9f3eee66c76d7fe4c14570f35ed2152a.ppt
- Количество слайдов: 44

Xeloda and Xeloda-based combinations for the first-line treatment of MCRC Chris Twelves University of Leeds and Bradford NHS Trust UK
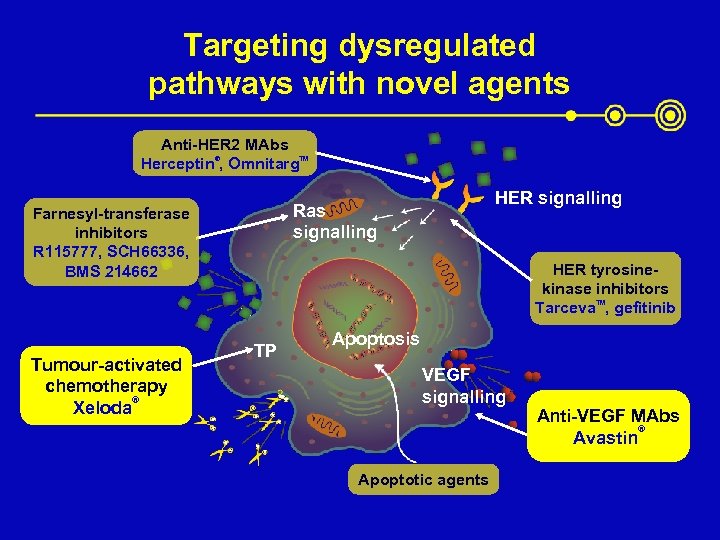
Targeting dysregulated pathways with novel agents Anti-HER 2 MAbs Herceptin®, Omnitarg. TM Tumour-activated chemotherapy ® Xeloda HER signalling Ras signalling Farnesyl-transferase inhibitors R 115777, SCH 66336, BMS 214662 HER tyrosinekinase inhibitors Tarceva. TM, gefitinib TP Apoptosis VEGF signalling Apoptotic agents Anti-VEGF MAbs ® Avastin
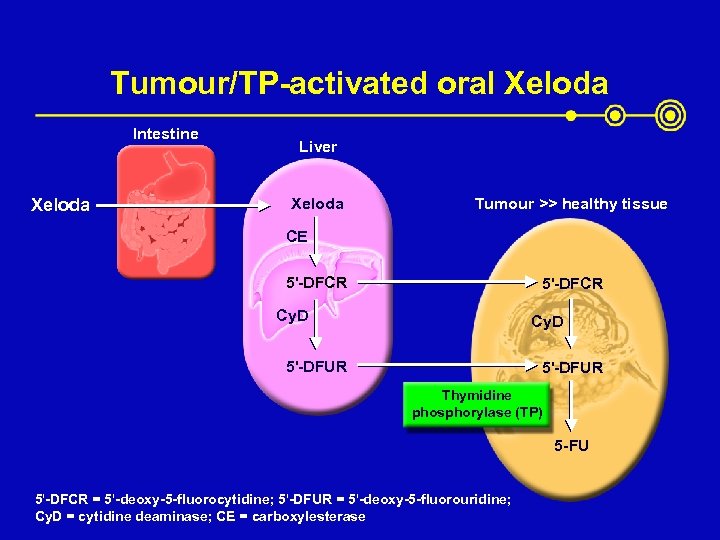
Tumour/TP-activated oral Xeloda Intestine Xeloda Liver Xeloda Tumour >> healthy tissue CE 5'-DFCR Cy. D 5'-DFUR Thymidine phosphorylase (TP) 5 -FU 5'-DFCR = 5'-deoxy-5 -fluorocytidine; 5'-DFUR = 5'-deoxy-5 -fluorouridine; Cy. D = cytidine deaminase; CE = carboxylesterase
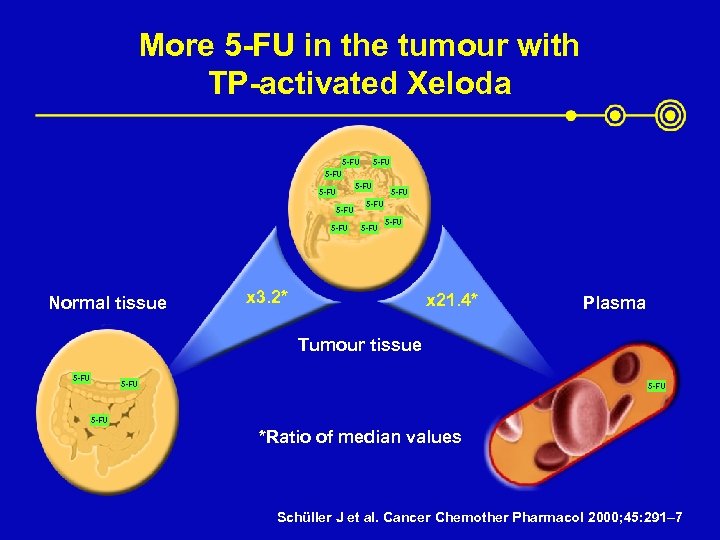
More 5 -FU in the tumour with TP-activated Xeloda 5 -FU 5 -FU 5 -FU Normal tissue x 3. 2* x 21. 4* Plasma Tumour tissue 5 -FU *Ratio of median values Schüller J et al. Cancer Chemother Pharmacol 2000; 45: 291– 7
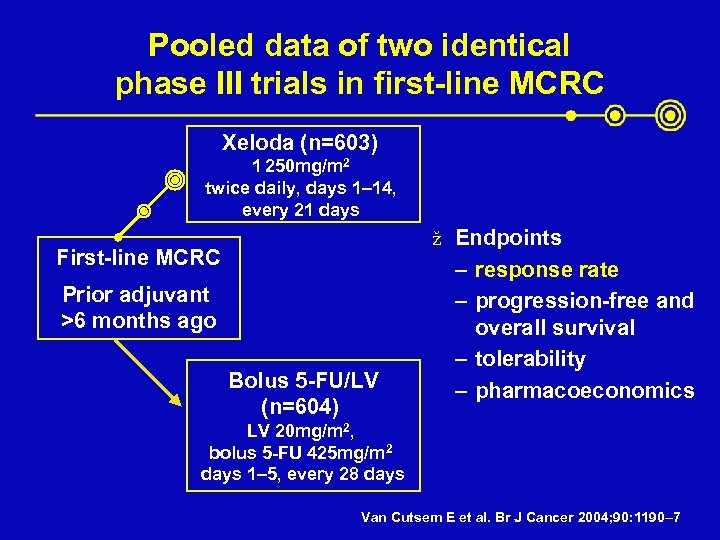
Pooled data of two identical phase III trials in first-line MCRC Xeloda (n=603) 1 250 mg/m 2 twice daily, days 1– 14, every 21 days ž Endpoints First-line MCRC Prior adjuvant >6 months ago Bolus 5 -FU/LV (n=604) – response rate – progression-free and overall survival – tolerability – pharmacoeconomics LV 20 mg/m 2, bolus 5 -FU 425 mg/m 2 days 1– 5, every 28 days Van Cutsem E et al. Br J Cancer 2004; 90: 1190– 7
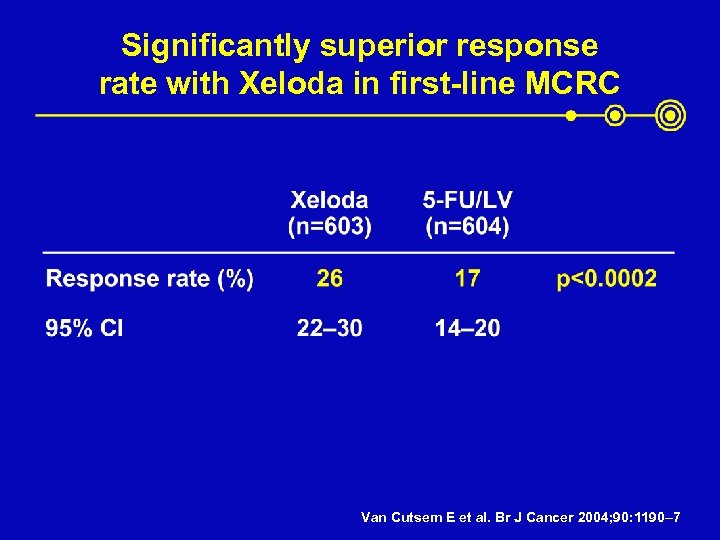
Significantly superior response rate with Xeloda in first-line MCRC Van Cutsem E et al. Br J Cancer 2004; 90: 1190– 7
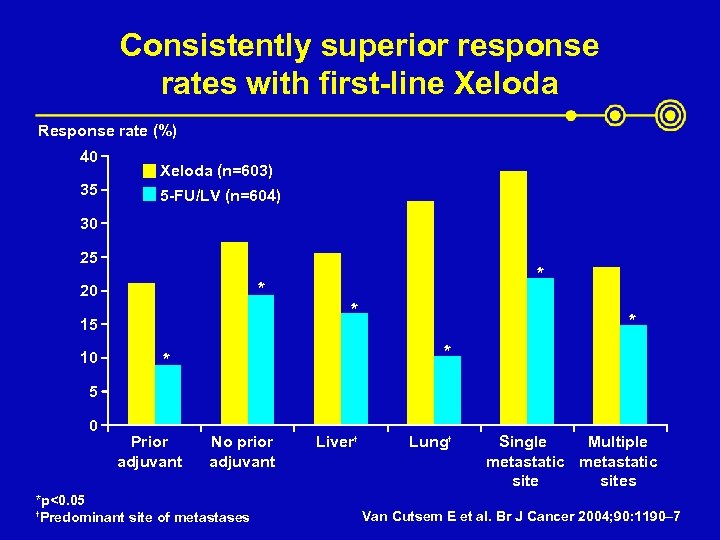
