a4d3b0469659318090381893588a0631.ppt
- Количество слайдов: 40
 www. crc. gov. my
www. crc. gov. my
 National Transplant Registry Methodology Dr Jamaiyah Haniff Head, Disease & Treatment Registry Unit
National Transplant Registry Methodology Dr Jamaiyah Haniff Head, Disease & Treatment Registry Unit
 Methodology • • Background Components Organisation Operations
Methodology • • Background Components Organisation Operations
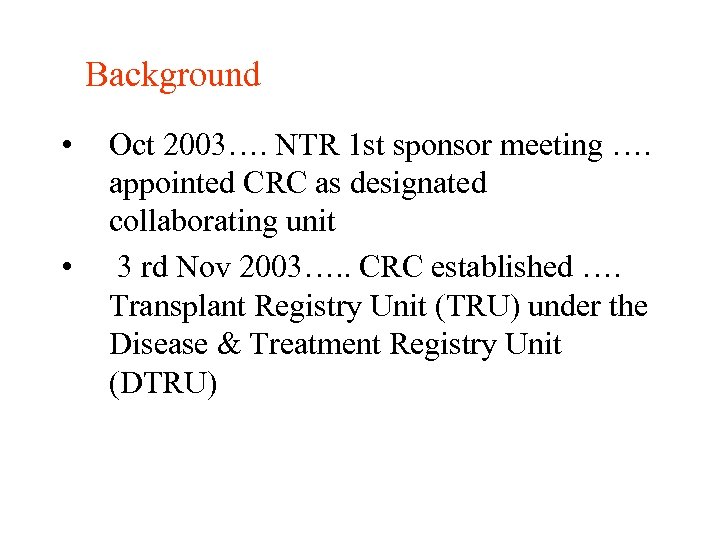 Background • • Oct 2003…. NTR 1 st sponsor meeting …. appointed CRC as designated collaborating unit 3 rd Nov 2003…. . CRC established …. Transplant Registry Unit (TRU) under the Disease & Treatment Registry Unit (DTRU)
Background • • Oct 2003…. NTR 1 st sponsor meeting …. appointed CRC as designated collaborating unit 3 rd Nov 2003…. . CRC established …. Transplant Registry Unit (TRU) under the Disease & Treatment Registry Unit (DTRU)
 CRC in NIH CARTA ORGANISASI RANGKAIAN PUSAT PENYELIDIKAN KLINIKAL INSTITUT KESIHATAN KEBANGSAAN (NATIONAL INSTITUTES OF HEALTH) MALAYSIA Institut Kesihatan Kebangsaan Rangkaian Institut Kesihatan Umum (IKU) Institut Penyelidikan Perubatan (IMR) CRC Hospital Ipoh Perak Institut Pengurusan Kesihatan (IHM) CRC Hospital Pulau Pinang Rangkaian Pusat Penyelidikan Klinikal (CRC) CRC Hospital Sultanah Aminah JB Institut Promosi Kesihatan (IHP) CRC Hospital Kota Bharu Kelantan Institut Penyelidikan Sistem Kesihatan (IHSR) CRC Hospital Kuching Sarawak Institut Kebangsaan Produk Asli dan Vaksinologi CRC Hospital Kuantan Pahang
CRC in NIH CARTA ORGANISASI RANGKAIAN PUSAT PENYELIDIKAN KLINIKAL INSTITUT KESIHATAN KEBANGSAAN (NATIONAL INSTITUTES OF HEALTH) MALAYSIA Institut Kesihatan Kebangsaan Rangkaian Institut Kesihatan Umum (IKU) Institut Penyelidikan Perubatan (IMR) CRC Hospital Ipoh Perak Institut Pengurusan Kesihatan (IHM) CRC Hospital Pulau Pinang Rangkaian Pusat Penyelidikan Klinikal (CRC) CRC Hospital Sultanah Aminah JB Institut Promosi Kesihatan (IHP) CRC Hospital Kota Bharu Kelantan Institut Penyelidikan Sistem Kesihatan (IHSR) CRC Hospital Kuching Sarawak Institut Kebangsaan Produk Asli dan Vaksinologi CRC Hospital Kuantan Pahang
