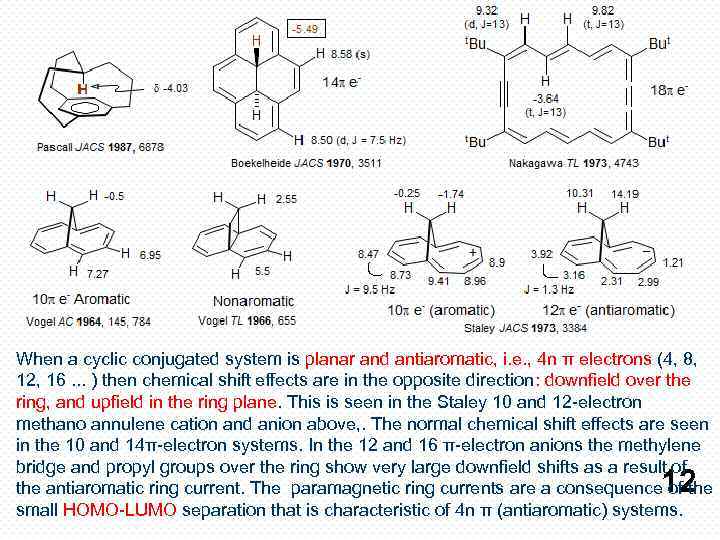 When a cyclic conjugated system is planar and antiaromatic, i. e. , 4 n π electrons (4, 8, 12, 16. . . ) then chemical shift effects are in the opposite direction: downfield over the ring, and upfield in the ring plane. This is seen in the Staley 10 and 12 -electron methano annulene cation and anion above, . The normal chemical shift effects are seen in the 10 and 14π-electron systems. In the 12 and 16 π-electron anions the methylene bridge and propyl groups over the ring show very large downfield shifts as a result of the antiaromatic ring current. The paramagnetic ring currents are a consequence of the small HOMO-LUMO separation that is characteristic of 4 n π (antiaromatic) systems. 12
When a cyclic conjugated system is planar and antiaromatic, i. e. , 4 n π electrons (4, 8, 12, 16. . . ) then chemical shift effects are in the opposite direction: downfield over the ring, and upfield in the ring plane. This is seen in the Staley 10 and 12 -electron methano annulene cation and anion above, . The normal chemical shift effects are seen in the 10 and 14π-electron systems. In the 12 and 16 π-electron anions the methylene bridge and