Consistently superior response rates with first-line Xeloda Response rate (%) 40 35 Xeloda (n=603) 5 -FU/LV (n=604) 30 25 * 20 * 15 10 * * 5 0 Prior adjuvant No prior adjuvant *p<0. 05 † Predominant site of metastases Liver† Lung† Single Multiple metastatic sites Van Cutsem E et al. Br J Cancer 2004; 90: 1190– 7
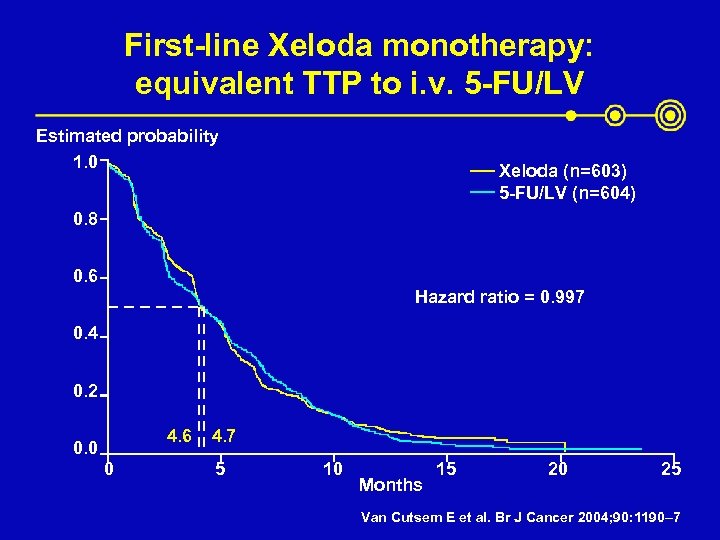
First-line Xeloda monotherapy: equivalent TTP to i. v. 5 -FU/LV Estimated probability 1. 0 Xeloda (n=603) 5 -FU/LV (n=604) 0. 8 0. 6 Hazard ratio = 0. 997 0. 4 0. 2 4. 6 0. 0 0 4. 7 5 10 Months 15 20 25 Van Cutsem E et al. Br J Cancer 2004; 90: 1190– 7
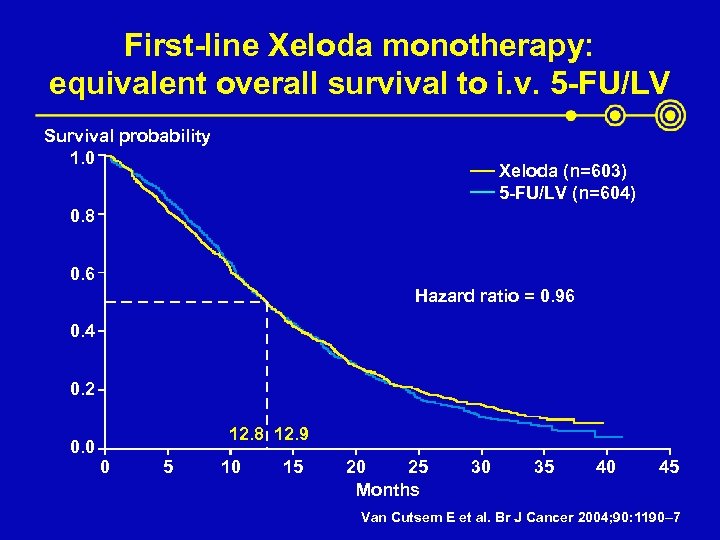
First-line Xeloda monotherapy: equivalent overall survival to i. v. 5 -FU/LV Survival probability 1. 0 Xeloda (n=603) 5 -FU/LV (n=604) 0. 8 0. 6 Hazard ratio = 0. 96 0. 4 0. 2 0. 0 12. 8 12. 9 0 5 10 15 20 25 Months 30 35 40 45 Van Cutsem E et al. Br J Cancer 2004; 90: 1190– 7
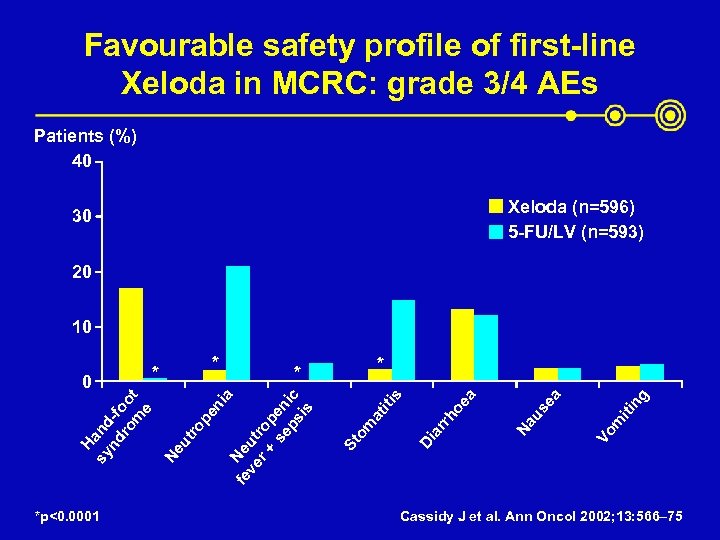
Favourable safety profile of first-line Xeloda in MCRC: grade 3/4 AEs Patients (%) 40 Xeloda (n=596) 5 -FU/LV (n=593) 30 20 10 *p<0. 0001 ng iti m Vo us ea Na ar rh Di St om at iti s oe a * * ia Ne fe ve ut r + rop se en ps ic is pe n ut ro Ne H sy and nd -f ro oo m t e 0 * * Cassidy J et al. Ann Oncol 2002; 13: 566– 75
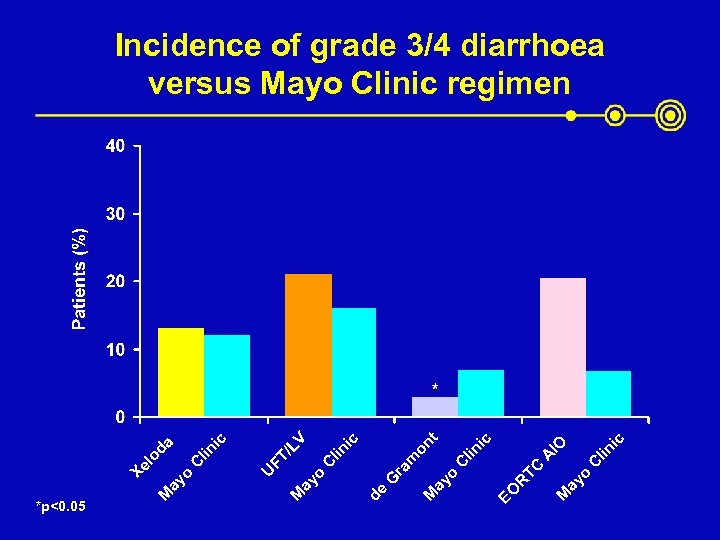
*p<0. 05 ic lin ic IO A C TC ay o M EO R lin C t ic V on lin C /L FT ic da lin C G ra m ay o M de ay o M U ay o M Xe lo Incidence of grade 3/4 diarrhoea versus Mayo Clinic regimen
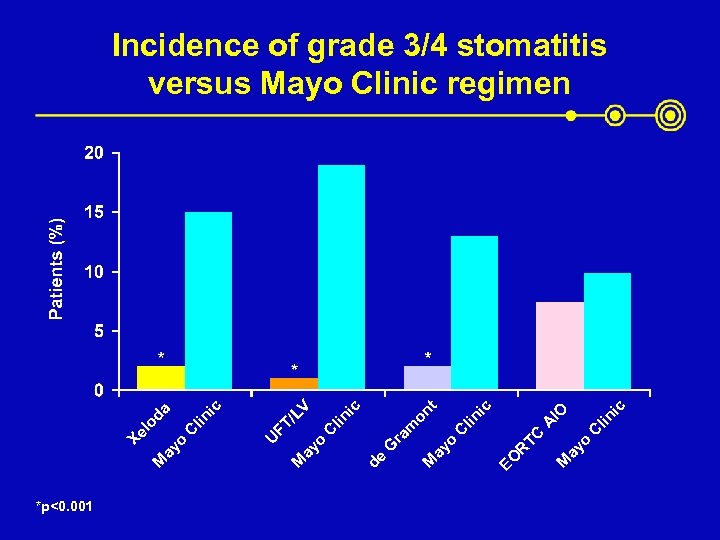
*p<0. 001 ic lin ic IO A C TC ay o M EO R lin C t ic V on lin C /L FT ic da lin C G ra m ay o M de ay o M U ay o M Xe lo Incidence of grade 3/4 stomatitis versus Mayo Clinic regimen
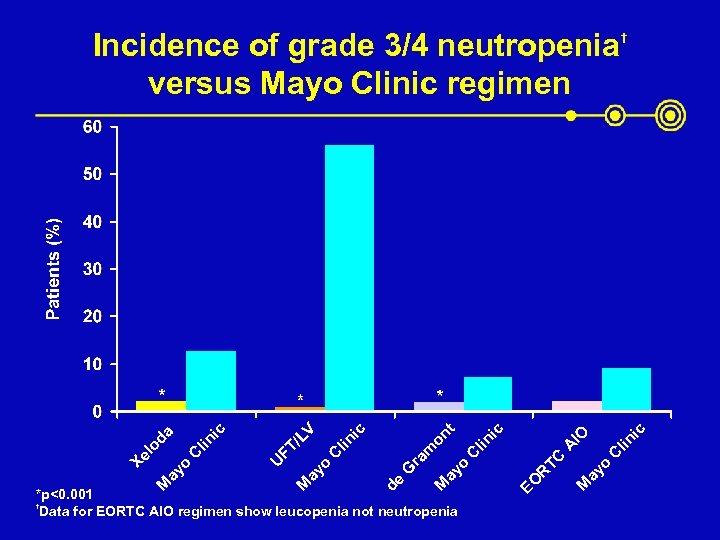