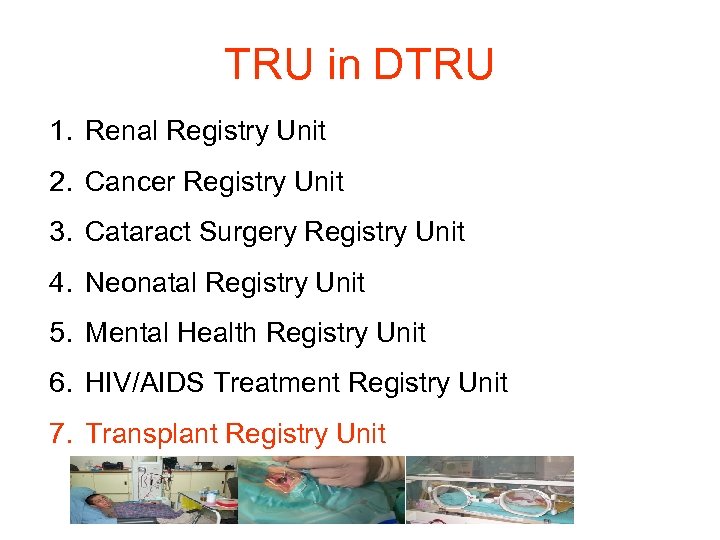 TRU in DTRU 1. Renal Registry Unit 2. Cancer Registry Unit 3. Cataract Surgery Registry Unit 4. Neonatal Registry Unit 5. Mental Health Registry Unit 6. HIV/AIDS Treatment Registry Unit 7. Transplant Registry Unit
TRU in DTRU 1. Renal Registry Unit 2. Cancer Registry Unit 3. Cataract Surgery Registry Unit 4. Neonatal Registry Unit 5. Mental Health Registry Unit 6. HIV/AIDS Treatment Registry Unit 7. Transplant Registry Unit

 “DATA IN, REPORT OUT” DTRU Mission statement • The DTRU aims to serve as the national centre for key diseases and treatment registration in the country by providing a range of services starting “from concept to operations” of these registries and through ensuring high quality standards right “from data to report” which would be used to finally effect patients’ outcome improvement programmes.
“DATA IN, REPORT OUT” DTRU Mission statement • The DTRU aims to serve as the national centre for key diseases and treatment registration in the country by providing a range of services starting “from concept to operations” of these registries and through ensuring high quality standards right “from data to report” which would be used to finally effect patients’ outcome improvement programmes.
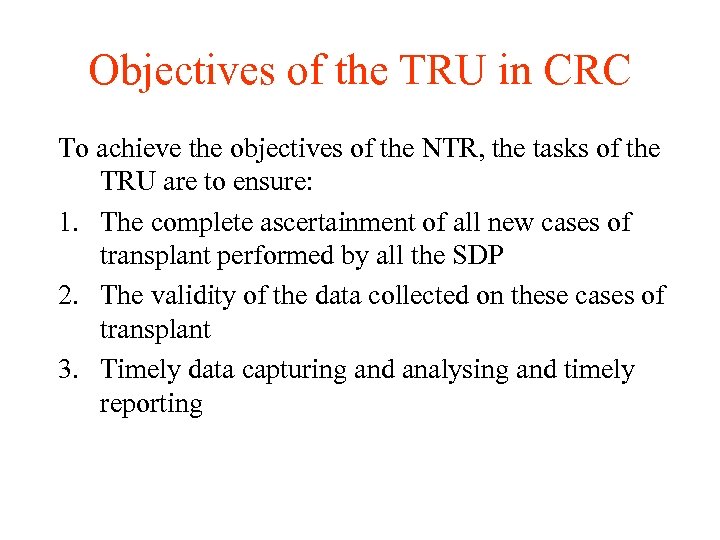 Objectives of the TRU in CRC To achieve the objectives of the NTR, the tasks of the TRU are to ensure: 1. The complete ascertainment of all new cases of transplant performed by all the SDP 2. The validity of the data collected on these cases of transplant 3. Timely data capturing and analysing and timely reporting
