29e23a0cebe7a550b55c83e41b83c25d.ppt
- Количество слайдов: 40

Wortham Laboratories Fast. Act Presentation IN-PROCESS COPY ® Presentation NOT FOR RELEASE! – 10. 2007 © 2007 Wortham Laboratories, Inc.

n. Wortham Training This presentation is intended to inform our sales network and professional medical practitioners about the next generation of coagulation products. This presentation is made available with the following important conditionsinherent to the delivery of product information to the medical community and our sales network: While the information prepared herein is presented in good faith, changes may occur which affect the intended result of this presentation. Please understand that literature and training regarding the proper utilization of Wortham products may be updated at any time. Material changes in product information will be made available as changes are made. This presentation was prepared in October 2007. Each WLI Presentation is presented with version date. The use of any medical product requires specific instruction which may exceed the scope of this presentation. Please consult Wortham Laboratories product inserts, your distributor and/or our support desk with any product questions. We are devoted to your satisfaction and success. While this presentation represents Wortham products as suitable for a particular purpose, the presentation alone cannot act as a sole source of information for introducing this product into your laboratory or other setting. We will provide a full complement of supporting tools and documentation to assist you. The contents of this presentation are Copyright © 2007 Wortham Laboratories. This presentation contains internationally recognized trademark of Wortham Laboratories, Incorporated. Other trademarks referenced herein are the property of their specific owners, and are incorporated throughout for the exclusive purpose of information, education and training. THIS PRESENTATION IS FORMATTED FOR NARRATIVE HOSTING, and cannot serve as a sole product insert or instructional reference document. WELCOME TO WORTHAM LABORATORIES – TOMORROW’S TECHNOLOGY TODAY

Introduction
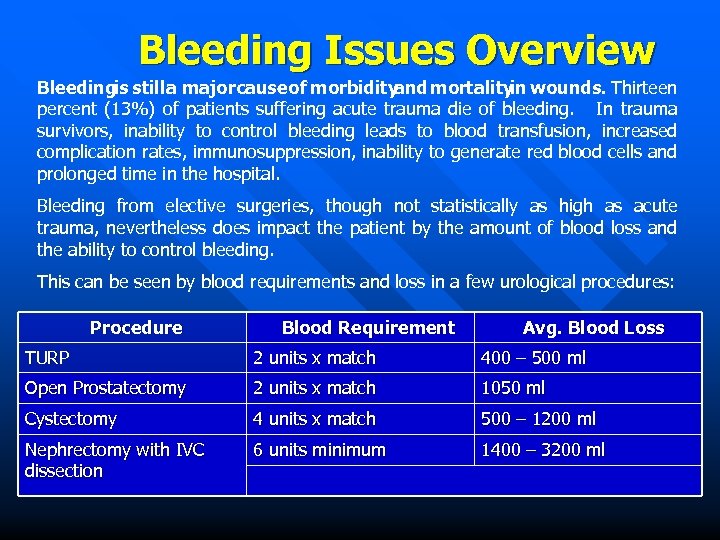
Bleeding Issues Overview Bleedingis still a majorcause of morbidity and mortality wounds. Thirteen in percent (13%) of patients suffering acute trauma die of bleeding. In trauma survivors, inability to control bleeding leads to blood transfusion, increased complication rates, immunosuppression, inability to generate red blood cells and prolonged time in the hospital. Bleeding from elective surgeries, though not statistically as high as acute trauma, nevertheless does impact the patient by the amount of blood loss and the ability to control bleeding. This can be seen by blood requirements and loss in a few urological procedures: Procedure Blood Requirement Avg. Blood Loss TURP 2 units x match 400 – 500 ml Open Prostatectomy 2 units x match 1050 ml Cystectomy 4 units x match 500 – 1200 ml Nephrectomy with IVC dissection 6 units minimum 1400 – 3200 ml
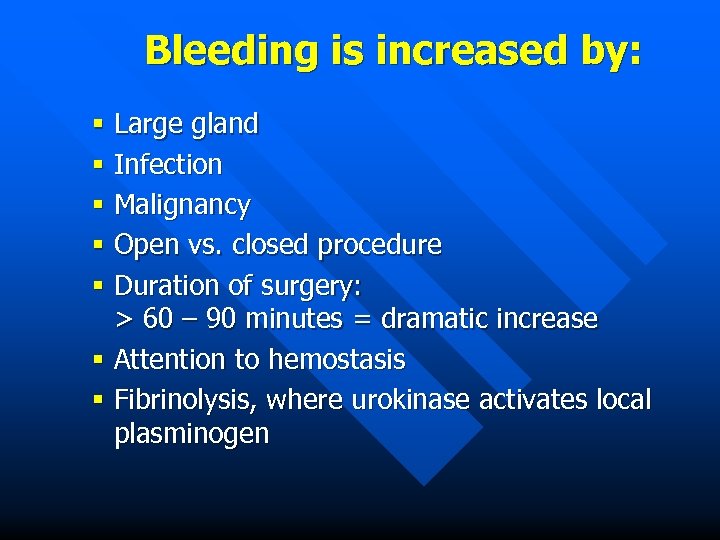
Bleeding is increased by: § Large gland § Infection § Malignancy § Open vs. closed procedure § Duration of surgery: > 60 – 90 minutes = dramatic increase § Attention to hemostasis § Fibrinolysis, where urokinase activates local plasminogen