ic † lin C ay o M EO R TC A lin C *p<0. 001 † Data for EORTC AIO regimen show leucopenia not neutropenia IO ic t ay o M de G ra m on ic lin C ay o M U FT /L ic C lin da ay o M Xe lo V Incidence of grade 3/4 neutropenia versus Mayo Clinic regimen
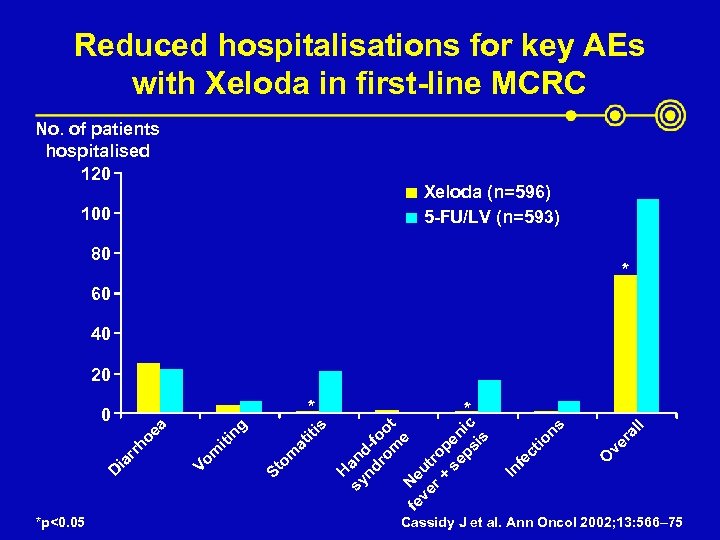
Reduced hospitalisations for key AEs with Xeloda in first-line MCRC No. of patients hospitalised 120 Xeloda (n=596) 5 -FU/LV (n=593) 100 80 * 60 40 20 * *p<0. 05 O ve r al l ns ct io fe In sy an nd d-f ro oo m t e fe Ne ve ut r + ro se pen ps ic is at om St * H s iti ng iti m Vo D ia rr ho ea 0 Cassidy J et al. Ann Oncol 2002; 13: 566– 75
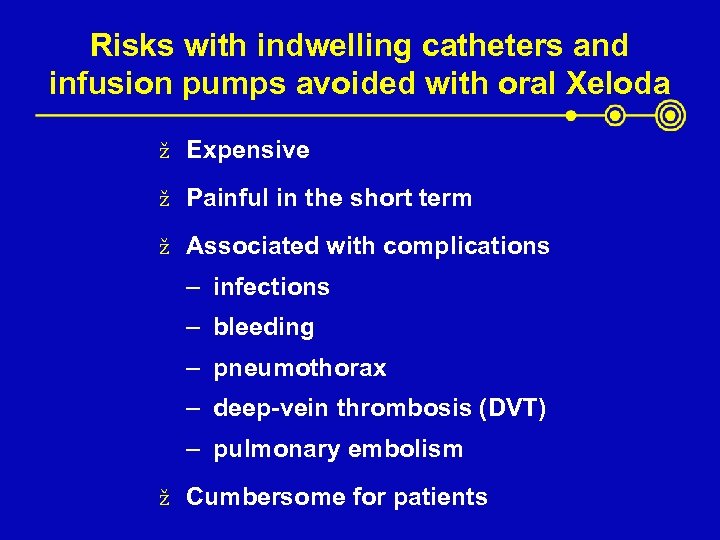
Risks with indwelling catheters and infusion pumps avoided with oral Xeloda ž Expensive ž Painful in the short term ž Associated with complications – infections – bleeding – pneumothorax – deep-vein thrombosis (DVT) – pulmonary embolism ž Cumbersome for patients
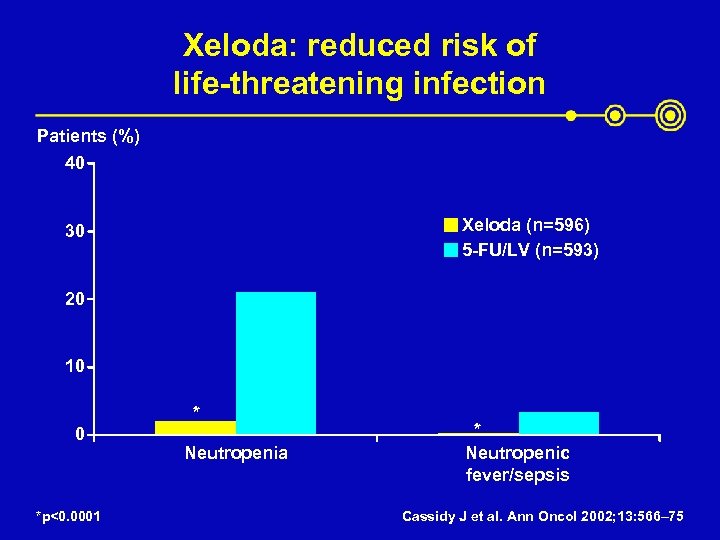
Xeloda: reduced risk of life-threatening infection Patients (%) 40 Xeloda (n=596) 5 -FU/LV (n=593) 30 20 10 * 0 *p<0. 0001 Neutropenia * Neutropenic fever/sepsis Cassidy J et al. Ann Oncol 2002; 13: 566– 75

Xeloda is substantially resource-saving versus Mayo Clinic in first-line MCRC ž Data prospectively collected (n=602) on drug administration, hospital admissions, drugs and consultations for AEs ž Compared with 5 -FU/LV, Xeloda patients required – fewer hospital visits for drug administration – fewer days in hospital for treatment-related AEs – fewer expensive drugs (antimicrobials fluconazole, 5 -HT 3 -antagonists) for AEs – more frequent unscheduled home, day care, office and telephone consultations Twelves C et al. Eur J Cancer 2001; 37: 597– 604