propyl groups over the ring show very large downfield shifts as a result of the antiaromatic ring current. The paramagnetic ring currents are a consequence of the small HOMO-LUMO separation that is characteristic of 4 n π (antiaromatic) systems. 12
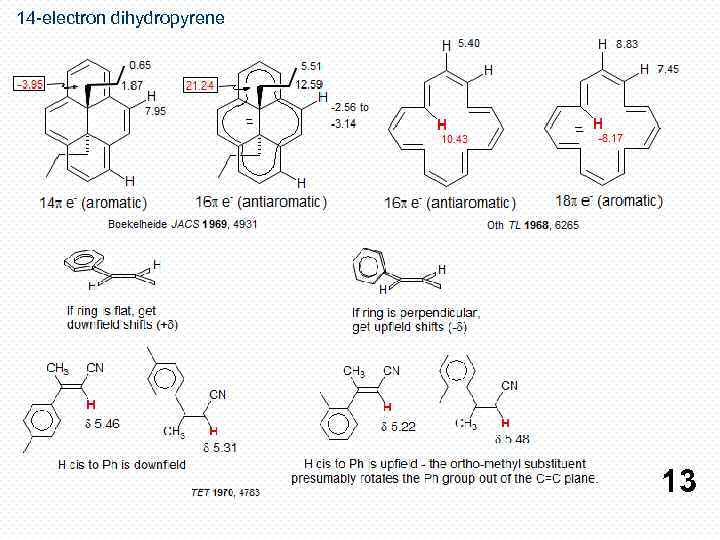 14 -electron dihydropyrene 13
14 -electron dihydropyrene 13
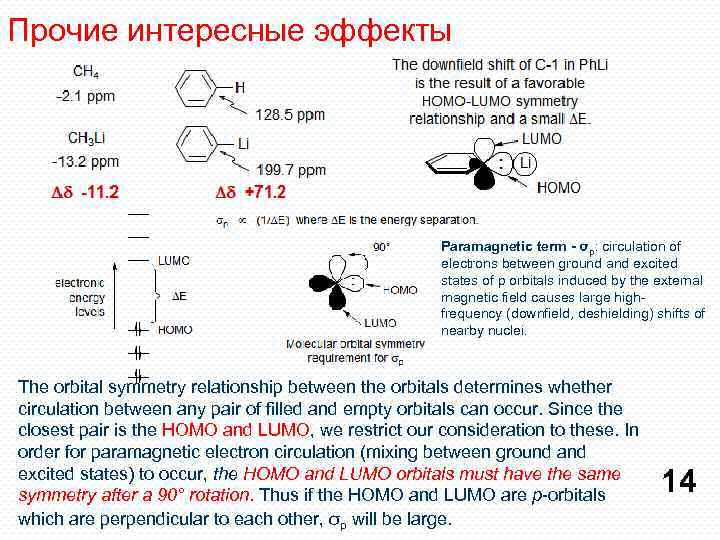 Прочие интересные эффекты Paramagnetic term - σp: circulation of electrons between ground and excited states of p orbitals induced by the external magnetic field causes large highfrequency (downfield, deshielding) shifts of nearby nuclei. The orbital symmetry relationship between the orbitals determines whether circulation between any pair of filled and empty orbitals can occur. Since the closest pair is the HOMO and LUMO, we restrict our consideration to these. In order for paramagnetic electron circulation (mixing between ground and excited states) to occur, the HOMO and LUMO orbitals must have the same symmetry after a 90° rotation. Thus if the HOMO and LUMO are p-orbitals which are perpendicular to each other, σp will be large. 14
