2a803111da095830d18359db5832220b.ppt
- Количество слайдов: 92
 WWW. ICAD-CISD. COM New Prevention Technologies Workshop Module 6: Ethics
WWW. ICAD-CISD. COM New Prevention Technologies Workshop Module 6: Ethics
 HUMAN RIGHTS AND ETHICAL CHALLENGES IN NPT RESEARCH
HUMAN RIGHTS AND ETHICAL CHALLENGES IN NPT RESEARCH
 OUTLINE Overview of research ethics: origins, guidelines, principles Overview of role of various players in biomedical prevention research Community engagement: models, challenges, vulnerability and targeted populations Dynamics of North-South research Case study: Cambodia, Cameroon, Thai trials Standard of prevention and care for trial participants Use of ARVs for prevention vs treatment
OUTLINE Overview of research ethics: origins, guidelines, principles Overview of role of various players in biomedical prevention research Community engagement: models, challenges, vulnerability and targeted populations Dynamics of North-South research Case study: Cambodia, Cameroon, Thai trials Standard of prevention and care for trial participants Use of ARVs for prevention vs treatment
 What is “Ethics”? Ethics is a way of understanding and examining what is “right” and what is “wrong” Bioethics is a way of understanding and examining what is “right” and what is “wrong” in biomedical research and practice.
What is “Ethics”? Ethics is a way of understanding and examining what is “right” and what is “wrong” Bioethics is a way of understanding and examining what is “right” and what is “wrong” in biomedical research and practice.
 Activity What is your understanding of these words ? Respect Harm Fairness
Activity What is your understanding of these words ? Respect Harm Fairness
 Principles of Research Ethics Respect for Persons Beneficence/Non-Maleficence Justice/Non-Exploitation
Principles of Research Ethics Respect for Persons Beneficence/Non-Maleficence Justice/Non-Exploitation
 Respect for Persons Autonomy Says that each individual: § § Is unique and free; Has the right and capacity to decide; Has value and dignity; and Has the right to informed consent. Protection for vulnerable persons Special protections must be in place for those whose decision–making capacity is impaired or diminished, whether due to physical or social factors
Respect for Persons Autonomy Says that each individual: § § Is unique and free; Has the right and capacity to decide; Has value and dignity; and Has the right to informed consent. Protection for vulnerable persons Special protections must be in place for those whose decision–making capacity is impaired or diminished, whether due to physical or social factors
 Beneficence/Non-Maleficence Protection of the study participants is the most important responsibility of the researcher Researchers must: Protect the physical, mental and social well- being of each research participant; Minimizes physical and social risks; Maximize the possible benefits; and Retain the community perspective.
Beneficence/Non-Maleficence Protection of the study participants is the most important responsibility of the researcher Researchers must: Protect the physical, mental and social well- being of each research participant; Minimizes physical and social risks; Maximize the possible benefits; and Retain the community perspective.
 Beneficence/Non-Maleficence ON BALANCE: The research should generate more good than harm; and Risks of research should be reasonable in light of the expected benefits to the individual and to society.
Beneficence/Non-Maleficence ON BALANCE: The research should generate more good than harm; and Risks of research should be reasonable in light of the expected benefits to the individual and to society.
 Justice/Non-Exploitation The principle that calls for fairness in the conduct of research is the principle of justice/non-exploitation Research must: § Ensure a fair distribution of risks and benefits § Research should not be done in a community that is not likely to benefit from the result § Conduct equitable recruitment of research participants; and § Provide special protection for vulnerable groups.
Justice/Non-Exploitation The principle that calls for fairness in the conduct of research is the principle of justice/non-exploitation Research must: § Ensure a fair distribution of risks and benefits § Research should not be done in a community that is not likely to benefit from the result § Conduct equitable recruitment of research participants; and § Provide special protection for vulnerable groups.
 RISKS and BENEFITS DISCUSSION QUESTIONS What are the potential RISKS of becoming involved in a prevention trial? For participants? For communities? What are the potential BENEFITS of becoming involved in a prevention trial? For participants? For communities?
RISKS and BENEFITS DISCUSSION QUESTIONS What are the potential RISKS of becoming involved in a prevention trial? For participants? For communities? What are the potential BENEFITS of becoming involved in a prevention trial? For participants? For communities?
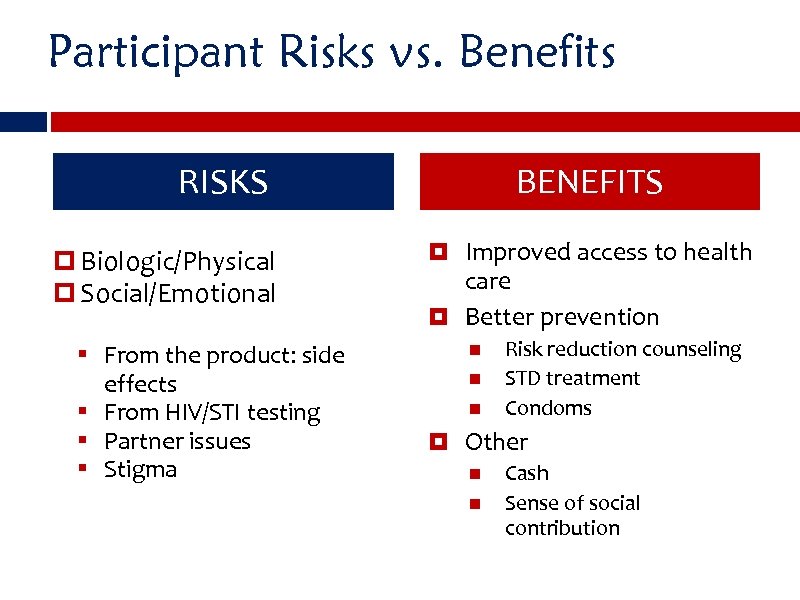 Participant Risks vs. Benefits RISKS Biologic/Physical Social/Emotional § From the product: side effects § From HIV/STI testing § Partner issues § Stigma BENEFITS Improved access to health care Better prevention Risk reduction counseling STD treatment Condoms Other Cash Sense of social contribution