Impact on Surgeon Bleeding intrinsicallyimpacts the surgeon, too, by a state of stress and anxietythat beginsprior to surgeryand lasts at least throughthe first 24 -48 hours post-op. The ideal solution to this problems is to control the bleeding in the first plac A quick, effective and safe device to control bleeding is found in a new ® hemostatic agent, Fast. Act.
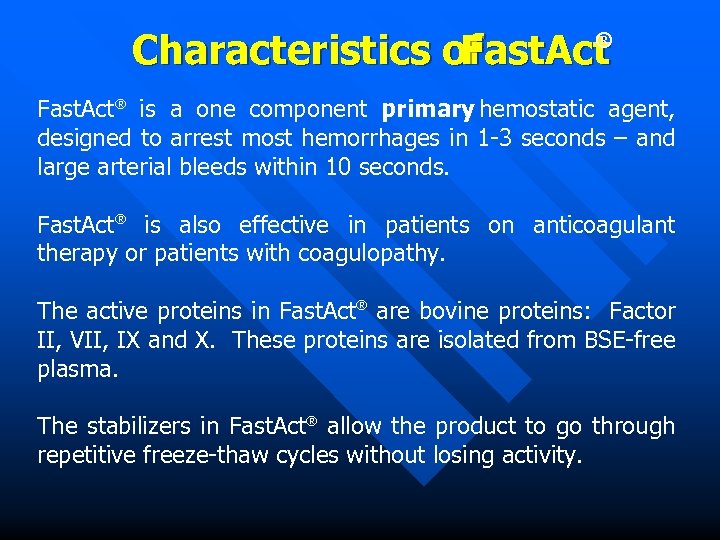
Characteristics of Fast. Act ® Fast. Act® is a one component primary hemostatic agent, designed to arrest most hemorrhages in 1 -3 seconds – and large arterial bleeds within 10 seconds. Fast. Act® is also effective in patients on anticoagulant therapy or patients with coagulopathy. The active proteins in Fast. Act® are bovine proteins: Factor II, VII, IX and X. These proteins are isolated from BSE-free plasma. The stabilizers in Fast. Act® allow the product to go through repetitive freeze-thaw cycles without losing activity.