Patients prefer oral therapy: randomised study in first-line MCRC (n=97) ž Xeloda i. v. 5 -FU/LV (Mayo Clinic, in- or out-patient de Gramont regimens) or i. v. 5 -FU/LV Xeloda ž Before treatment 95% preferred oral ž Most still preferred oral after treatment: 64% overall – 86%, 63% and 50% in the Mayo, in- and out-patient de Gramont groups, respectively – preference strength depended on comparator i. v. regimen ž Principal reasons for oral preference were – increased convenience – home-based administration – tablet formulation

First-line Xeloda monotherapy versus 5 -FU/LV ž Significantly superior response rate ž Equivalent median TTP and overall survival ž Favourable safety profile ž More resource saving ž Patient preference for oral therapy
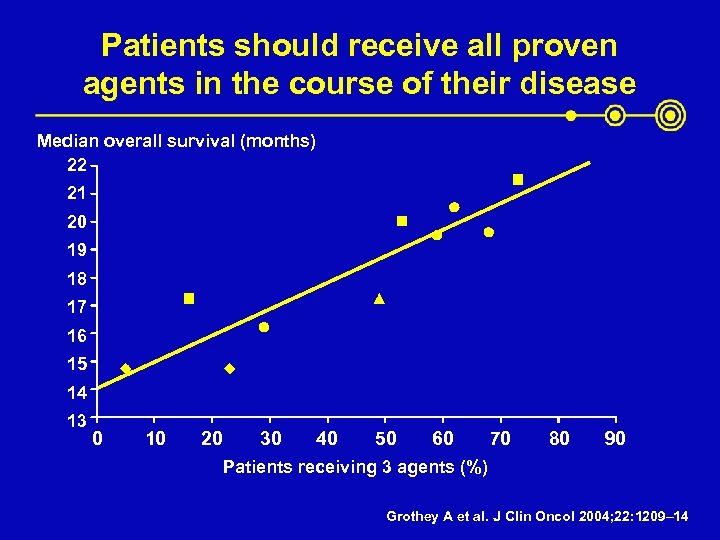
Patients should receive all proven agents in the course of their disease Median overall survival (months) 22 21 20 19 18 17 16 15 14 13 0 10 20 30 40 50 60 70 80 90 Patients receiving 3 agents (%) Grothey A et al. J Clin Oncol 2004; 22: 1209– 14
Xeloda is an ideal combination partner Xeloda generates 5 -FU preferentially in tumour tissue and has high single-agent, first-line activity Synergistic antitumour activity in human colon cancer xenografts – Xeloda + oxaliplatin (XELOX)1 – Xeloda + irinotecan (XELIRI)2 1 Cassidy 2 Cao J et al. J Clin Oncol 2004; 22: 2084– 91 S et al. Clin Colorectal Cancer 2005; 4: 336– 43

Xeloda-based combinations: XELOX
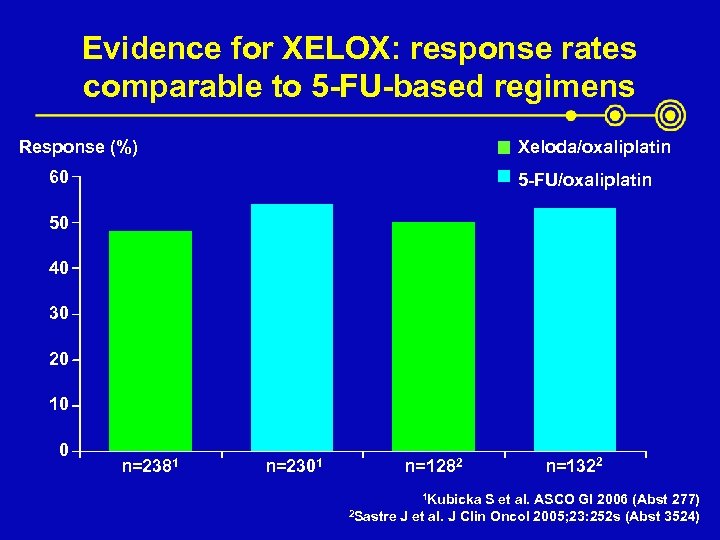
Evidence for XELOX: response rates comparable to 5 -FU-based regimens Response (%) Xeloda/oxaliplatin 60 5 -FU/oxaliplatin 50 40 30 20 10 0 n=2381 n=2301 n=1282 1 Kubicka 2 Sastre n=1322 S et al. ASCO GI 2006 (Abst 277) J et al. J Clin Oncol 2005; 23: 252 s (Abst 3524)
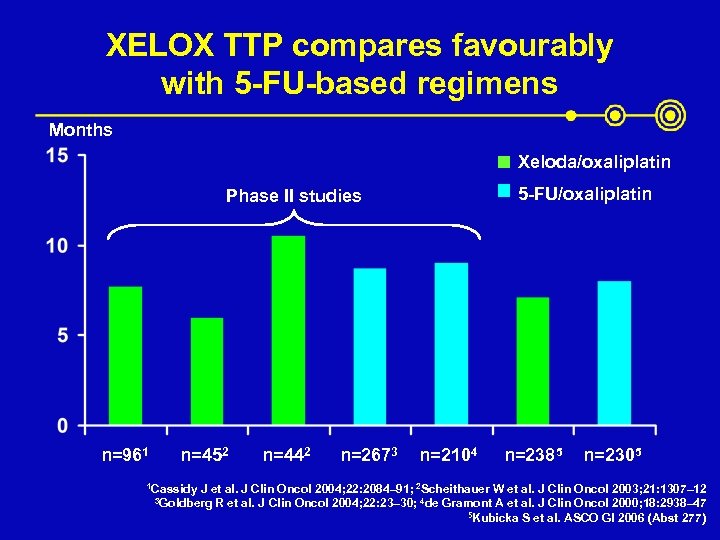