Participant Risks vs. Benefits RISKS Biologic/Physical Social/Emotional § From the product: side effects § From HIV/STI testing § Partner issues § Stigma BENEFITS Improved access to health care Better prevention Risk reduction counseling STD treatment Condoms Other Cash Sense of social contribution
 Participants Have Said the Benefits of Participating in Microbicide Trials Include: Access to medical services and regular health checks is considered the biggest benefit Counseling about women’s bodies, sexuality, reproductive tract infections, STIs, and HIV Good relationships with study staff Feeling empowered; improved communication with male partners and children Access to study gel; improved sex due to gel Contributing to a women’s health cause: “One is helped but is also helping others”
Participants Have Said the Benefits of Participating in Microbicide Trials Include: Access to medical services and regular health checks is considered the biggest benefit Counseling about women’s bodies, sexuality, reproductive tract infections, STIs, and HIV Good relationships with study staff Feeling empowered; improved communication with male partners and children Access to study gel; improved sex due to gel Contributing to a women’s health cause: “One is helped but is also helping others”
 Participants Have Said the Risks and Burdens of Microbicide Trials Include: HIV testing considered biggest burden Discomfort during pelvic exam Long waiting times at clinic Feeling of loss at end of study Worries about side effects
Participants Have Said the Risks and Burdens of Microbicide Trials Include: HIV testing considered biggest burden Discomfort during pelvic exam Long waiting times at clinic Feeling of loss at end of study Worries about side effects
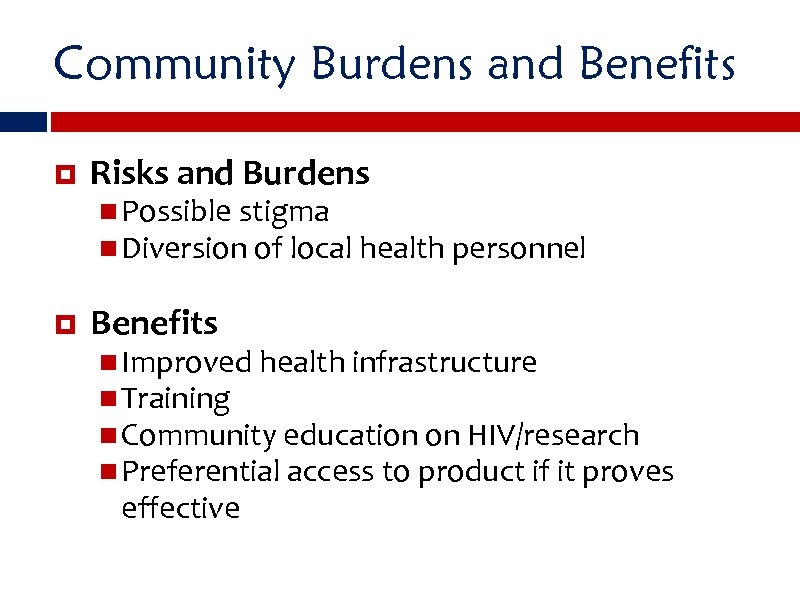 Community Burdens and Benefits Risks and Burdens Possible stigma Diversion of local health personnel Benefits Improved health infrastructure Training Community education on HIV/research Preferential access to product if it proves effective
Community Burdens and Benefits Risks and Burdens Possible stigma Diversion of local health personnel Benefits Improved health infrastructure Training Community education on HIV/research Preferential access to product if it proves effective
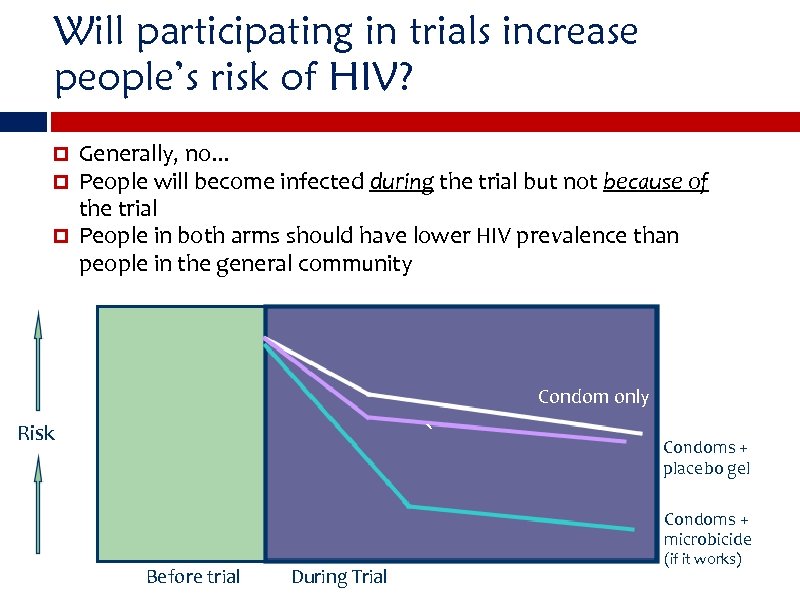 Will participating in trials increase people’s risk of HIV? Generally, no. . . People will become infected during the trial but not because of the trial People in both arms should have lower HIV prevalence than people in the general community Condoms only Condom only ` Risk Condoms + placebo gel Condoms + microbicide Before trial During Trial (if it works)
Will participating in trials increase people’s risk of HIV? Generally, no. . . People will become infected during the trial but not because of the trial People in both arms should have lower HIV prevalence than people in the general community Condoms only Condom only ` Risk Condoms + placebo gel Condoms + microbicide Before trial During Trial (if it works)
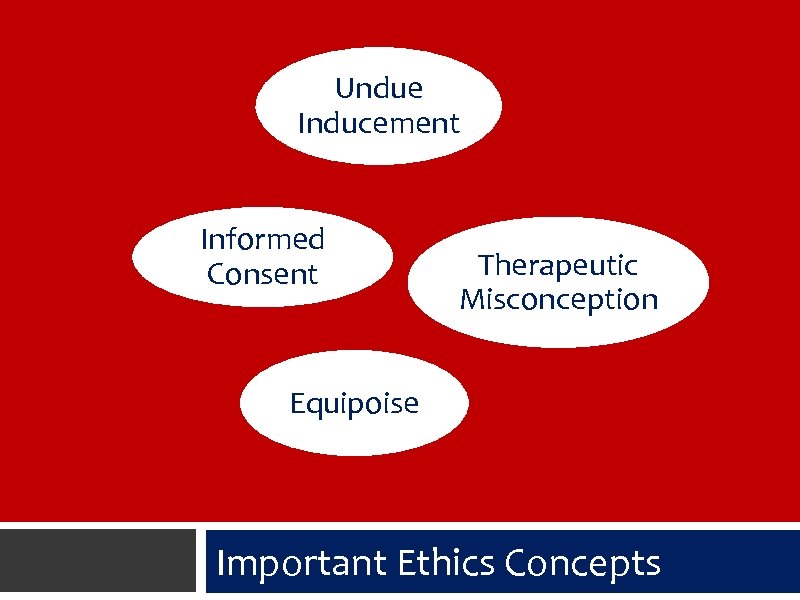 Undue Inducement Informed Consent Therapeutic Misconception Equipoise Important Ethics Concepts
Undue Inducement Informed Consent Therapeutic Misconception Equipoise Important Ethics Concepts
 Equipoise is a state of genuine uncertainty or doubt about whether one intervention or treatment is superior to another Equipoise is a necessary condition for clinical research to be morally acceptable If the scientific community “knows” that one treatment is better than another, it would be considered unethical to withhold it Questions remain, however, about how to decide when “scientific or clinical consensus” exists about the relative merits of different treatments
Equipoise is a state of genuine uncertainty or doubt about whether one intervention or treatment is superior to another Equipoise is a necessary condition for clinical research to be morally acceptable If the scientific community “knows” that one treatment is better than another, it would be considered unethical to withhold it Questions remain, however, about how to decide when “scientific or clinical consensus” exists about the relative merits of different treatments
 Therapeutic Misconception “Therapeutic misconception” refers to the tendency of some research participants to wrongly assume that whatever drug or intervention they are offered must work or be beneficial (or why would it be offered? ) It occurs when the goals of research and those of therapy or “health care” become confused in the participants mind. The therapeutic misconception is a major threat to “informed consent. ”
Therapeutic Misconception “Therapeutic misconception” refers to the tendency of some research participants to wrongly assume that whatever drug or intervention they are offered must work or be beneficial (or why would it be offered? ) It occurs when the goals of research and those of therapy or “health care” become confused in the participants mind. The therapeutic misconception is a major threat to “informed consent. ”
 Voluntary Informed Consent Voluntary informed consent is the agreement given by a well-informed person who: Has received the necessary information expressed in spoken words and in writing; Has adequately understood the information; and Has made the choice to participate (or not participate) without coercion.