Contrast: ® Fast. Act vs. Standard Surgical Methods -and. Other Hemostatic Agents
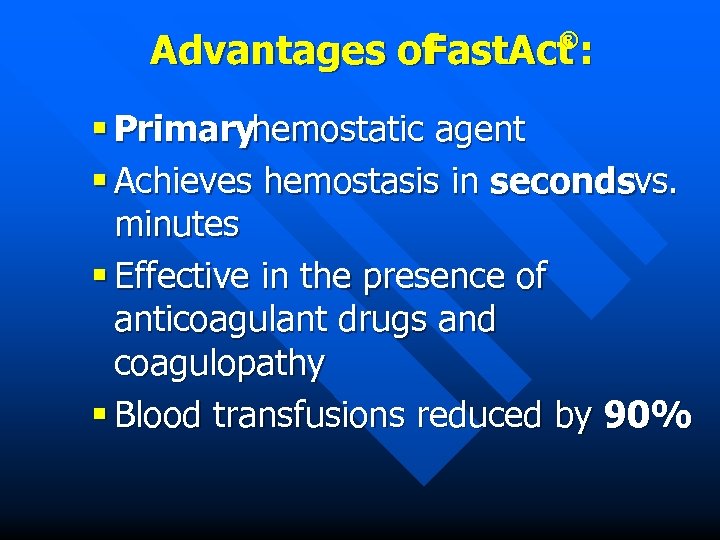
Advantages of Fast. Act : ® § Primaryhemostatic agent § Achieves hemostasis in secondsvs. minutes § Effective in the presence of anticoagulant drugs and coagulopathy § Blood transfusions reduced by 90%