Прочие интересные эффекты Paramagnetic term - σp: circulation of electrons between ground and excited states of p orbitals induced by the external magnetic field causes large highfrequency (downfield, deshielding) shifts of nearby nuclei. The orbital symmetry relationship between the orbitals determines whether circulation between any pair of filled and empty orbitals can occur. Since the closest pair is the HOMO and LUMO, we restrict our consideration to these. In order for paramagnetic electron circulation (mixing between ground and excited states) to occur, the HOMO and LUMO orbitals must have the same symmetry after a 90° rotation. Thus if the HOMO and LUMO are p-orbitals which are perpendicular to each other, σp will be large. 14
 15
15
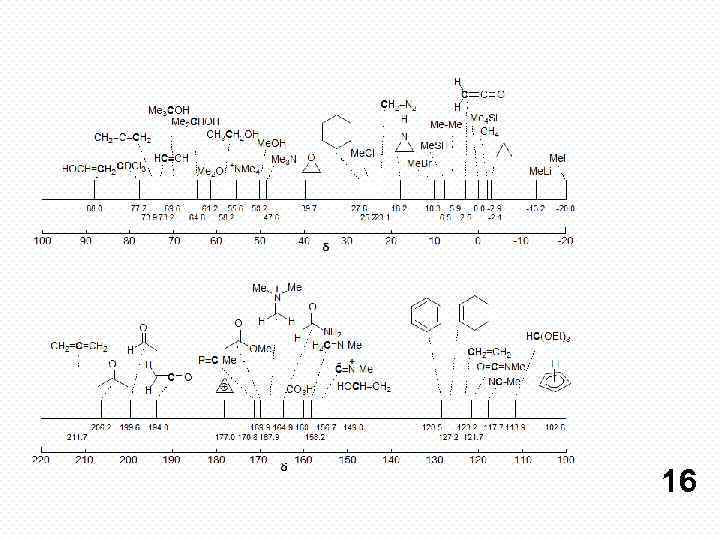 16
16
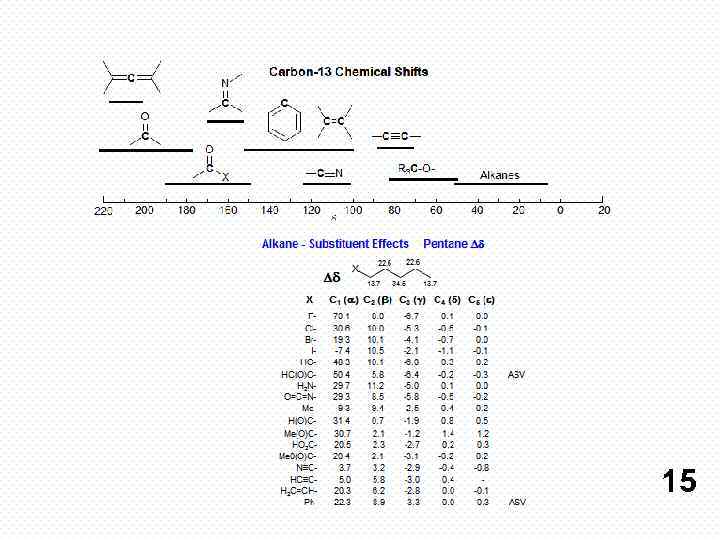
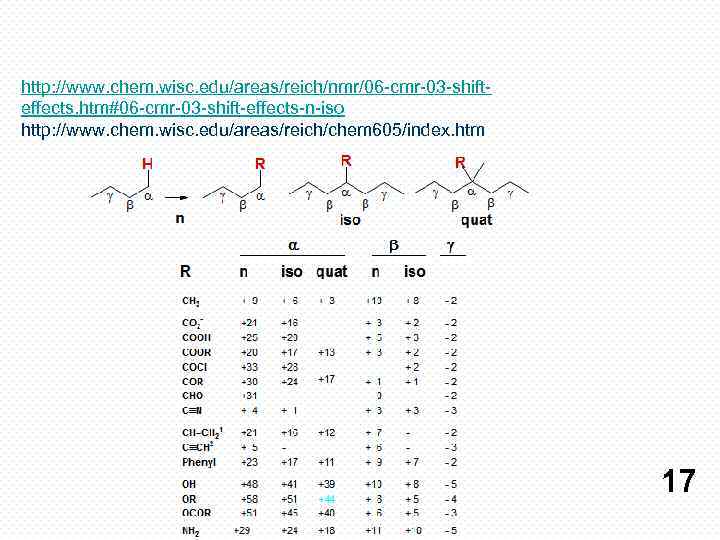 http: //www. chem. wisc. edu/areas/reich/nmr/06 -cmr-03 -shifteffects. htm#06 -cmr-03 -shift-effects-n-iso http: //www. chem. wisc. edu/areas/reich/chem 605/index. htm 17
http: //www. chem. wisc. edu/areas/reich/nmr/06 -cmr-03 -shifteffects. htm#06 -cmr-03 -shift-effects-n-iso http: //www. chem. wisc. edu/areas/reich/chem 605/index. htm 17
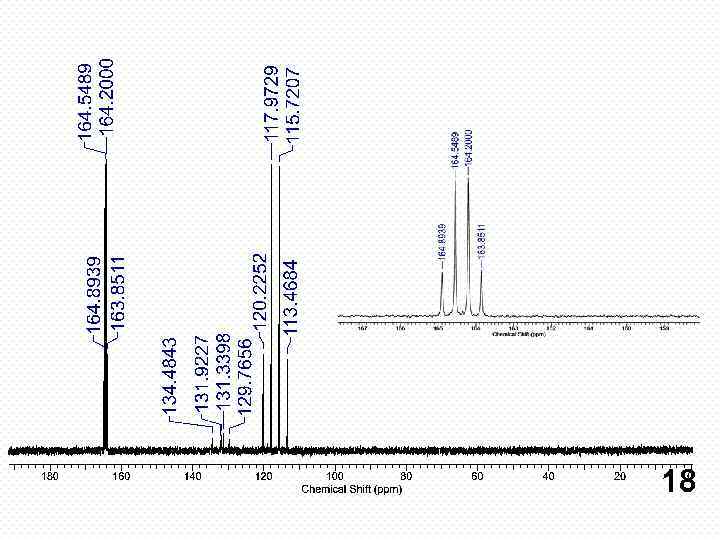 18