XELOX TTP compares favourably with 5 -FU-based regimens Months Xeloda/oxaliplatin 5 -FU/oxaliplatin Phase II studies n=961 n=452 1 Cassidy n=442 n=2673 n=2104 n=2385 n=2305 J et al. J Clin Oncol 2004; 22: 2084– 91; 2 Scheithauer W et al. J Clin Oncol 2003; 21: 1307– 12 R et al. J Clin Oncol 2004; 22: 23– 30; 4 de Gramont A et al. J Clin Oncol 2000; 18: 2938– 47 5 Kubicka S et al. ASCO GI 2006 (Abst 277) 3 Goldberg
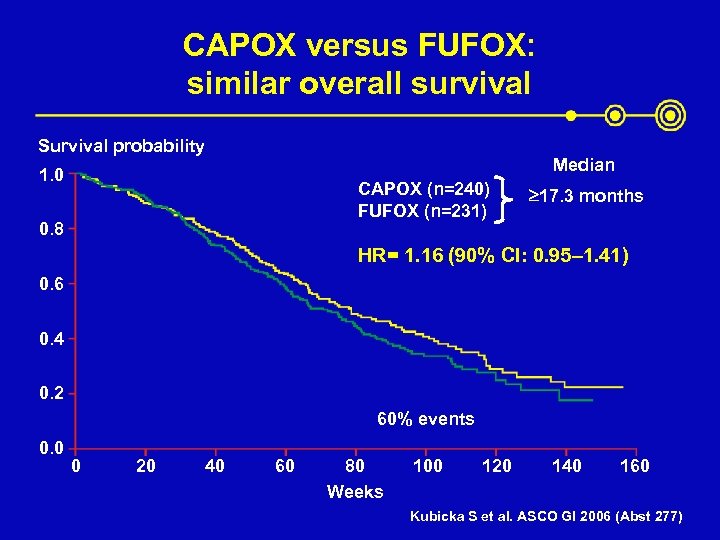
CAPOX versus FUFOX: similar overall survival Survival probability Median 1. 0 CAPOX (n=240) FUFOX (n=231) 0. 8 ³ 17. 3 months HR= 1. 16 (90% CI: 0. 95– 1. 41) 0. 6 0. 4 0. 2 60% events 0. 0 0 20 40 60 80 Weeks 100 120 140 160 Kubicka S et al. ASCO GI 2006 (Abst 277)
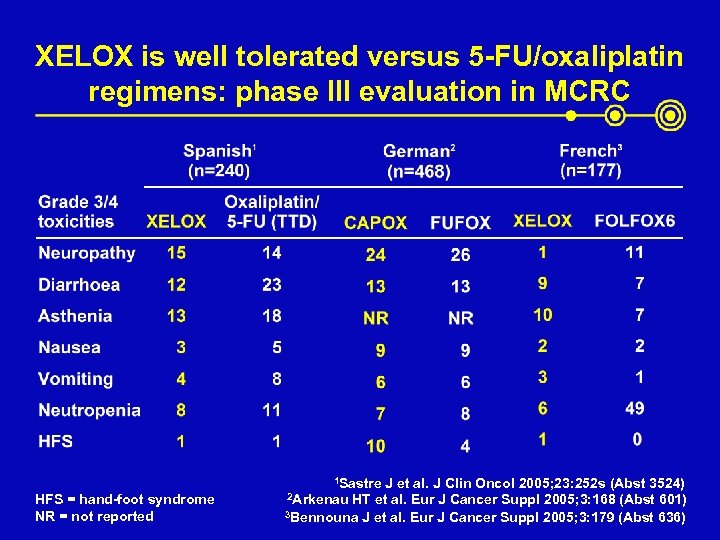
XELOX is well tolerated versus 5 -FU/oxaliplatin regimens: phase III evaluation in MCRC 1 Sastre HFS = hand-foot syndrome NR = not reported J et al. J Clin Oncol 2005; 23: 252 s (Abst 3524) 2 Arkenau HT et al. Eur J Cancer Suppl 2005; 3: 168 (Abst 601) 3 Bennouna J et al. Eur J Cancer Suppl 2005; 3: 179 (Abst 636)
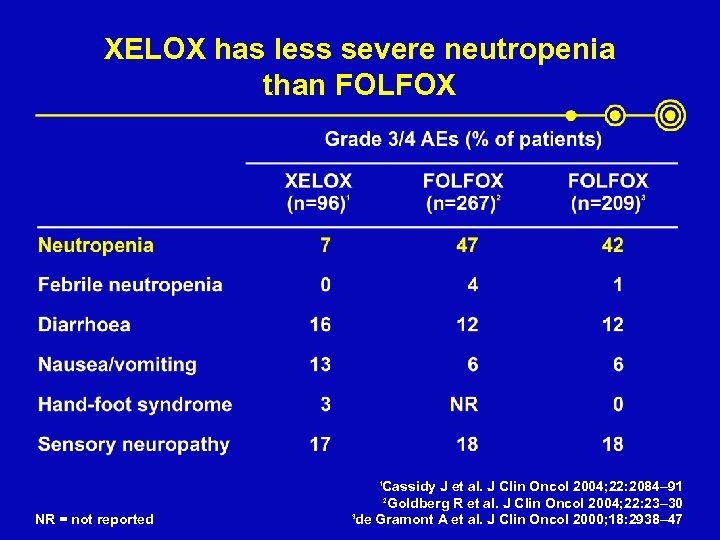
XELOX has less severe neutropenia than FOLFOX Cassidy J et al. J Clin Oncol 2004; 22: 2084– 91 2 Goldberg R et al. J Clin Oncol 2004; 22: 23– 30 3 de Gramont A et al. J Clin Oncol 2000; 18: 2938– 47 1 NR = not reported
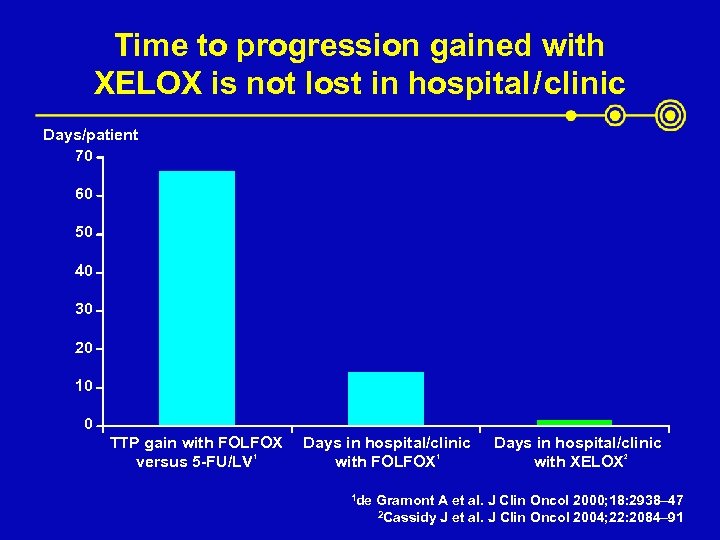
Time to progression gained with XELOX is not lost in hospital / clinic Days/patient 70 60 50 40 30 20 10 0 TTP gain with FOLFOX versus 5 -FU/LV 1 Days in hospital/clinic with FOLFOX 1 1 de Days in hospital/clinic with XELOX 2 Gramont A et al. J Clin Oncol 2000; 18: 2938– 47 2 Cassidy J et al. J Clin Oncol 2004; 22: 2084– 91
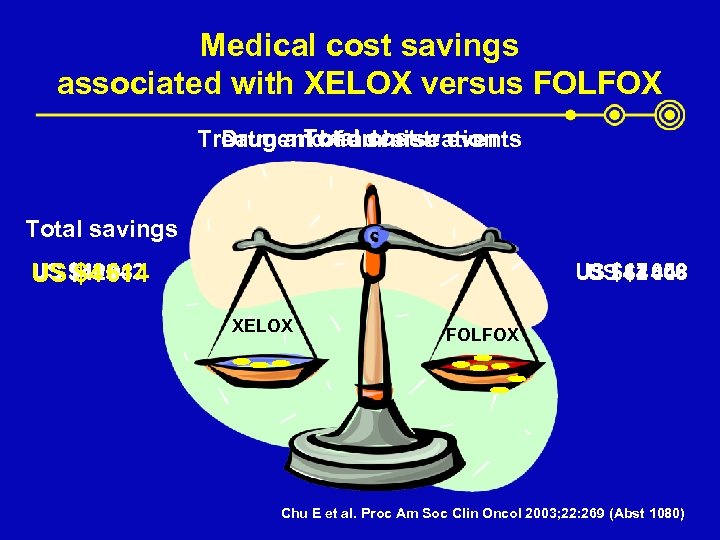
Medical cost savings associated with XELOX versus FOLFOX Total cost Treatment of adverse events Drug and administration Total savings US $405 037 US $42 614 $4 442 US $47 056 US $2 608 $44 448 XELOX FOLFOX Chu E et al. Proc Am Soc Clin Oncol 2003; 22: 269 (Abst 1080)