Objectives of the TRU in CRC To achieve the objectives of the NTR, the tasks of the TRU are to ensure: 1. The complete ascertainment of all new cases of transplant performed by all the SDP 2. The validity of the data collected on these cases of transplant 3. Timely data capturing and analysing and timely reporting
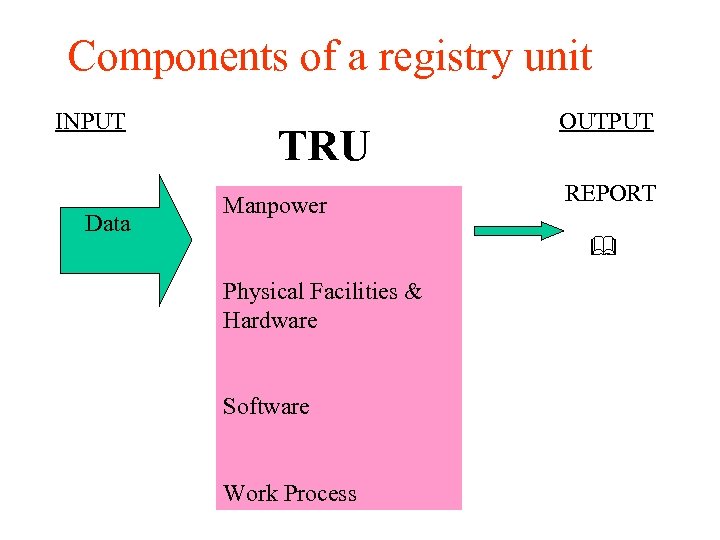 Components of a registry unit INPUT Data TRU Manpower OUTPUT REPORT Physical Facilities & Hardware Software Work Process
Components of a registry unit INPUT Data TRU Manpower OUTPUT REPORT Physical Facilities & Hardware Software Work Process
 Manpower 1. 2. 3. 4. 5. 6. 7. 8. Clinical Registry Manager (CRM) Clinical Registry Coordinator (CRC) Clinical Registry Assistant (CRA) Epidemiologist Biostatistician Report editor Desktop publisher Webmaster 8. 9. 10. 11. 12. 13. Clinical Data Manager Database Administrator Programmer Network administrator Security officer Regulatory / Compliance officer
Manpower 1. 2. 3. 4. 5. 6. 7. 8. Clinical Registry Manager (CRM) Clinical Registry Coordinator (CRC) Clinical Registry Assistant (CRA) Epidemiologist Biostatistician Report editor Desktop publisher Webmaster 8. 9. 10. 11. 12. 13. Clinical Data Manager Database Administrator Programmer Network administrator Security officer Regulatory / Compliance officer
 Physical Facilities & Hardware 1. Office space 2. IT infra: Server, VPN , network, workstation, etc 3. Communication infra - direct lines, broad band 4. Archive 5. Security infrastructure
Physical Facilities & Hardware 1. Office space 2. IT infra: Server, VPN , network, workstation, etc 3. Communication infra - direct lines, broad band 4. Archive 5. Security infrastructure
 Office
Office
 Security measures (1)
Security measures (1)
 Security measures (2)
Security measures (2)
 Security measure (3) – Fire safety facilities
Security measure (3) – Fire safety facilities