18
 2 D ЯМР эксперименты • в общем выявляют спиновую связь между ядрами (гомо/гетеро) в пределах одного эксперимента • COSY • HSQC (heteronuclear single quantum correlation) • HMBC (Heteronuclear Multiple Bond Correlation) • INADEQUATE 19
2 D ЯМР эксперименты • в общем выявляют спиновую связь между ядрами (гомо/гетеро) в пределах одного эксперимента • COSY • HSQC (heteronuclear single quantum correlation) • HMBC (Heteronuclear Multiple Bond Correlation) • INADEQUATE 19
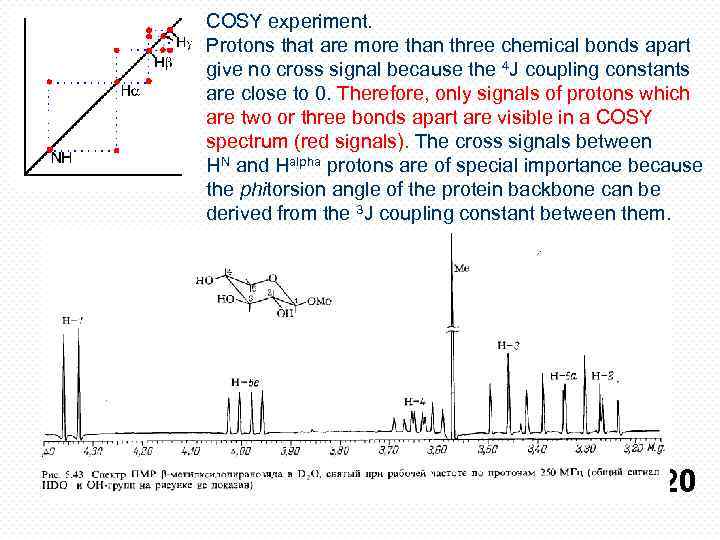 COSY experiment. Protons that are more than three chemical bonds apart give no cross signal because the 4 J coupling constants are close to 0. Therefore, only signals of protons which are two or three bonds apart are visible in a COSY spectrum (red signals). The cross signals between HN and Halpha protons are of special importance because the phitorsion angle of the protein backbone can be derived from the 3 J coupling constant between them. 20
COSY experiment. Protons that are more than three chemical bonds apart give no cross signal because the 4 J coupling constants are close to 0. Therefore, only signals of protons which are two or three