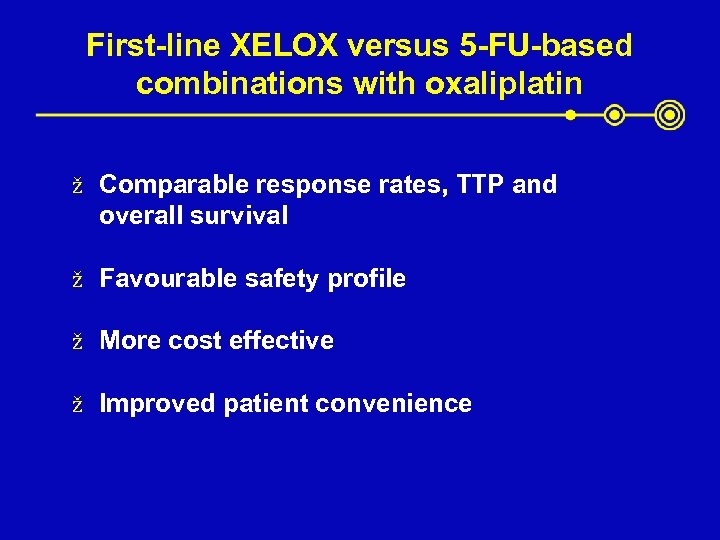
First-line XELOX versus 5 -FU-based combinations with oxaliplatin ž Comparable response rates, TTP and overall survival ž Favourable safety profile ž More cost effective ž Improved patient convenience

Xeloda-based combinations: XELIRI
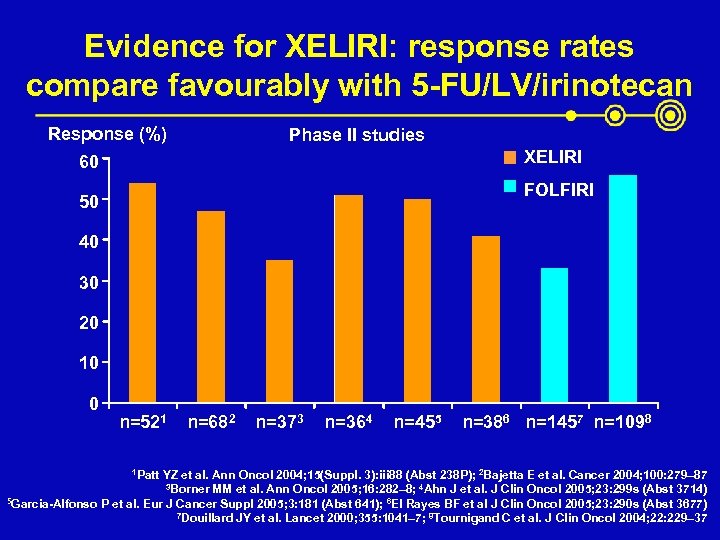
Evidence for XELIRI: response rates compare favourably with 5 -FU/LV/irinotecan Response (%) Phase II studies XELIRI 60 FOLFIRI 50 40 30 20 10 0 n=521 1 Patt n=682 n=373 n=364 n=455 n=386 n=1457 n=1098 YZ et al. Ann Oncol 2004; 15(Suppl. 3): iii 88 (Abst 238 P); 2 Bajetta E et al. Cancer 2004; 100: 279– 87 3 Borner MM et al. Ann Oncol 2005; 16: 282– 8; 4 Ahn J et al. J Clin Oncol 2005; 23: 299 s (Abst 3714) 5 Garcia-Alfonso P et al. Eur J Cancer Suppl 2005; 3: 181 (Abst 641); 6 El Rayes BF et al J Clin Oncol 2005; 23: 290 s (Abst 3677) 7 Douillard JY et al. Lancet 2000; 355: 1041– 7; 8 Tournigand C et al. J Clin Oncol 2004; 22: 229– 37
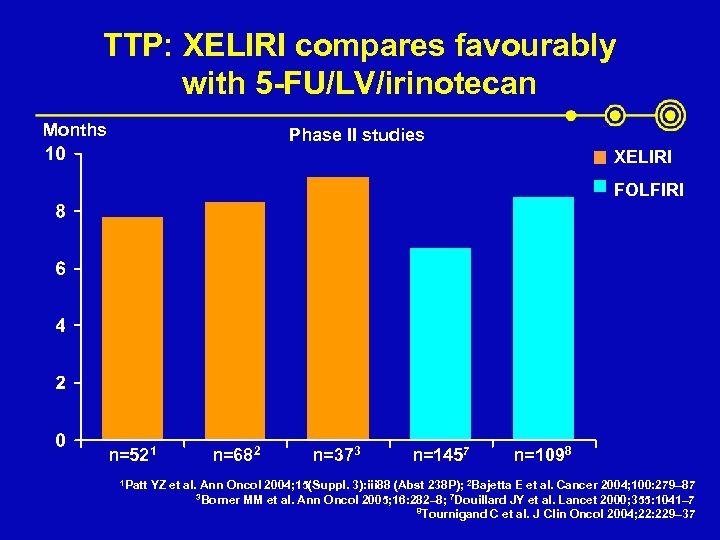
TTP: XELIRI compares favourably with 5 -FU/LV/irinotecan Months Phase II studies XELIRI FOLFIRI n=521 1 Patt n=682 n=373 n=1457 n=1098 YZ et al. Ann Oncol 2004; 15(Suppl. 3): iii 88 (Abst 238 P); 2 Bajetta E et al. Cancer 2004; 100: 279– 87 3 Borner MM et al. Ann Oncol 2005; 16: 282– 8; 7 Douillard JY et al. Lancet 2000; 355: 1041– 7 8 Tournigand C et al. J Clin Oncol 2004; 22: 229– 37
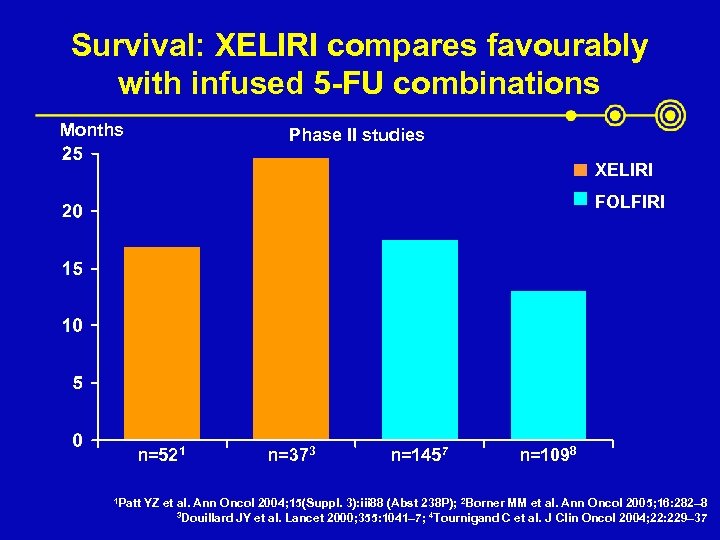
Survival: XELIRI compares favourably with infused 5 -FU combinations Months Phase II studies XELIRI FOLFIRI n=521 1 Patt n=373 n=1457 n=1098 YZ et al. Ann Oncol 2004; 15(Suppl. 3): iii 88 (Abst 238 P); 2 Borner MM et al. Ann Oncol 2005; 16: 282– 8 3 Douillard JY et al. Lancet 2000; 355: 1041– 7; 4 Tournigand C et al. J Clin Oncol 2004; 22: 229– 37
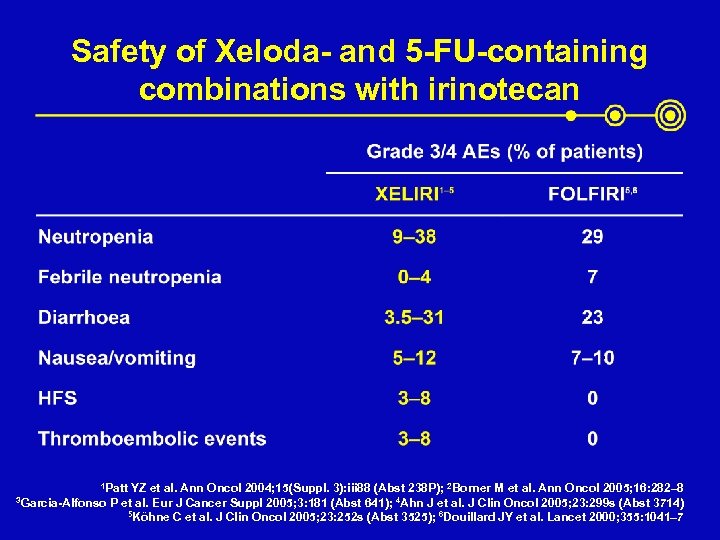