 Office space
Office space
 On-site Server
On-site Server
 Archive Facilities
Archive Facilities
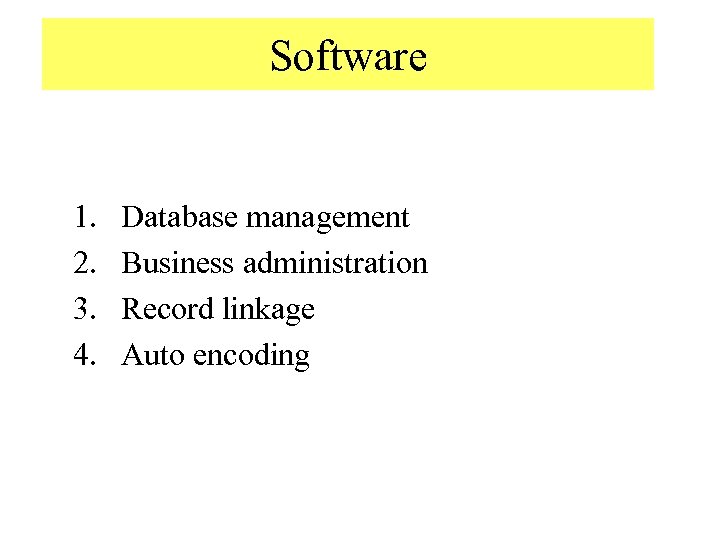 Software 1. 2. 3. 4. Database management Business administration Record linkage Auto encoding
Software 1. 2. 3. 4. Database management Business administration Record linkage Auto encoding
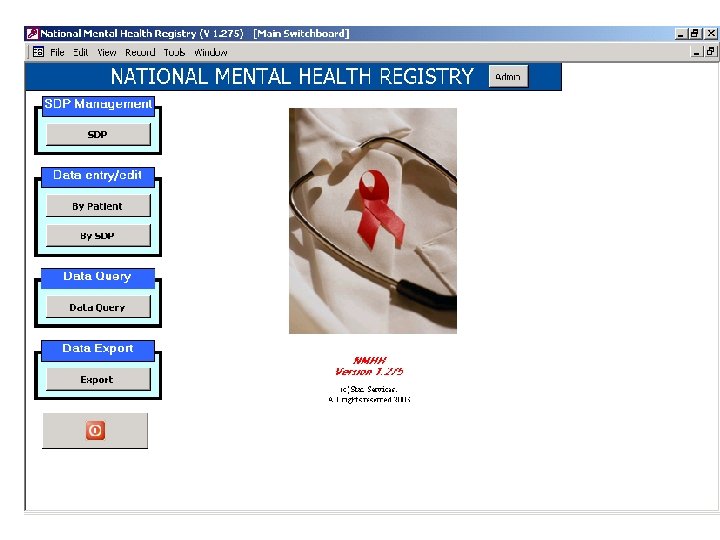
 Contact / Hospital Management
Contact / Hospital Management
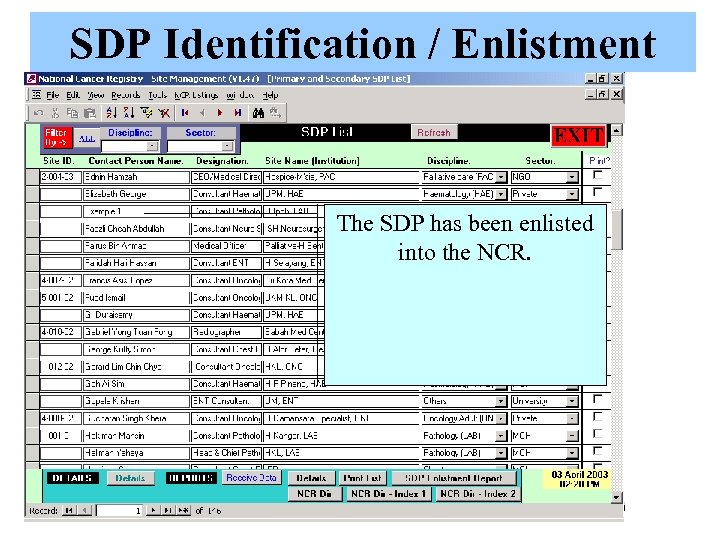 SDP Identification / Enlistment Click Exit to Save this SDP record Identified a new SDP: Department of If response is not received from the Pathology, Hospital Ipoh identified prospective SDP, data manager will The SDP has been enlisted To add a contact (SDP / Donor / EPM / Sponsor / etc), click on try other means to enlist site. into the NCR. Contacts. Send an ‘Invitation to Participate Letter” to this Tick Confirm SDP as ‘Yes’ to If response is received from the SDP. register the SDP as participant. Identified prospective SDP, The SDP will appear in Select the SDP as register the SDP as participant. SDP Evaluation list ‘Yes’ for Prospective SDP Click on Add New button to enlist a new SDP.
SDP Identification / Enlistment Click Exit to Save this SDP record Identified a new SDP: Department of If response is not received from the Pathology, Hospital Ipoh identified prospective SDP, data manager will The SDP has been enlisted To add a contact (SDP / Donor / EPM / Sponsor / etc), click on try other means to enlist site. into the NCR. Contacts. Send an ‘Invitation to Participate Letter” to this Tick Confirm SDP as ‘Yes’ to If response is received from the SDP. register the SDP as participant. Identified prospective SDP, The SDP will appear in Select the SDP as register the SDP as participant. SDP Evaluation list ‘Yes’ for Prospective SDP Click on Add New button to enlist a new SDP.