Voluntary Informed Consent Voluntary informed consent is the agreement given by a well-informed person who: Has received the necessary information expressed in spoken words and in writing; Has adequately understood the information; and Has made the choice to participate (or not participate) without coercion.
 Essential Elements for Informed Consent Research description (what is being studied, what is the procedure, who is sponsoring the study? ); Risks of participating; Benefits of participating; Alternatives to participation, such as other studies or services in the area; Assurance that information will be kept confidential; Compensation for time, travel or possible harm; Contacts (whom to contact with questions/concerns); and Voluntary participation and withdrawal.
Essential Elements for Informed Consent Research description (what is being studied, what is the procedure, who is sponsoring the study? ); Risks of participating; Benefits of participating; Alternatives to participation, such as other studies or services in the area; Assurance that information will be kept confidential; Compensation for time, travel or possible harm; Contacts (whom to contact with questions/concerns); and Voluntary participation and withdrawal.
 Informed Consent, Cont’d Adequate understanding includes the difference between research and health care related concept: “therapeutic misconception” After thinking seriously about the information, the person can arrive at a decision without being forced, threatened or offered something so valuable that free choice is impossible related concepts: “coercion” and “undue inducement”
Informed Consent, Cont’d Adequate understanding includes the difference between research and health care related concept: “therapeutic misconception” After thinking seriously about the information, the person can arrive at a decision without being forced, threatened or offered something so valuable that free choice is impossible related concepts: “coercion” and “undue inducement”
 Legal and moral agenda can sometimes conflict Indemnify the research institution VERSUS Facilitate collaborative decision making Length of forms Degree of technical information imparted Written versus oral consent Emphasis on right to withdraw
Legal and moral agenda can sometimes conflict Indemnify the research institution VERSUS Facilitate collaborative decision making Length of forms Degree of technical information imparted Written versus oral consent Emphasis on right to withdraw
 Informed Consent is a Process Informed consent is a process of collaborative communication and decision making, not the signing of a form Informed consent requires that prospective participants: Be appropriately informed about the nature of the research Adequately understand this information and its implications Voluntarily decide to participate, without coercion Explicitly consent to participate, orally or in writing
Informed Consent is a Process Informed consent is a process of collaborative communication and decision making, not the signing of a form Informed consent requires that prospective participants: Be appropriately informed about the nature of the research Adequately understand this information and its implications Voluntarily decide to participate, without coercion Explicitly consent to participate, orally or in writing
 Activity Discussion questions: What are good ways to convey this kind of information to people to ensure that if they agree to participate in a study, they are giving informed consent? How do you know if people have understood the information and are making a free choice to participate?
Activity Discussion questions: What are good ways to convey this kind of information to people to ensure that if they agree to participate in a study, they are giving informed consent? How do you know if people have understood the information and are making a free choice to participate?
 Balancing respect for culture and respect for persons concept of “individual autonomy” may be in conflict with entrenched cultural norms or expectations example: may be expected that a woman’s husband has the right and authority to make decisions regarding her health care While recognizing local value and ethical pluralisms, ethics is also concerned with universal principles of conduct
Balancing respect for culture and respect for persons concept of “individual autonomy” may be in conflict with entrenched cultural norms or expectations example: may be expected that a woman’s husband has the right and authority to make decisions regarding her health care While recognizing local value and ethical pluralisms, ethics is also concerned with universal principles of conduct
 Case Study – Informed Consent A microbicide study is taking place in an African country. Focus groups in the community have shown that many women are interested in a microbicide because they are not able to negotiate condom use with their partners. Many women are coming to the study clinic to enroll in the trial. A community advisory group is formed with community leaders and representatives. A male member of the group says that he does not approve of the study because the women are not required to get the consent of their partners to enroll. A local women’s group expresses concern that a woman who enrolls in the trial without telling their partner risks being harmed if her partner finds out she is participating.
Case Study – Informed Consent A microbicide study is taking place in an African country. Focus groups in the community have shown that many women are interested in a microbicide because they are not able to negotiate condom use with their partners. Many women are coming to the study clinic to enroll in the trial. A community advisory group is formed with community leaders and representatives. A male member of the group says that he does not approve of the study because the women are not required to get the consent of their partners to enroll. A local women’s group expresses concern that a woman who enrolls in the trial without telling their partner risks being harmed if her partner finds out she is participating.
 ACTIVITY In some settings it is generally expected that a woman’s husband has the right and authority to make decisions regarding her health care § In this instance, how should one balance respect for persons with respect for culture? § Should sexual partners be involved? Are there creative strategies for encouraging partner engagement? § What might you recommend as an appropriate way to respect both of these values in this instance?
ACTIVITY In some settings it is generally expected that a woman’s husband has the right and authority to make decisions regarding her health care § In this instance, how should one balance respect for persons with respect for culture? § Should sexual partners be involved? Are there creative strategies for encouraging partner engagement? § What might you recommend as an appropriate way to respect both of these values in this instance?
 OVERVIEW OF ROLE OF VARIOUS PLAYERS
OVERVIEW OF ROLE OF VARIOUS PLAYERS
 Who? Ethics Committees and Review Boards Ethical Community and Research Advocates Researchers and Sponsors
Who? Ethics Committees and Review Boards Ethical Community and Research Advocates Researchers and Sponsors
 Who Decides? Decisions have to be made about what the acceptable balance is between risks and benefits CABs and ethics committees can help judge acceptability of risk: benefit ratio overall The informed consent process helps an individual make his/her own judgment about the risks and benefits The health and well-being of the participant can never be sacrificed for “research’s sake” or the “greater global good”