Safety of Xeloda- and 5 -FU-containing combinations with irinotecan 1 Patt 3 Garcia-Alfonso YZ et al. Ann Oncol 2004; 15(Suppl. 3): iii 88 (Abst 238 P); 2 Borner M et al. Ann Oncol 2005; 16: 282– 8 P et al. Eur J Cancer Suppl 2005; 3: 181 (Abst 641); 4 Ahn J et al. J Clin Oncol 2005; 23: 299 s (Abst 3714) 5 Köhne C et al. J Clin Oncol 2005; 23: 252 s (Abst 3525); 6 Douillard JY et al. Lancet 2000; 355: 1041– 7
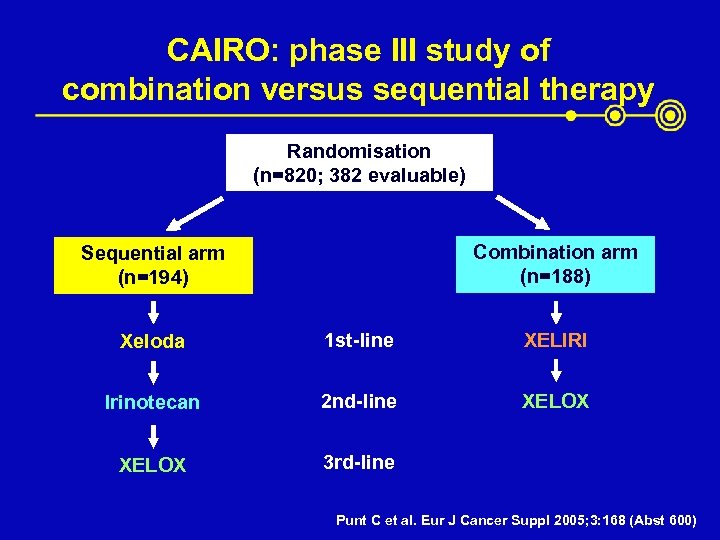
CAIRO: phase III study of combination versus sequential therapy Randomisation (n=820; 382 evaluable) Combination arm (n=188) Sequential arm (n=194) Xeloda 1 st-line XELIRI Irinotecan 2 nd-line XELOX 3 rd-line Punt C et al. Eur J Cancer Suppl 2005; 3: 168 (Abst 600)
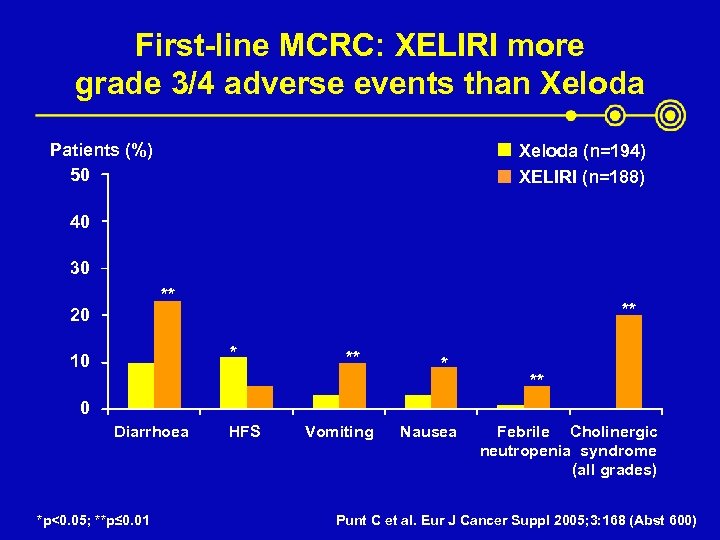
First-line MCRC: XELIRI more grade 3/4 adverse events than Xeloda Patients (%) 50 Xeloda (n=194) XELIRI (n=188) 40 30 ** 20 ** * 10 ** * ** 0 Diarrhoea *p<0. 05; **p≤ 0. 01 HFS Vomiting Nausea Febrile Cholinergic neutropenia syndrome (all grades) Punt C et al. Eur J Cancer Suppl 2005; 3: 168 (Abst 600)
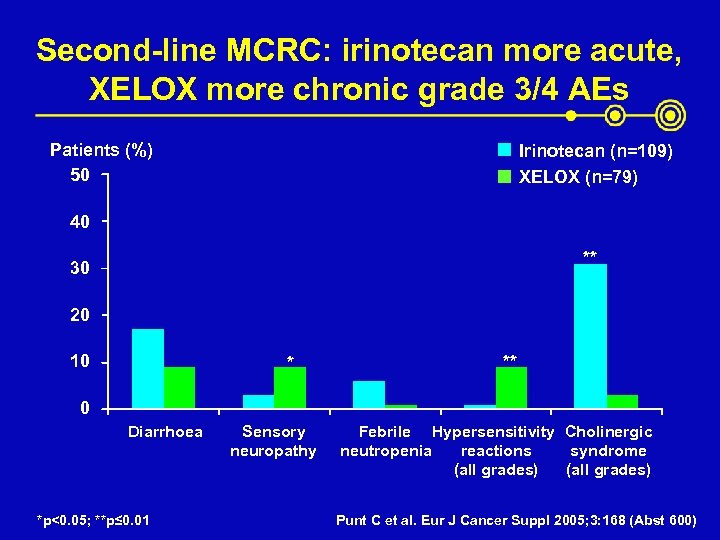
Second-line MCRC: irinotecan more acute, XELOX more chronic grade 3/4 AEs Patients (%) 50 Irinotecan (n=109) XELOX (n=79) 40 ** 30 20 10 * ** 0 Diarrhoea *p<0. 05; **p≤ 0. 01 Sensory neuropathy Febrile Hypersensitivity Cholinergic neutropenia reactions syndrome (all grades) Punt C et al. Eur J Cancer Suppl 2005; 3: 168 (Abst 600)
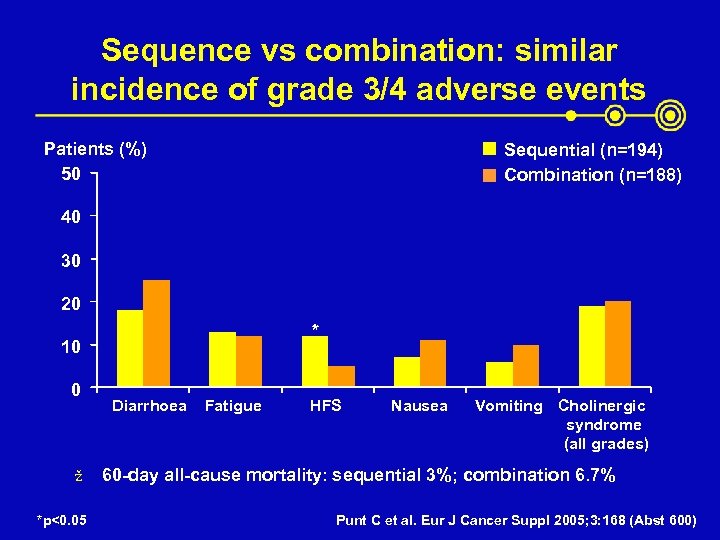
Sequence vs combination: similar incidence of grade 3/4 adverse events Patients (%) 50 Sequential (n=194) Combination (n=188) 40 30 20 * 10 0 ž *p<0. 05 Diarrhoea Fatigue HFS Nausea Vomiting Cholinergic syndrome (all grades) 60 -day all-cause mortality: sequential 3%; combination 6. 7% Punt C et al. Eur J Cancer Suppl 2005; 3: 168 (Abst 600)