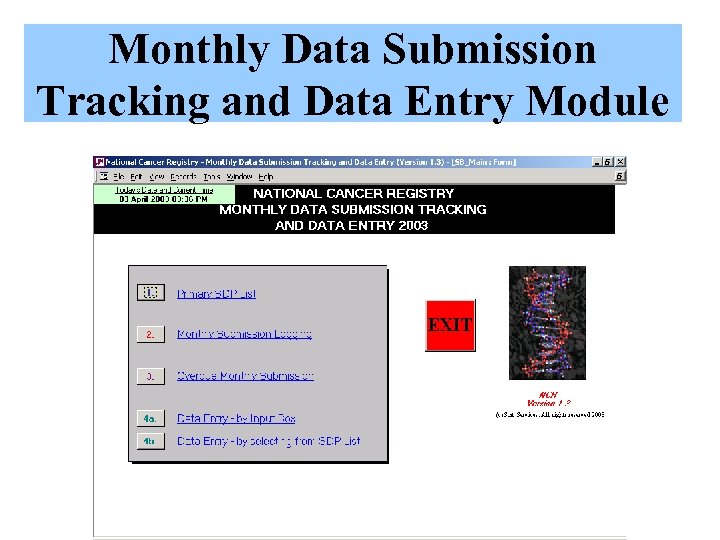 Monthly Data Submission Tracking and Data Entry Module
Monthly Data Submission Tracking and Data Entry Module
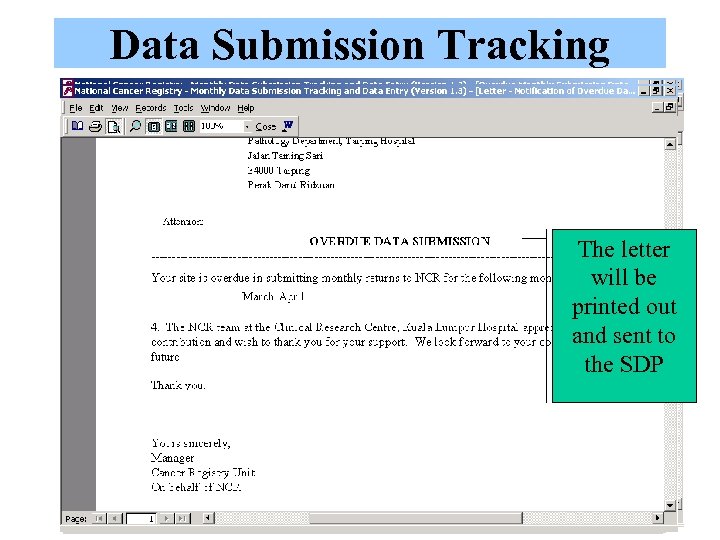 Data Submission Tracking Click Exit to save Type the data was received An acknowledgement The letter When the letter is Data manager will Select ‘Yes’ for the of data submission will be sent, the date will also review the At the end of each month, At the beginning of month when data is Click this to log a letter will appear. printed out be recorded number of data this report will be reviewed each month, data received monthly and sent to Click on the SDP link within the system returns (# of patient by Data Manager to identify manager will submission by This letter is printed and a popup to log records) to date. the progress of data the SDP identify who are the SDP out and sent to the monthly data submission SDPs that have not SDP. submission will appear submitted their data
Data Submission Tracking Click Exit to save Type the data was received An acknowledgement The letter When the letter is Data manager will Select ‘Yes’ for the of data submission will be sent, the date will also review the At the end of each month, At the beginning of month when data is Click this to log a letter will appear. printed out be recorded number of data this report will be reviewed each month, data received monthly and sent to Click on the SDP link within the system returns (# of patient by Data Manager to identify manager will submission by This letter is printed and a popup to log records) to date. the progress of data the SDP identify who are the SDP out and sent to the monthly data submission SDPs that have not SDP. submission will appear submitted their data
 Data Review and Coding Module
Data Review and Coding Module
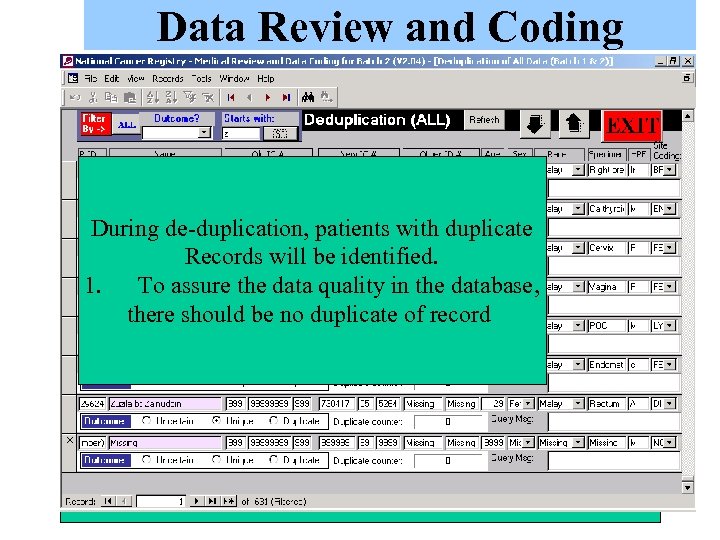 Data Review and Coding Here, CRC’s medical offier will verify the codes that has been autocoded by the system. They may select one of the following During de-duplication, patients with duplicate In the meantime while The data will be printed out Options: (1) Verified (2)Recode to another For records that were not autocoded Records will be identified. Upon clicking this button, waiting for data to be and sent to Expert Panel major site code (3)Uncertain (4)Query data After data has been received from EPM, data will be by the system, the reviewer will code 1. To assure the data quality in the database, the system will reviewed by EPM, data Members for verification, review entered into the system. specimen/HPE based on there should be no duplicate of record automatically code the Manager will resolve queries and coding. their judgement specimen and HPE data arising from patient data. to ICD-10 Major site code based on a predefined keyword dictionary.