Who Decides? Decisions have to be made about what the acceptable balance is between risks and benefits CABs and ethics committees can help judge acceptability of risk: benefit ratio overall The informed consent process helps an individual make his/her own judgment about the risks and benefits The health and well-being of the participant can never be sacrificed for “research’s sake” or the “greater global good”
 Who are the Players in HIV NPT Research? Academic researchers and universities Community members and organizations, community advisory boards Private sector – pharmaceutical and biotech companies Government funders and regulators Health care providers
Who are the Players in HIV NPT Research? Academic researchers and universities Community members and organizations, community advisory boards Private sector – pharmaceutical and biotech companies Government funders and regulators Health care providers
 Academic Researchers Basic Researchers lead the scientific discovery and development of NPT candidate concepts and products Clinical Researchers lead the clinical testing of candidate NPT products, testing efficacy as well as issues of acceptance and accessibility establish and maintain the highest standards of ethical conduct of clinical trials Social Researchers conduct research on acceptability, preparedness, access and delivery issues work alongside clinical research to understand usability and acceptance of NPTs
Academic Researchers Basic Researchers lead the scientific discovery and development of NPT candidate concepts and products Clinical Researchers lead the clinical testing of candidate NPT products, testing efficacy as well as issues of acceptance and accessibility establish and maintain the highest standards of ethical conduct of clinical trials Social Researchers conduct research on acceptability, preparedness, access and delivery issues work alongside clinical research to understand usability and acceptance of NPTs
 Community Roles develop community acceptance and preparedness for NPTs anticipate and mitigate stigma associated with trial raise awareness about the role community based organizations can play before, during and after trials facilitate clinical trial recruitment incorporate NPTs into prevention education and training programs for specific vulnerable populations develop strategies for promoting and distributing NPTs once available advocate for investment in NPT research and development
Community Roles develop community acceptance and preparedness for NPTs anticipate and mitigate stigma associated with trial raise awareness about the role community based organizations can play before, during and after trials facilitate clinical trial recruitment incorporate NPTs into prevention education and training programs for specific vulnerable populations develop strategies for promoting and distributing NPTs once available advocate for investment in NPT research and development
 Private Sector invest in research and development, manufacturing and production technical innovation establish clinical infrastructure (e. g. , epidemiological laboratories, trials infrastructure) during the pre-clinical development of the NPT that will be needed in clinical research translational research: generate data, clinical materials
Private Sector invest in research and development, manufacturing and production technical innovation establish clinical infrastructure (e. g. , epidemiological laboratories, trials infrastructure) during the pre-clinical development of the NPT that will be needed in clinical research translational research: generate data, clinical materials
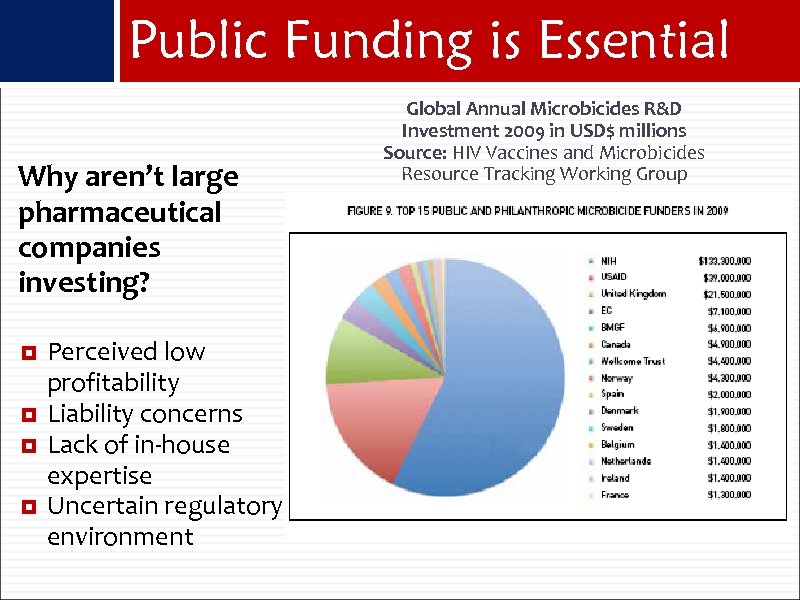 Public Funding is Essential Why aren’t large pharmaceutical companies investing? Perceived low profitability Liability concerns Lack of in-house expertise Uncertain regulatory environment Global Annual Microbicides R&D Investment 2009 in USD$ millions Source: HIV Vaccines and Microbicides Resource Tracking Working Group
Public Funding is Essential Why aren’t large pharmaceutical companies investing? Perceived low profitability Liability concerns Lack of in-house expertise Uncertain regulatory environment Global Annual Microbicides R&D Investment 2009 in USD$ millions Source: HIV Vaccines and Microbicides Resource Tracking Working Group
 Government Funders and Regulators provide funding for NPT research programs, academic researchers, conferences coordinate domestic and global efforts ensure that adequate clinical research facilities exist ensure availability of properly trained staff help build public awareness and support for research and development achieve speedy and appropriate access once a NPT becomes available
Government Funders and Regulators provide funding for NPT research programs, academic researchers, conferences coordinate domestic and global efforts ensure that adequate clinical research facilities exist ensure availability of properly trained staff help build public awareness and support for research and development achieve speedy and appropriate access once a NPT becomes available
 Health Care Providers Monitoring, prevention and control of HIV/AIDS and STIs Help with NPT delivery, education and access With ARV-based NPTs, may need to be prescribers
Health Care Providers Monitoring, prevention and control of HIV/AIDS and STIs Help with NPT delivery, education and access With ARV-based NPTs, may need to be prescribers
 COMMUNITY ENGAGEMENT
COMMUNITY ENGAGEMENT
 WHAT DO WE MEAN BY COMMUNITY?
WHAT DO WE MEAN BY COMMUNITY?
 Competing & Changing Definitions of Community “…separate and overlapping groups of people who are infected and affected by HIV in various ways” Good Participatory Practice, UNAIDS/AVAC “ …trial participants, their families and partners, other local stakeholders, and service providers/community groups within the geographic parameters of the clinical trial location. MDS Civil Society Working Group Report “…the group of people who will participate in or are likely to be affected by or have an influence on the conduct of the research. ” HIV Prevention Trials Network, Community Program FAQs
Competing & Changing Definitions of Community “…separate and overlapping groups of people who are infected and affected by HIV in various ways” Good Participatory Practice, UNAIDS/AVAC “ …trial participants, their families and partners, other local stakeholders, and service providers/community groups within the geographic parameters of the clinical trial location. MDS Civil Society Working Group Report “…the group of people who will participate in or are likely to be affected by or have an influence on the conduct of the research. ” HIV Prevention Trials Network, Community Program FAQs
 …Or No Definition At All In addition to many competing definitions, often times people talk about “community” without defining what they mean or who they are specifically referring to
…Or No Definition At All In addition to many competing definitions, often times people talk about “community” without defining what they mean or who they are specifically referring to
 Locating Community When we talk about community, it is important to frame the discussion in terms of: Who is included in the particular “community” we are discussing? And distinguish which “level” we are referring to
Locating Community When we talk about community, it is important to frame the discussion in terms of: Who is included in the particular “community” we are discussing? And distinguish which “level” we are referring to
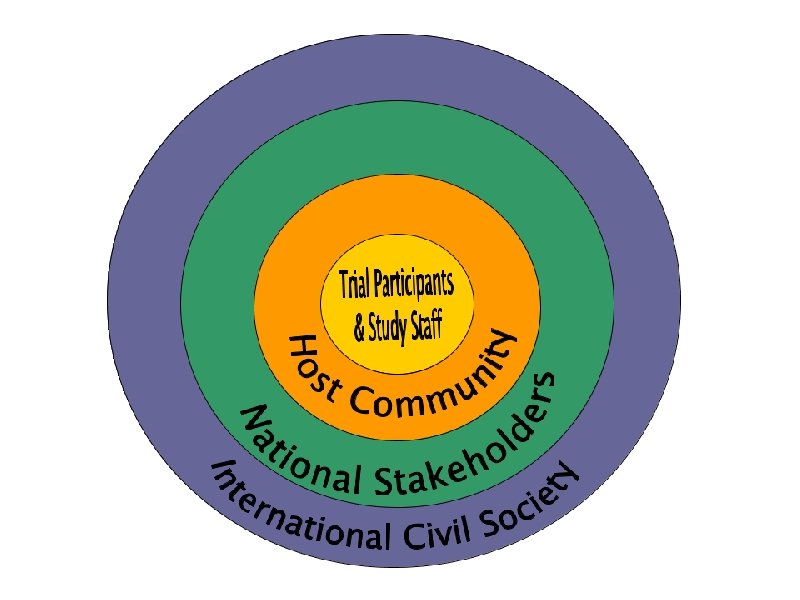
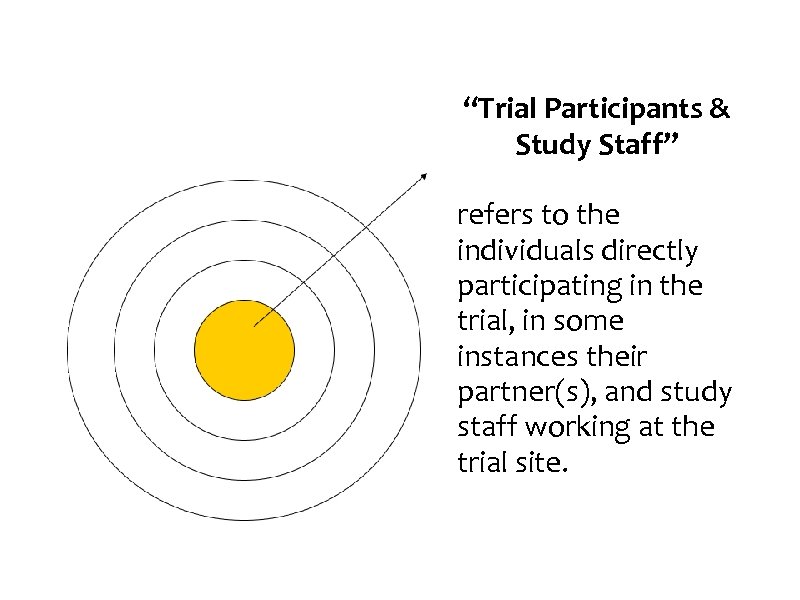 “Trial Participants & Study Staff” refers to the individuals directly participating in the trial, in some instances their partner(s), and study staff working at the trial site.