First-line XELIRI versus 5 -FU-based combinations with irinotecan ž Comparable response rates, TTP and overall survival ž Favourable safety profile ž Improved patient convenience

Oral Xeloda offers freedom without a loss of effective management ž Proven compliance in clinical trials ž Patients prefer oral chemotherapy ž Home-based treatment: patients lead more normal life ž Less time in hospital = more time with family ž Patient education is a priority – time allocated before treatment begins – patients advised on side-effect management upfront – telephone follow-up ž Oral Xeloda simplifies increasingly complex regimens
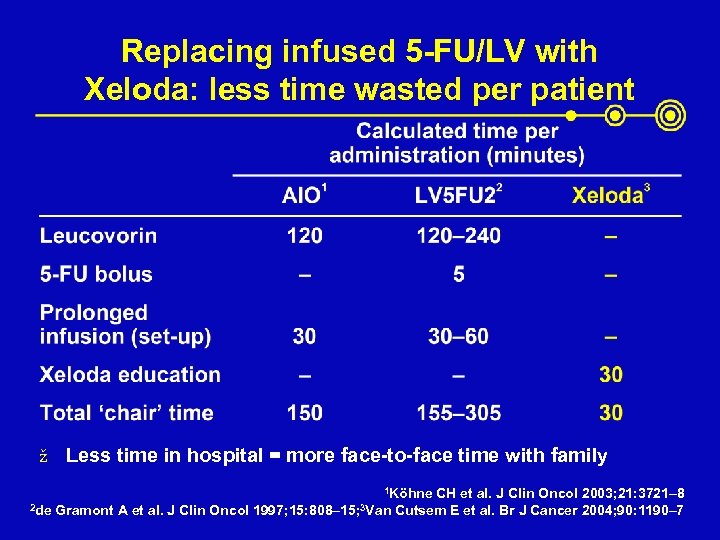
Replacing infused 5 -FU/LV with Xeloda: less time wasted per patient ž Less time in hospital = more face-to-face time with family 1 Köhne 2 de Gramont A et al. J Clin Oncol 1997; 15: 808– 15; 3 Van CH et al. J Clin Oncol 2003; 21: 3721– 8 Cutsem E et al. Br J Cancer 2004; 90: 1190– 7
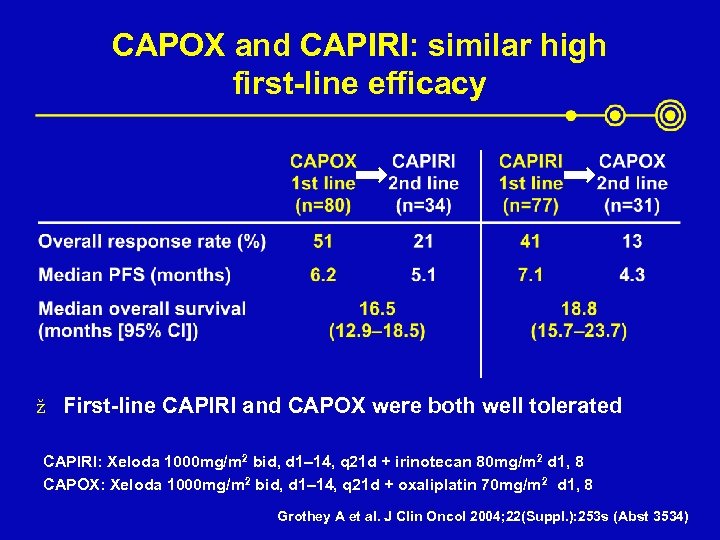
CAPOX and CAPIRI: similar high first-line efficacy ž First-line CAPIRI and CAPOX were both well tolerated CAPIRI: Xeloda 1000 mg/m 2 bid, d 1– 14, q 21 d + irinotecan 80 mg/m 2 d 1, 8 CAPOX: Xeloda 1000 mg/m 2 bid, d 1– 14, q 21 d + oxaliplatin 70 mg/m 2 d 1, 8 Grothey A et al. J Clin Oncol 2004; 22(Suppl. ): 253 s (Abst 3534)

Xeloda: the backbone of first-line therapy for MCRC Xeloda: superior response rate, similar TTP and OS, favourable safety compared with 5 -FU/LV XELOX: ideal combination, effective and well tolerated XELIRI: effective, long-term treatment, with appropriate management of adverse events After discontinuation of combination, Xeloda allows continued oral treatment – maximising response duration first-line – freedom from infusions, continued efficacy with minimal side effects
9f3eee66c76d7fe4c14570f35ed2152a.ppt