Data Review and Coding Here, CRC’s medical offier will verify the codes that has been autocoded by the system. They may select one of the following During de-duplication, patients with duplicate In the meantime while The data will be printed out Options: (1) Verified (2)Recode to another For records that were not autocoded Records will be identified. Upon clicking this button, waiting for data to be and sent to Expert Panel major site code (3)Uncertain (4)Query data After data has been received from EPM, data will be by the system, the reviewer will code 1. To assure the data quality in the database, the system will reviewed by EPM, data Members for verification, review entered into the system. specimen/HPE based on there should be no duplicate of record automatically code the Manager will resolve queries and coding. their judgement specimen and HPE data arising from patient data. to ICD-10 Major site code based on a predefined keyword dictionary.
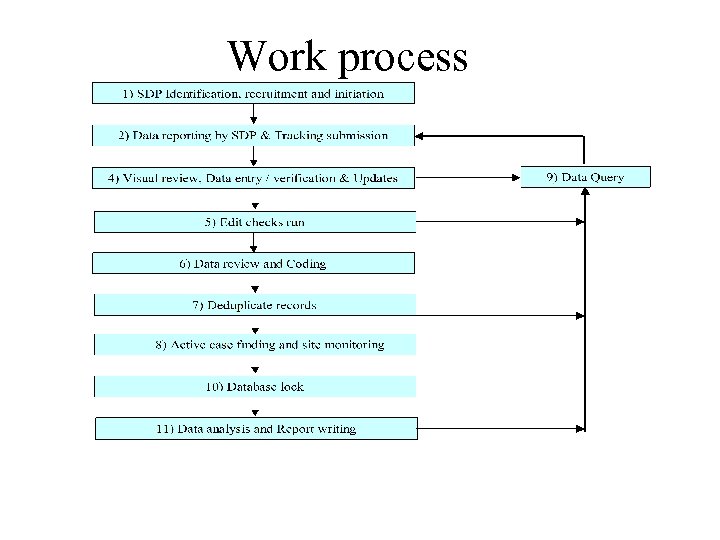 Work process
Work process
 1) SDP Identification, recruitment and initiation 1. Definition of SDP: Treating physicians or surgeons who manage cases of transplant (organ and tissue) 2. TRU would identify and enlist all SDP 3. TRU would ensure continuous flow of data by continuing motivation: - KOL, peer and Professional society - Meaningful involvement: Governance Board, Expert panel, NTR supported Transplant Research group - Timely feedback and report - Marketing: web, NTR events etc
1) SDP Identification, recruitment and initiation 1. Definition of SDP: Treating physicians or surgeons who manage cases of transplant (organ and tissue) 2. TRU would identify and enlist all SDP 3. TRU would ensure continuous flow of data by continuing motivation: - KOL, peer and Professional society - Meaningful involvement: Governance Board, Expert panel, NTR supported Transplant Research group - Timely feedback and report - Marketing: web, NTR events etc
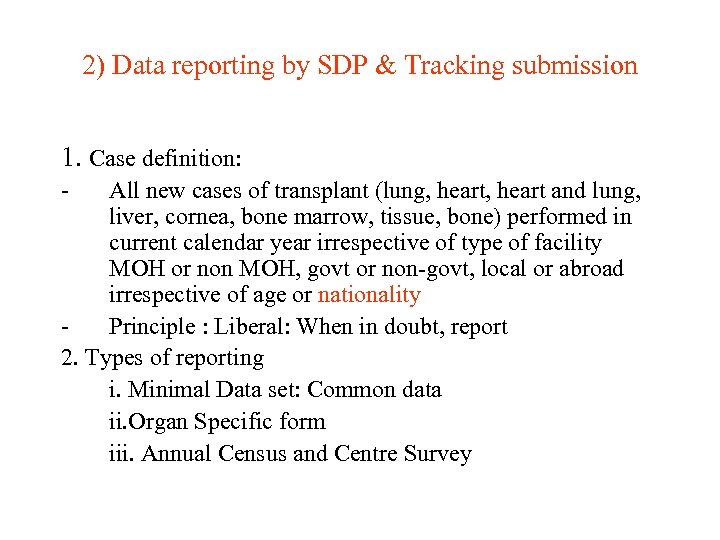 2) Data reporting by SDP & Tracking submission 1. Case definition: - All new cases of transplant (lung, heart and lung, liver, cornea, bone marrow, tissue, bone) performed in current calendar year irrespective of type of facility MOH or non MOH, govt or non-govt, local or abroad irrespective of age or nationality Principle : Liberal: When in doubt, report 2. Types of reporting i. Minimal Data set: Common data ii. Organ Specific form iii. Annual Census and Centre Survey