“Trial Participants & Study Staff” refers to the individuals directly participating in the trial, in some instances their partner(s), and study staff working at the trial site.
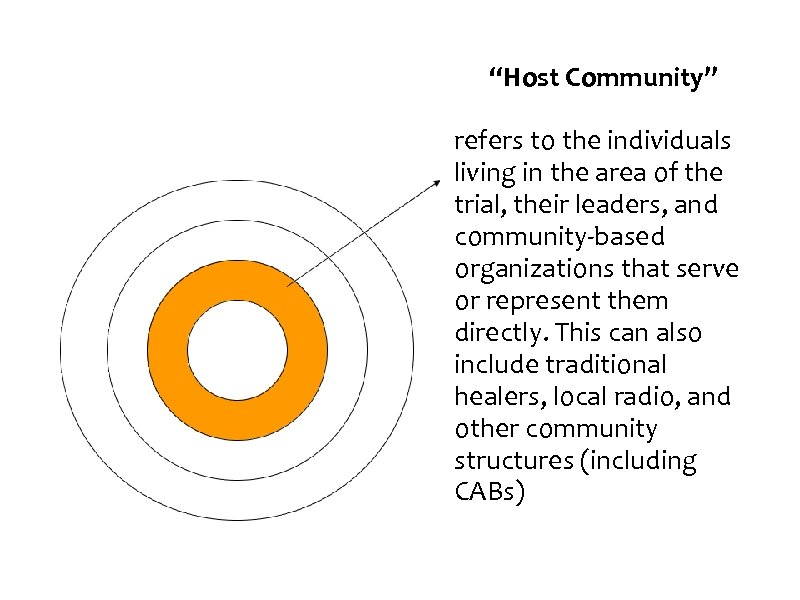 “Host Community” refers to the individuals living in the area of the trial, their leaders, and community-based organizations that serve or represent them directly. This can also include traditional healers, local radio, and other community structures (including CABs)
“Host Community” refers to the individuals living in the area of the trial, their leaders, and community-based organizations that serve or represent them directly. This can also include traditional healers, local radio, and other community structures (including CABs)
 “National Stakeholders” describes anyone who has a role to play in the political, scientific, and social enterprise of microbicide development in the larger, national community. It includes political decisionmakers, Mo. H, regulatory bodies, ethical review committees, national NGOs, donors, national media, etc.
“National Stakeholders” describes anyone who has a role to play in the political, scientific, and social enterprise of microbicide development in the larger, national community. It includes political decisionmakers, Mo. H, regulatory bodies, ethical review committees, national NGOs, donors, national media, etc.
 “International Civil Society” refers to non-profit, organized, citizen-led movements or groups interested in the goals, process, and outcomes of microbicide research, and/or in the rights of communities or research participants. Civil society includes international or regional NGOs (GCM/UNAIDS), international or media.
“International Civil Society” refers to non-profit, organized, citizen-led movements or groups interested in the goals, process, and outcomes of microbicide research, and/or in the rights of communities or research participants. Civil society includes international or regional NGOs (GCM/UNAIDS), international or media.
 Issues of Power • Power imbalances exist across multiple lines: Principal investigators versus field staff, Northern researchers versus Southern; community versus research enterprise; within communities and CABs • One goal of community involvement and NPT advocacy is to work towards reduced power disparities • Pretending that power imbalances do not exist, however, breeds the worst form of tokenism
Issues of Power • Power imbalances exist across multiple lines: Principal investigators versus field staff, Northern researchers versus Southern; community versus research enterprise; within communities and CABs • One goal of community involvement and NPT advocacy is to work towards reduced power disparities • Pretending that power imbalances do not exist, however, breeds the worst form of tokenism
 Why do we need Community Involvement? § Ethical principle of beneficence § Maximize benefits and minimizes risks for participants and for host communities. § Minimize exposure to controversy and risk of disruption § Increase the transparency and accountability of the research to the community § Improve quality of trials, participant retention, adherence and accurate self-reporting: ensuring trial procedures are acceptable to participants and other decision-makers § Strengthen local capacity and infrastructure
Why do we need Community Involvement? § Ethical principle of beneficence § Maximize benefits and minimizes risks for participants and for host communities. § Minimize exposure to controversy and risk of disruption § Increase the transparency and accountability of the research to the community § Improve quality of trials, participant retention, adherence and accurate self-reporting: ensuring trial procedures are acceptable to participants and other decision-makers § Strengthen local capacity and infrastructure
 Community Involvement Strategies Community advisory structures (CABs, CAGs, CACs, participant representatives) Community preparedness Community mappings Radio & local media Dramas and community events Network community working groups Cross-network community involvement
Community Involvement Strategies Community advisory structures (CABs, CAGs, CACs, participant representatives) Community preparedness Community mappings Radio & local media Dramas and community events Network community working groups Cross-network community involvement
 Community Advisory Groups Also Community Advisory Boards (CABs) CAGs are now required by many research sponsors and trial networks A CAG is a group of volunteers from the general public and from the diverse communities affected by a condition like HIV/AIDS A CAG is organized to assist and advise researchers within a given network or site
Community Advisory Groups Also Community Advisory Boards (CABs) CAGs are now required by many research sponsors and trial networks A CAG is a group of volunteers from the general public and from the diverse communities affected by a condition like HIV/AIDS A CAG is organized to assist and advise researchers within a given network or site

 • Why is so much blood taken? • What do you do with the left over blood? • Are the needles safe/clean?
• Why is so much blood taken? • What do you do with the left over blood? • Are the needles safe/clean?
