fbbb8aaee61b554663c038943b2b204c.ppt
- Количество слайдов: 10

y=A/x Boyle’s Law Pressure = A PV = constant Volume P 1 V 1 = P 2 V 2 Inverse relationship ↑P ↓V ↓P ↑V

Practice Problem V 1 P 1 A gas occupies a volume of 3. 86 L at 0. 750 atm. At what pressure will the volume be 4. 86 L? ? P 2 V 2 P 1 V 1 = P 2 V 2 (0. 750 atm)(3. 86 L) = P 2(4. 86 L) (0. 750 atm)(3. 86 L) = P 2 = 0. 596 atm (4. 86 L)
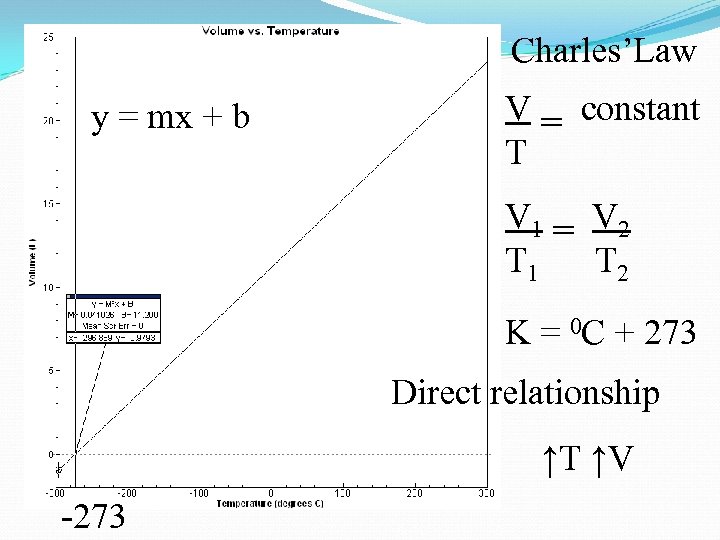
Charles’Law y = mx + b V = constant T V 1 = V 2 T 1 T 2 K = 0 C + 273 Direct relationship ↑T ↑V -273

Practice Problem T 1 V 1 A 4. 50 L container of nitrogen gas at 28. 0 0 C is heated to 56. 0 0 C. Assuming the volume of the container can vary, what is the new volume of the gas? T 2 ? V 2 V 1 = V 2 T 1 T 2 K = 0 C + 273 4. 50 L = V 2 301 K 329 K V 2 = 4. 92 L

y = mx + b Gay-Lussac’s Law P = constant T P 1 = P 2 T 1 T 2 K = 0 C + 273 Direct relationship -273 0 C ↑T ↑P

Practice Problem T 1 A gas cylinder contains 40. 0 L of gas at 45. 0 0 C and has a pressure of 650. torr. What will the pressure be if the temperature is changed to 100. 0 C? ? P 2 P 1 = P 2 T 1 T 2 K = 0 C + 273 T 2 650. torr = P 2 318 K 373 K P 2 = 762 torr
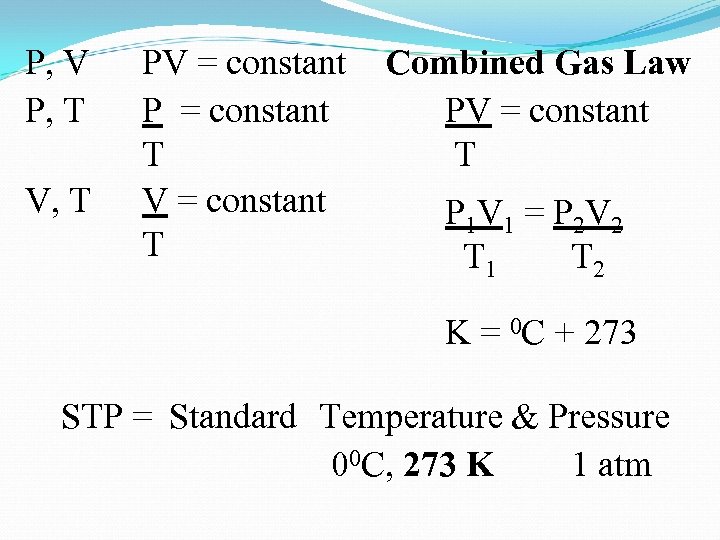
P, V P, T V, T PV = constant P = constant T V = constant T Combined Gas Law PV = constant T P 1 V 1 = P 2 V 2 T 1 T 2 K = 0 C + 273 STP = Standard Temperature & Pressure 00 C, 273 K 1 atm

Practice Problem V 1 T 1 P 1 15. 00 L of gas at 45. 0 0 C and 800. torr is heated to 400. 0 C, and the pressure changed to 300. torr. What is the new volume? P 2 T 2 ? V 2 P 1 V 1 = P 2 V 2 T 1 T 2 K = 0 C + 273 (800. torr)(15. 00 L) = (300. torr) V 2 318 K 673 K V 2 = 84. 7 L

Practice Problem ? T 2 V 1 To what temperature must 5. 00 L of oxygen at 50. 0 C and 600. torr be heated in order to have a volume of 10. 0 L and a pressure of 800. torr? T 1 P 1 V 2 P 1 V 1 = P 2 V 2 T 1 T 2 K = 0 C + 273 (600. torr)(5. 00 L) = (800. torr)(10. 0 L) 323 K T 2 = 861 K = 588 0 C

Do problems: 1 & 2 4 -6 8&9 11& 12 pg 443 pg 446 pg 448 pg 450
fbbb8aaee61b554663c038943b2b204c.ppt