2) Data reporting by SDP & Tracking submission 1. Case definition: - All new cases of transplant (lung, heart and lung, liver, cornea, bone marrow, tissue, bone) performed in current calendar year irrespective of type of facility MOH or non MOH, govt or non-govt, local or abroad irrespective of age or nationality Principle : Liberal: When in doubt, report 2. Types of reporting i. Minimal Data set: Common data ii. Organ Specific form iii. Annual Census and Centre Survey
 2) Data reporting by SDP & Tracking submission 3. Frequency of submission For i and ii minimally monthly submission is requested otherwise if not possible ad hoc reporting. For iii. Annual at year end 4. Methods of reporting: -electronic data capture -paper based
2) Data reporting by SDP & Tracking submission 3. Frequency of submission For i and ii minimally monthly submission is requested otherwise if not possible ad hoc reporting. For iii. Annual at year end 4. Methods of reporting: -electronic data capture -paper based
 3. Data management (steps 4 -10) Visual review for error 2. Login into an automated data tracking system (prompt and reminders) 3. Data entry / verification & updates 4. Run edit checks for non-allowed codes, extreme values, inconsistent data etc 5. Data review and coding 6. Search for duplicate records 7. Periodic intensive Active case finding and site monitoring to round up all the unreported cases & late notifications and other Data Query (need for Direct access to source data and SDP cooperation) 8. Database lock 1.
3. Data management (steps 4 -10) Visual review for error 2. Login into an automated data tracking system (prompt and reminders) 3. Data entry / verification & updates 4. Run edit checks for non-allowed codes, extreme values, inconsistent data etc 5. Data review and coding 6. Search for duplicate records 7. Periodic intensive Active case finding and site monitoring to round up all the unreported cases & late notifications and other Data Query (need for Direct access to source data and SDP cooperation) 8. Database lock 1.
 Conclusion • The challenge ……. . to commence data collection from 1. 1. 2004 • The deliverable ……. . 1 st first National Transplant Registry Report by July 2005
Conclusion • The challenge ……. . to commence data collection from 1. 1. 2004 • The deliverable ……. . 1 st first National Transplant Registry Report by July 2005
 Thank you www. crc. gov. my
Thank you www. crc. gov. my