 “No one wins when a trial is stopped for non-scientific reasons. But the only way to prevent this is to invest the time and resources needed to build the kind of mutual trust on which collaborative partnerships can be based. ” Anna Forbes & Sanushka Mudaliar Preventing Prevention Trial Failures: A Case Study and Lessons for Future Trials from the 2004 Tenofovir Trial in Cambodia
“No one wins when a trial is stopped for non-scientific reasons. But the only way to prevent this is to invest the time and resources needed to build the kind of mutual trust on which collaborative partnerships can be based. ” Anna Forbes & Sanushka Mudaliar Preventing Prevention Trial Failures: A Case Study and Lessons for Future Trials from the 2004 Tenofovir Trial in Cambodia
 “We will not let Cambodians be used as guinea pigs…” Cambodian prime minister
“We will not let Cambodians be used as guinea pigs…” Cambodian prime minister
 Case Study: Cambodia Tenofovir Study 2003: Preparations begin for the conduct of a tenofovir Pr. EP study among sex workers in Phnom Penh Many miscommunications and misunderstandings between community groups and researchers Protests at the International AIDS Conference, Bangkok Press release by activist groups denounce trial Media storm & negative reaction from Cambodia PM 2004: Trial halted by Cambodia government
Case Study: Cambodia Tenofovir Study 2003: Preparations begin for the conduct of a tenofovir Pr. EP study among sex workers in Phnom Penh Many miscommunications and misunderstandings between community groups and researchers Protests at the International AIDS Conference, Bangkok Press release by activist groups denounce trial Media storm & negative reaction from Cambodia PM 2004: Trial halted by Cambodia government
 Cameroon falls next
Cameroon falls next
 Lessons Learned: Community Consultation Must extend beyond local trial community to include NGOs and other opinion leaders and stakeholders Requires adequate lead time and a specialized skill set; Must begin early when input can still effect change Demands separate line item in the budget Formative research cannot substitute for a consultative process
Lessons Learned: Community Consultation Must extend beyond local trial community to include NGOs and other opinion leaders and stakeholders Requires adequate lead time and a specialized skill set; Must begin early when input can still effect change Demands separate line item in the budget Formative research cannot substitute for a consultative process
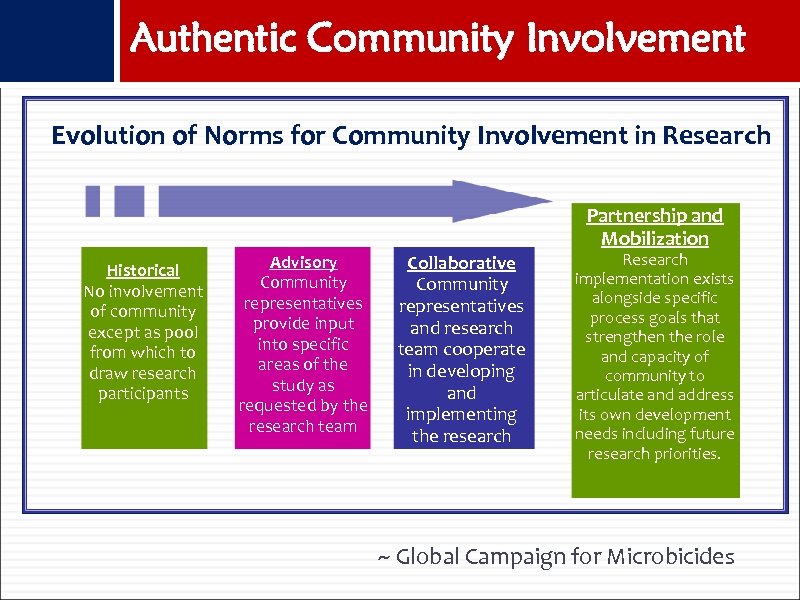 Authentic Community Involvement Evolution of Norms for Community Involvement in Research Partnership and Mobilization Historical No involvement of community except as pool from which to draw research participants Advisory Community representatives provide input into specific areas of the study as requested by the research team Collaborative Community representatives and research team cooperate in developing and implementing the research Research implementation exists alongside specific process goals that strengthen the role and capacity of community to articulate and address its own development needs including future research priorities. ~ Global Campaign for Microbicides
Authentic Community Involvement Evolution of Norms for Community Involvement in Research Partnership and Mobilization Historical No involvement of community except as pool from which to draw research participants Advisory Community representatives provide input into specific areas of the study as requested by the research team Collaborative Community representatives and research team cooperate in developing and implementing the research Research implementation exists alongside specific process goals that strengthen the role and capacity of community to articulate and address its own development needs including future research priorities. ~ Global Campaign for Microbicides
 DYNAMICS OF NORTH-SOUTH RESEARCH
DYNAMICS OF NORTH-SOUTH RESEARCH
 Researcher Obligations Ethically, researchers must provide participants with medical care and compensation for study-related injuries Legally, researchers may not have to provide treatment and compensation For example, US law only requires that study participants be told what types of compensation or treatment will be available
Researcher Obligations Ethically, researchers must provide participants with medical care and compensation for study-related injuries Legally, researchers may not have to provide treatment and compensation For example, US law only requires that study participants be told what types of compensation or treatment will be available
 “Can” implies “Ought” A person’s duty to benefit another is related to his or her capacity to do so, whether financial or practical. If a benefit cannot be provided for reasons of practical constraint, the duty to do so is weakened. Conversely, if a country’s wealth allows it to confer a benefit on the inhabitants of another country, the wealthier country has a stronger duty to provide that benefit
“Can” implies “Ought” A person’s duty to benefit another is related to his or her capacity to do so, whether financial or practical. If a benefit cannot be provided for reasons of practical constraint, the duty to do so is weakened. Conversely, if a country’s wealth allows it to confer a benefit on the inhabitants of another country, the wealthier country has a stronger duty to provide that benefit
 An alternative articulation of Core Ethical Principles The duty to alleviate suffering The duty to show respect for persons The duty to be sensitive to cultural difference The duty not to exploit the vulnerable or less powerful (Nuffield Council on Bioethics, 2002)
An alternative articulation of Core Ethical Principles The duty to alleviate suffering The duty to show respect for persons The duty to be sensitive to cultural difference The duty not to exploit the vulnerable or less powerful (Nuffield Council on Bioethics, 2002)
 PUTTING IT ALL TOGETHER: WHAT MAKES RESEARCH ETHICAL?
PUTTING IT ALL TOGETHER: WHAT MAKES RESEARCH ETHICAL?
 What Makes Research Ethical? Social or scientific value Scientific validity Fair subject selection Favorable risk-benefit ratio Independent review Informed consent Respect for potential and enrolled subjects Collaborative partnership (Emanual et al. , JAMA, 283, 2000)
What Makes Research Ethical? Social or scientific value Scientific validity Fair subject selection Favorable risk-benefit ratio Independent review Informed consent Respect for potential and enrolled subjects Collaborative partnership (Emanual et al. , JAMA, 283, 2000)
 Seven Steps for Ethical Research 1. Priorities: Did the study address a priority issue? Whose? 2. Planning: Was the study well designed to optimize the chances of 3. 4. 5. 6. 7. generating useful knowledge and protecting subjects? Permission: Was the project reviewed and cleared by the relevant institutions? Did the investigators obtain informed consent? Performance: Was the study conducted in a way that respected the rights of the subjects and minimized the risks to them? Processing: Were the results correctly analyzed and interpreted? Publication: Were the results published and disseminated? Programming: Have the findings been translated to policy and action?
Seven Steps for Ethical Research 1. Priorities: Did the study address a priority issue? Whose? 2. Planning: Was the study well designed to optimize the chances of 3. 4. 5. 6. 7. generating useful knowledge and protecting subjects? Permission: Was the project reviewed and cleared by the relevant institutions? Did the investigators obtain informed consent? Performance: Was the study conducted in a way that respected the rights of the subjects and minimized the risks to them? Processing: Were the results correctly analyzed and interpreted? Publication: Were the results published and disseminated? Programming: Have the findings been translated to policy and action?