bonds apart are visible in a COSY spectrum (red signals). The cross signals between HN and Halpha protons are of special importance because the phitorsion angle of the protein backbone can be derived from the 3 J coupling constant between them. 20
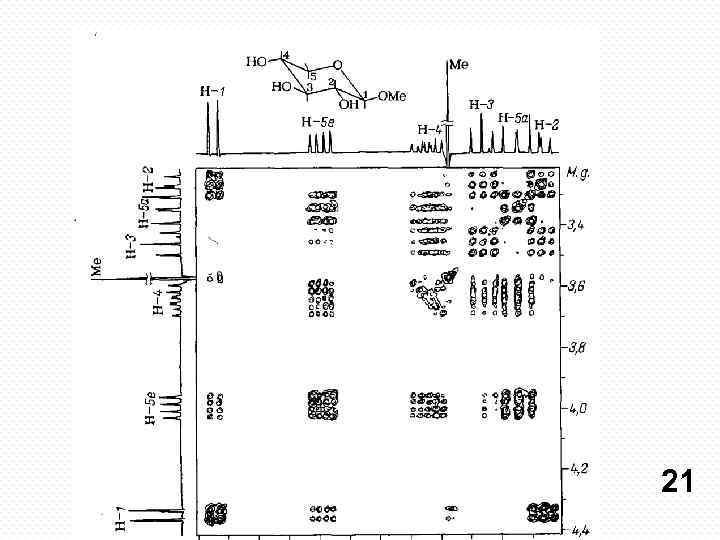 21
21
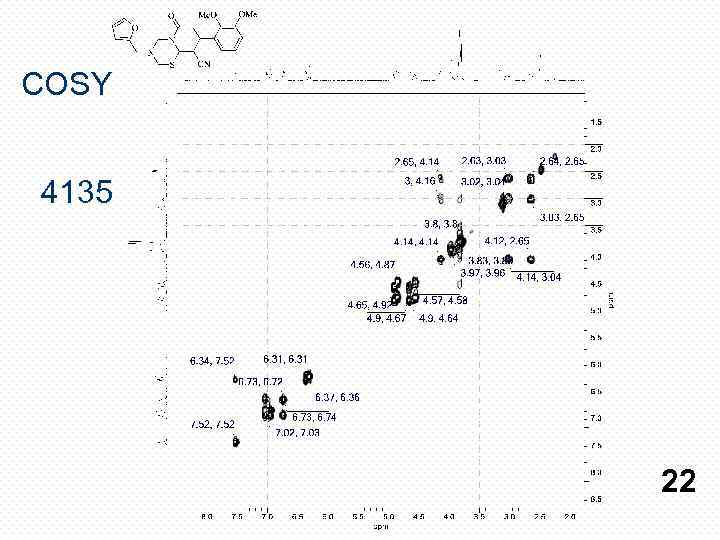 COSY 4135 22
COSY 4135 22
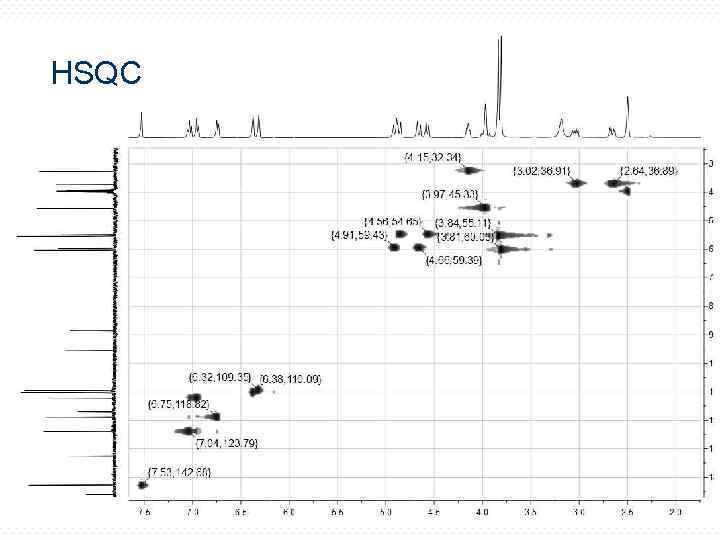 HSQC 23
HSQC 23
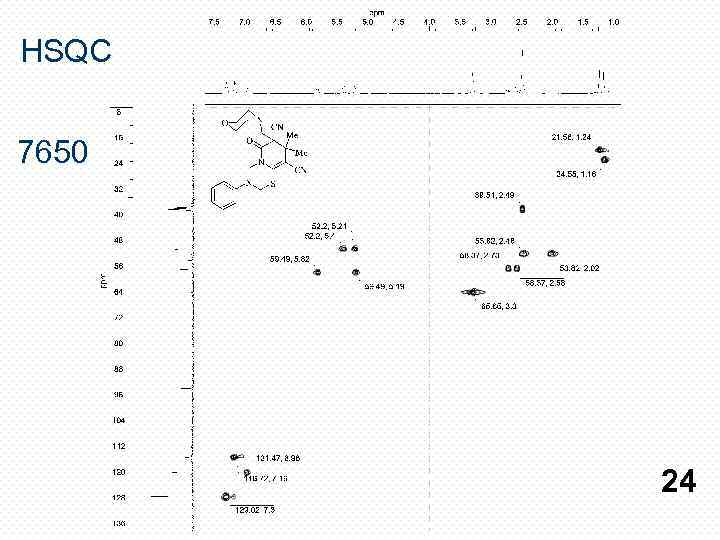 HSQC 7650 24
HSQC 7650 24
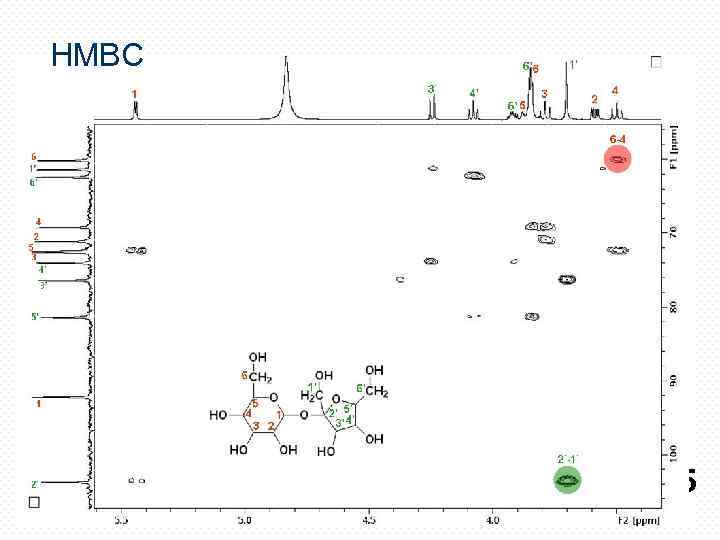 HMBC 25
HMBC 25
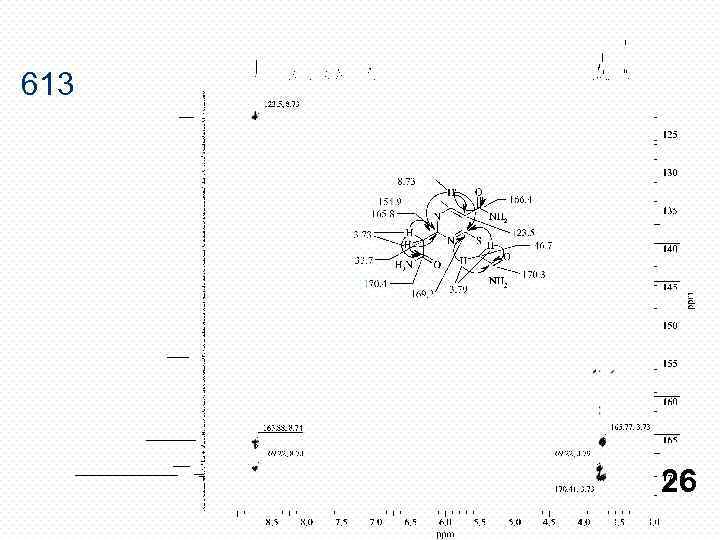 613 26
613 26
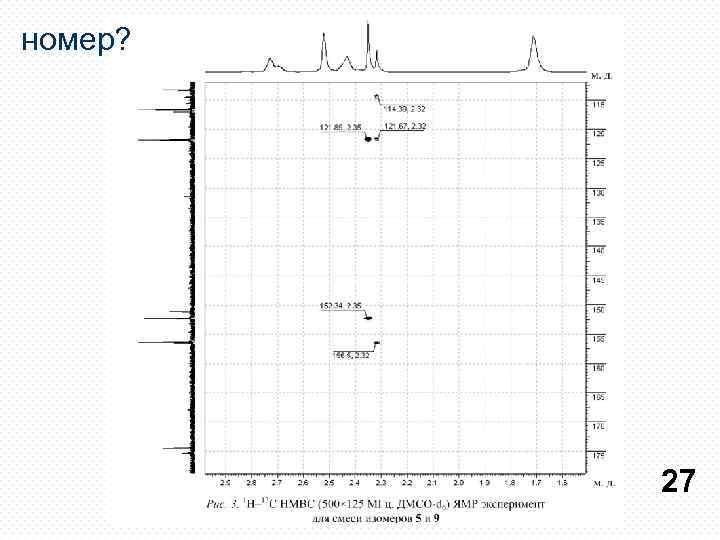 номер? 27
номер? 27
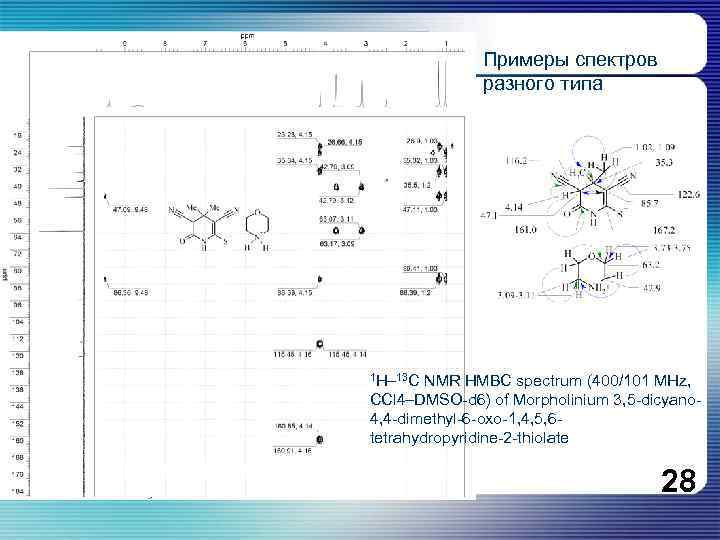 Примеры спектров разного типа 1 H– 13 C NMR HMBC spectrum (400/101 MHz, CCl 4–DMSO-d 6) of Morpholinium 3, 5 -dicyano 4, 4 -dimethyl-6 -oxo-1, 4, 5, 6 tetrahydropyridine-2 -thiolate 28
Примеры спектров разного типа 1 H– 13 C NMR HMBC spectrum (400/101 MHz, CCl 4–DMSO-d 6) of Morpholinium 3, 5 -dicyano 4, 4 -dimethyl-6 -oxo-1, 4, 5, 6 tetrahydropyridine-2 -thiolate 28