 STANDARD OF PREVENTION AND CARE FOR TRIAL PARTICIPANTS
STANDARD OF PREVENTION AND CARE FOR TRIAL PARTICIPANTS
 Standard of Care The term “Standard of care” refers to the nature of the prevention and/or care that will be provided to participants in research the general care and treatment that investigators agree to provide all participants in clinical research the quality of care that should be provided to people in the control arm of a RCT – i. e. those that are not receiving the experimental intervention
Standard of Care The term “Standard of care” refers to the nature of the prevention and/or care that will be provided to participants in research the general care and treatment that investigators agree to provide all participants in clinical research the quality of care that should be provided to people in the control arm of a RCT – i. e. those that are not receiving the experimental intervention
 The Standard of Care Debate The appropriate “Standard of Care” in international trials has been subject to intense discussion and debate Debate heated up around controversial HIV trials to prevent mother to child transmission in the developing world Commentators questioned the ethics of trials that used a “placebo” when an existing regimen 076 had been shown to reduce peri-natal transmission of HIV in the United States Defenders argued that the 076 protocol was not “relevant” to the health care needs or priorities of the developing world, because it could not viably be implemented
The Standard of Care Debate The appropriate “Standard of Care” in international trials has been subject to intense discussion and debate Debate heated up around controversial HIV trials to prevent mother to child transmission in the developing world Commentators questioned the ethics of trials that used a “placebo” when an existing regimen 076 had been shown to reduce peri-natal transmission of HIV in the United States Defenders argued that the 076 protocol was not “relevant” to the health care needs or priorities of the developing world, because it could not viably be implemented
 076/Placebo Controversy Is it ever acceptable to have different standards of health care in different parts of the world? Should the control arm receive a “universal” standard of care (i. e. the best available anywhere) or is some other standard morally acceptable? Pits principle of non exploitation of those who are vulnerable against the desire to generate findings that are relevant to and sustainable in the settings where they are needed
076/Placebo Controversy Is it ever acceptable to have different standards of health care in different parts of the world? Should the control arm receive a “universal” standard of care (i. e. the best available anywhere) or is some other standard morally acceptable? Pits principle of non exploitation of those who are vulnerable against the desire to generate findings that are relevant to and sustainable in the settings where they are needed
 Universal Standard Position “I believe that our ethical standards should not depend on where the research is performed. Furthermore I believe the nature of investigator’s responsibility for the welfare of their subjects should not be influenced by the political or economic conditions of the region. In practical terms any other position could lead to the exploitation of people in developing countries, in order to conduct research that could not be performed in the sponsoring country. ” Marcia Angell, Editor, NEJM
Universal Standard Position “I believe that our ethical standards should not depend on where the research is performed. Furthermore I believe the nature of investigator’s responsibility for the welfare of their subjects should not be influenced by the political or economic conditions of the region. In practical terms any other position could lead to the exploitation of people in developing countries, in order to conduct research that could not be performed in the sponsoring country. ” Marcia Angell, Editor, NEJM
 Ratcheting Up Standard “As it is unlikely that an overall universal standard of care can be rapidly achieved in research projects in developing countries, the goal should be to implement reasonable standards that are significantly higher than available in the host country and closer to standards in the sponsoring country. These ideas should be applied in a way that progressively ratchets SOC upwards, both for subsequent research projects and for local health care infrastructure through genuine partnerships and capacity building, leaving participants and their communities better off after the trial than before. ” Shapiro and Benatar, 2003
Ratcheting Up Standard “As it is unlikely that an overall universal standard of care can be rapidly achieved in research projects in developing countries, the goal should be to implement reasonable standards that are significantly higher than available in the host country and closer to standards in the sponsoring country. These ideas should be applied in a way that progressively ratchets SOC upwards, both for subsequent research projects and for local health care infrastructure through genuine partnerships and capacity building, leaving participants and their communities better off after the trial than before. ” Shapiro and Benatar, 2003
 What does ethics guidance say? Individuals in the control arm must receive: § “An established effective intervention” (CIOMS) § “The best current prophylactic, diagnostic and therapeutic method” (Declaration of Helsinki, 2002) § Ideal: “best proven therapy; ” Minimum: “highest level of care attainable in light of … the circumstances listed" (UNAIDS vaccine guidance) § Ideal: “best proven; ” Minimum: “the best intervention available for the disease as part of the national health system” (Nuffield Council) § “Highest achievable” standard should be the goal (Benatar & Singer, BMJ, 2000)
What does ethics guidance say? Individuals in the control arm must receive: § “An established effective intervention” (CIOMS) § “The best current prophylactic, diagnostic and therapeutic method” (Declaration of Helsinki, 2002) § Ideal: “best proven therapy; ” Minimum: “highest level of care attainable in light of … the circumstances listed" (UNAIDS vaccine guidance) § Ideal: “best proven; ” Minimum: “the best intervention available for the disease as part of the national health system” (Nuffield Council) § “Highest achievable” standard should be the goal (Benatar & Singer, BMJ, 2000)

 Standard of prevention and care in biomedical prevention trials
Standard of prevention and care in biomedical prevention trials
 (Undue) Inducement Informed consent can be undermined by incentives that lead to “undue pressure”, “coercion” or “undue inducement” to participate An inducement may persuade an individual to change his or her mind about entering a research project, but this in itself is not enough to make it inappropriate An “inducement” becomes inappropriate when it causes a person to assume risks that they would ordinarily view as unacceptable (Nuffield Council on Bioethics)
(Undue) Inducement Informed consent can be undermined by incentives that lead to “undue pressure”, “coercion” or “undue inducement” to participate An inducement may persuade an individual to change his or her mind about entering a research project, but this in itself is not enough to make it inappropriate An “inducement” becomes inappropriate when it causes a person to assume risks that they would ordinarily view as unacceptable (Nuffield Council on Bioethics)
 How Do You Decide If It’s “Undue”? Harmfulness: the nature of the potential risks to the participant’s health Proportionality: whether the inducement is in proportion to the risks and costs of research Vulnerability: whether prospective participants are especially vulnerable to influence Reciprocal Justice: someone who benefits from the investment and sacrifice of others owes them proportional recompense
How Do You Decide If It’s “Undue”? Harmfulness: the nature of the potential risks to the participant’s health Proportionality: whether the inducement is in proportion to the risks and costs of research Vulnerability: whether prospective participants are especially vulnerable to influence Reciprocal Justice: someone who benefits from the investment and sacrifice of others owes them proportional recompense
 “…access to all state of the art HIV risk reduction methods” Traditionally means sexual counseling and condoms New HIV risk reduction methods should be added as they are scientifically validated Would that include a partially effective vaccine or microbicide when available? Pr. EP? Male circumcision? Red herring: this requirement could make it difficult (impossible? ) to analyze results of HIV prevention trials Undue burden on researchers?
“…access to all state of the art HIV risk reduction methods” Traditionally means sexual counseling and condoms New HIV risk reduction methods should be added as they are scientifically validated Would that include a partially effective vaccine or microbicide when available? Pr. EP? Male circumcision? Red herring: this requirement could make it difficult (impossible? ) to analyze results of HIV prevention trials Undue burden on researchers?
 SOC Debate as Applied to Microbicides What package of prevention services should participants in the control arm of a trial be provided? High standard HIV counseling, condoms, STD screening, treatment? What other care should be provided during the trial? Pap tests? Family Planning? Malaria Rx? What HIV care should individuals who seroconvert during the trial be provided? TB prophylaxis, nutrition counseling, support groups, MTCT, ARVs? What care, if any is due women who are screened out of the trial because they are already HIV+?
SOC Debate as Applied to Microbicides What package of prevention services should participants in the control arm of a trial be provided? High standard HIV counseling, condoms, STD screening, treatment? What other care should be provided during the trial? Pap tests? Family Planning? Malaria Rx? What HIV care should individuals who seroconvert during the trial be provided? TB prophylaxis, nutrition counseling, support groups, MTCT, ARVs? What care, if any is due women who are screened out of the trial because they are already HIV+?
 STEP Trial Found enhanced susceptibility to HIV among those in the experimental arm = trial related harm Calls for enhanced obligation to patients for care and treatment follow-up – monitoring of viral loads and ARV BUT no time limit was discussed – generally accepted as 5 years
STEP Trial Found enhanced susceptibility to HIV among those in the experimental arm = trial related harm Calls for enhanced obligation to patients for care and treatment follow-up – monitoring of viral loads and ARV BUT no time limit was discussed – generally accepted as 5 years
 Balancing methodological and ethical gold standards Should future trials exclude uncircumcised men? Should trials offer/require/encourage circumcision among male participants?
Balancing methodological and ethical gold standards Should future trials exclude uncircumcised men? Should trials offer/require/encourage circumcision among male participants?
 THAI PREP TRIAL Ethics Case Study
THAI PREP TRIAL Ethics Case Study
 Thai Pr. EP Trial CDC trial in Thailand: examining the safety and efficacy of tenofovir as Pr. EP Conducted in collaboration with the Bangkok Metropolitan Administration and the Thailand Ministry of Public Health is enrolling 2, 400 HIV-negative intravenous drug users (IDUs) – male and female – at 17 drug treatment clinics in Bangkok Participants are recruited at the drug treatment clinics, at community outreach sites, and through a peer referral program. No clean needles or needle exchange being provided to participants
Thai Pr. EP Trial CDC trial in Thailand: examining the safety and efficacy of tenofovir as Pr. EP Conducted in collaboration with the Bangkok Metropolitan Administration and the Thailand Ministry of Public Health is enrolling 2, 400 HIV-negative intravenous drug users (IDUs) – male and female – at 17 drug treatment clinics in Bangkok Participants are recruited at the drug treatment clinics, at community outreach sites, and through a peer referral program. No clean needles or needle exchange being provided to participants
 Discussion Questions 1. Which ethical principles are potentially being violated in the Thai Pr. EP trial? 2. Should the researchers be expected to provide needle exchange when such programs are not available in Thailand? 3. How could the trial have been designed to be more ethical? 4. What impact do you think these ethical concerns have on the validity of the trial results?
Discussion Questions 1. Which ethical principles are potentially being violated in the Thai Pr. EP trial? 2. Should the researchers be expected to provide needle exchange when such programs are not available in Thailand? 3. How could the trial have been designed to be more ethical? 4. What impact do you think these ethical concerns have on the validity of the trial results?
 Standard of Care and Prevention How do we choose? Unilateral decision – FDA regulations? By following existing country standards? Consensus following debate – Helsinki In consultation with research participants By considering the reasons or motivations for the research—crucial introspection Using research to improve health care -- Solomon Benatar, University of Capetown
Standard of Care and Prevention How do we choose? Unilateral decision – FDA regulations? By following existing country standards? Consensus following debate – Helsinki In consultation with research participants By considering the reasons or motivations for the research—crucial introspection Using research to improve health care -- Solomon Benatar, University of Capetown
 Standard of Care How do we achieve new ideals? Heightened sensitivity to exploitation Aim for reasonable practical limits Ratchet the standard upwards Build capacity through real partnerships Follow the ‘spirit’ of Declarations Avoid ‘cook-book’ attitudes to ethics Consider: context / safety / logistics / harm benefit / sustainability
Standard of Care How do we achieve new ideals? Heightened sensitivity to exploitation Aim for reasonable practical limits Ratchet the standard upwards Build capacity through real partnerships Follow the ‘spirit’ of Declarations Avoid ‘cook-book’ attitudes to ethics Consider: context / safety / logistics / harm benefit / sustainability
 USE OF ARVS FOR PREVENTION VERSUS TREATMENT
USE OF ARVS FOR PREVENTION VERSUS TREATMENT
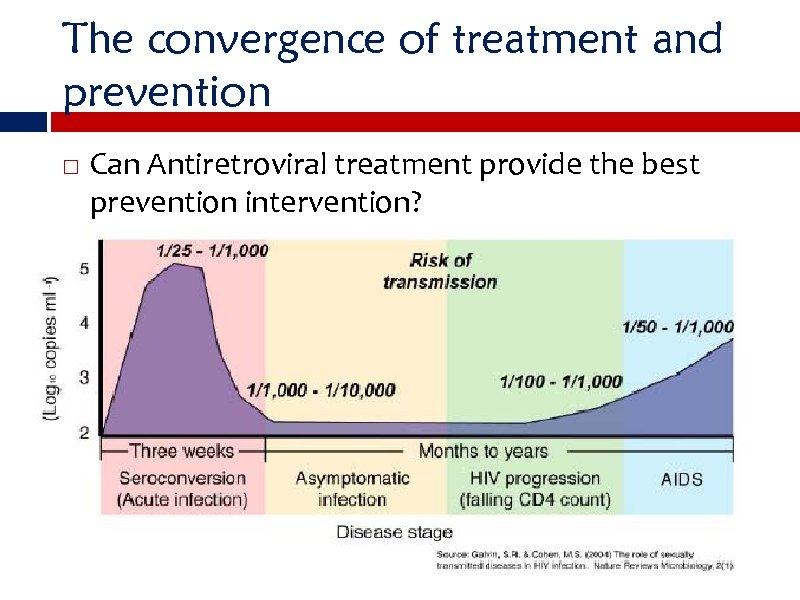 The convergence of treatment and prevention Can Antiretroviral treatment provide the best prevention intervention?
The convergence of treatment and prevention Can Antiretroviral treatment provide the best prevention intervention?
 “In view of the potential effect of HAART on HIV transmission, what would be the implications of an alternative prevention-centred strategy for the use of HAART? This approach would be based on the notion that new HIV infections are overwhelmingly contributed to by index HIV-infected individuals who are not on HAART. A prevention-centred approach would therefore argue that treating 100% of HIV-infected individuals at once could greatly reduce HIV transmission. While this would be costly in the short term, it could prove highly cost effective. The shortterm cost of treatment of all HIV-infected individuals would be more than offset by the number of new infections that it would prevent. ”
“In view of the potential effect of HAART on HIV transmission, what would be the implications of an alternative prevention-centred strategy for the use of HAART? This approach would be based on the notion that new HIV infections are overwhelmingly contributed to by index HIV-infected individuals who are not on HAART. A prevention-centred approach would therefore argue that treating 100% of HIV-infected individuals at once could greatly reduce HIV transmission. While this would be costly in the short term, it could prove highly cost effective. The shortterm cost of treatment of all HIV-infected individuals would be more than offset by the number of new infections that it would prevent. ”
 DEBATE SHOULD ARVS BE PRIORITIZED FOR PREVENTION OR FOR TREATMENT? Pr. EP Case Study
DEBATE SHOULD ARVS BE PRIORITIZED FOR PREVENTION OR FOR TREATMENT? Pr. EP Case Study
 Debate Group 1: Form three arguments for focusing ARV distribution globally on treating people already infected with HIV Group 2: Form three arguments for focusing ARV distribution globally as a prevention method with those who are not yet infected Plenary debrief: which argument is more convincing and why? Consensus statement
Debate Group 1: Form three arguments for focusing ARV distribution globally on treating people already infected with HIV Group 2: Form three arguments for focusing ARV distribution globally as a prevention method with those who are not yet infected Plenary debrief: which argument is more convincing and why? Consensus statement