 2 D 13 C-13 C INADEQUATE (Incredible Natural Abundance Doubl. E QUAntum Transfer Experiment) is useful for determining which signals arise from neighboring carbons. However, it is very insensitive as 0. 01% of the carbons are excited at natural abundance. Use this experiment as a last resort when all else fails. If you can enrich the sample to about 10% 13 C then the INADEQUATE experiment can be run with normal sample concentrations. At natural abundance, it is recommended to use between 100 and 500 mg of sample. If it will not completely dissolve in 0. 8 m. L of solvent then use a 10 mm NMR tube and 2. 5 m. L of solvent. A 2 D INADEQUATE spectrum yields one bond correlations via spin-spin coupling. There are no diagonal signals. Signals with similar chemical shifts loose sensitivity due to second order coupling. The one-bond coupling constants can be used to estimate carbon-carbon bond order. The constants are usually 35 to 45 for a single bond about 65 Hz for a double bond are increased by electronegative substituents. If the only purpose is to measure coupling constants then 1 D-INADEQUATE is a more suitable experiment. 29
2 D 13 C-13 C INADEQUATE (Incredible Natural Abundance Doubl. E QUAntum Transfer Experiment) is useful for determining which signals arise from neighboring carbons. However, it is very insensitive as 0. 01% of the carbons are excited at natural abundance. Use this experiment as a last resort when all else fails. If you can enrich the sample to about 10% 13 C then the INADEQUATE experiment can be run with normal sample concentrations. At natural abundance, it is recommended to use between 100 and 500 mg of sample. If it will not completely dissolve in 0. 8 m. L of solvent then use a 10 mm NMR tube and 2. 5 m. L of solvent. A 2 D INADEQUATE spectrum yields one bond correlations via spin-spin coupling. There are no diagonal signals. Signals with similar chemical shifts loose sensitivity due to second order coupling. The one-bond coupling constants can be used to estimate carbon-carbon bond order. The constants are usually 35 to 45 for a single bond about 65 Hz for a double bond are increased by electronegative substituents. If the only purpose is to measure coupling constants then 1 D-INADEQUATE is a more suitable experiment. 29
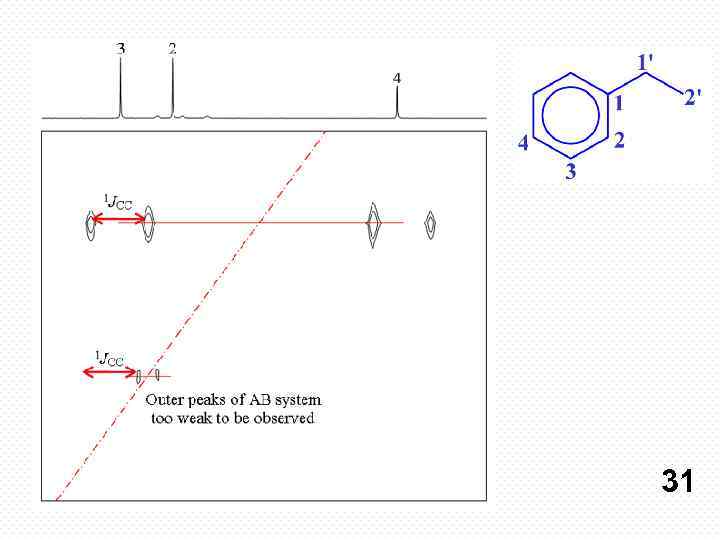
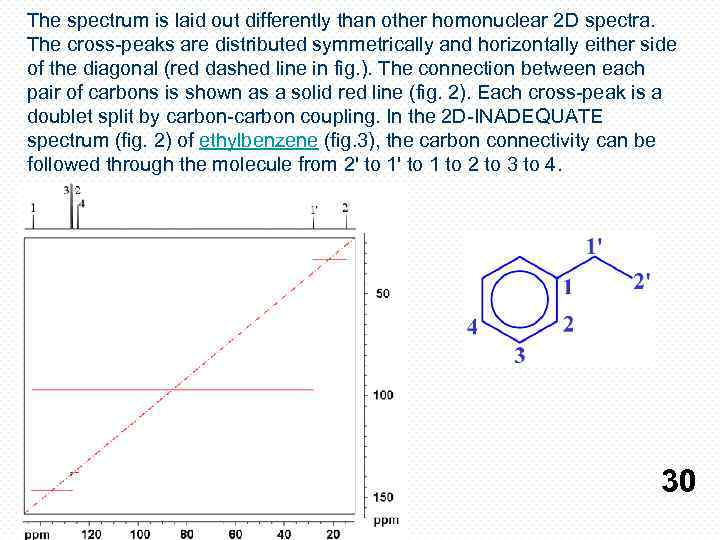 The spectrum is laid out differently than other homonuclear 2 D spectra. The cross-peaks are distributed symmetrically and horizontally either side of the diagonal (red dashed line in fig. ). The connection between each pair of carbons is shown as a solid red line (fig. 2). Each cross-peak is a doublet split by carbon-carbon coupling. In the 2 D-INADEQUATE spectrum (fig. 2) of ethylbenzene (fig. 3), the carbon connectivity can be followed through the molecule from 2' to 1 to 2 to 3 to 4. 30
The spectrum is laid out differently than other homonuclear 2 D spectra. The cross-peaks are distributed symmetrically and horizontally either side of the diagonal (red dashed line in fig. ). The connection between each pair of carbons is shown as a solid red line (fig. 2). Each cross-peak is a doublet split by carbon-carbon coupling. In the 2 D-INADEQUATE spectrum (fig. 2) of ethylbenzene (fig. 3), the carbon connectivity can be followed through the molecule from 2' to 1 to 2 to 3 to 4. 30
 31
31
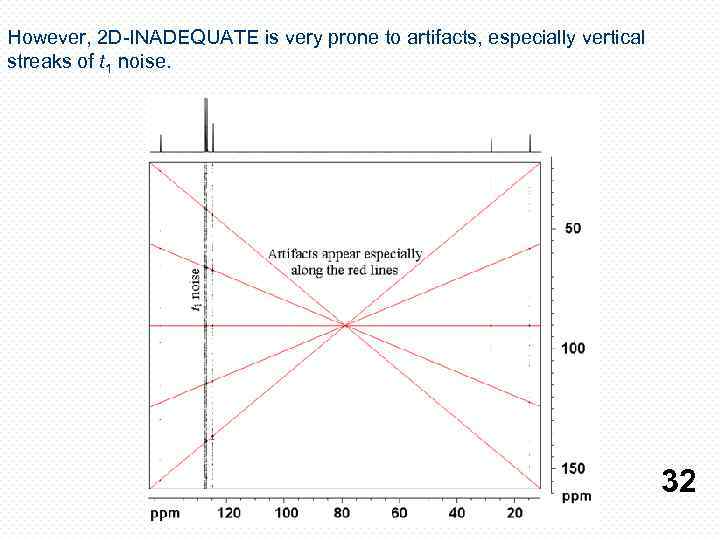 However, 2 D-INADEQUATE is very prone to artifacts, especially vertical streaks of t 1 noise. 32
However, 2 D-INADEQUATE is very prone to artifacts, especially vertical streaks of t 1 noise. 32
 Thanks for your patience and attention 33
Thanks for your patience and attention 33