Advantages of Fast. Act : ® § Surgical time reduced by 50% § No loss of tissue § Shorter healing time § No mixing, no preparation § Multiple delivery systems vs. one system § Reduces overall costs
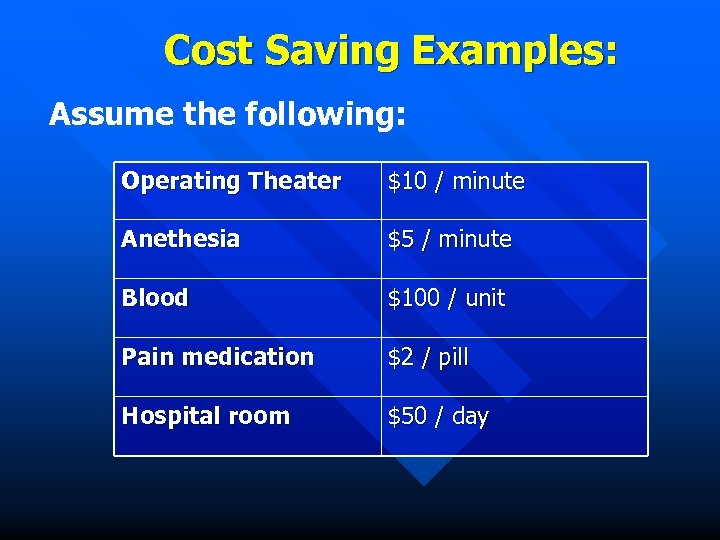
Cost Saving Examples: Assume the following: Operating Theater $10 / minute Anethesia $5 / minute Blood $100 / unit Pain medication $2 / pill Hospital room $50 / day
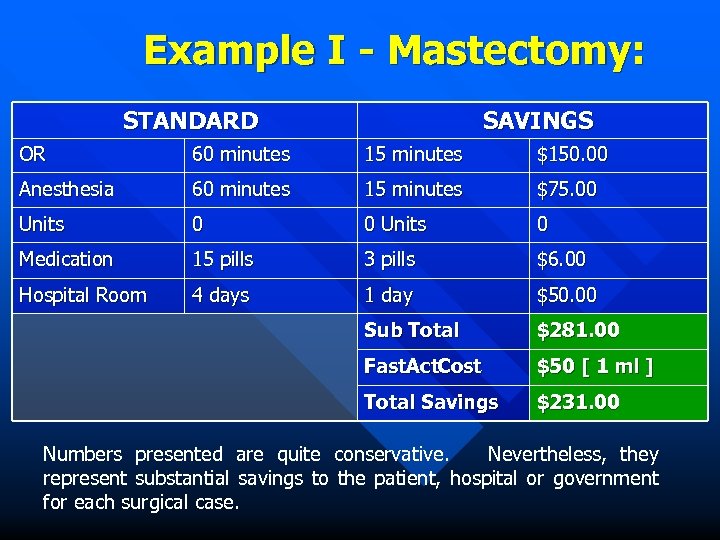
Example I - Mastectomy: STANDARD SAVINGS OR 60 minutes 15 minutes $150. 00 Anesthesia 60 minutes 15 minutes $75. 00 Units 0 Medication 15 pills 3 pills $6. 00 Hospital Room 4 days 1 day $50. 00 Sub Total $281. 00 Fast. Act. Cost $50 [ 1 ml ] Total Savings $231. 00 Numbers presented are quite conservative. Nevertheless, they represent substantial savings to the patient, hospital or government for each surgical case.
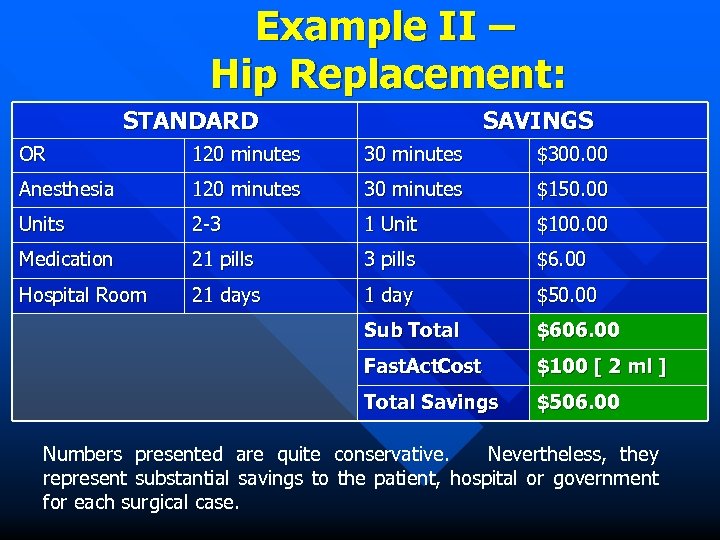
Example II – Hip Replacement: STANDARD SAVINGS OR 120 minutes 30 minutes $300. 00 Anesthesia 120 minutes 30 minutes $150. 00 Units 2 -3 1 Unit $100. 00 Medication 21 pills 3 pills $6. 00 Hospital Room 21 days 1 day $50. 00 Sub Total $606. 00 Fast. Act. Cost $100 [ 2 ml ] Total Savings $506. 00 Numbers presented are quite conservative. Nevertheless, they represent substantial savings to the patient, hospital or government for each surgical case.
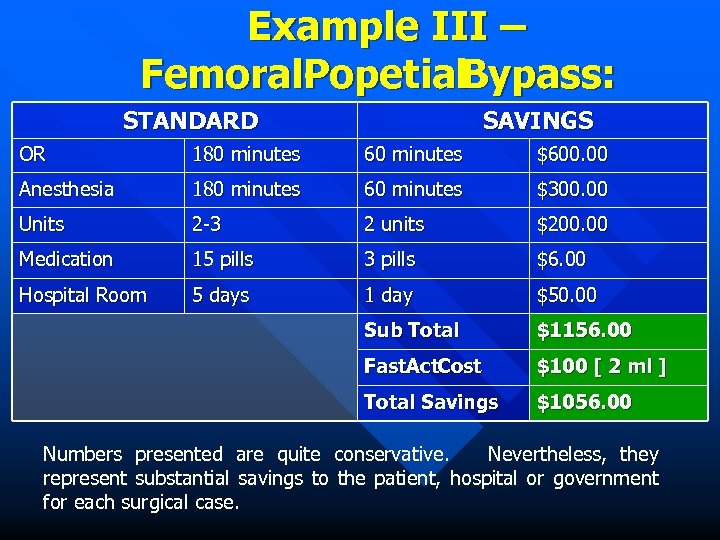
Example III – Femoral- opetial Popetial. Bypass: P STANDARD SAVINGS OR 180 minutes 60 minutes $600. 00 Anesthesia 180 minutes 60 minutes $300. 00 Units 2 -3 2 units $200. 00 Medication 15 pills 3 pills $6. 00 Hospital Room 5 days 1 day $50. 00 Sub Total $1156. 00 Fast. Act. Cost $100 [ 2 ml ] Total Savings $1056. 00 Numbers presented are quite conservative. Nevertheless, they represent substantial savings to the patient, hospital or government for each surgical case.

How Fast. Act Works ® Efficacy and Safety
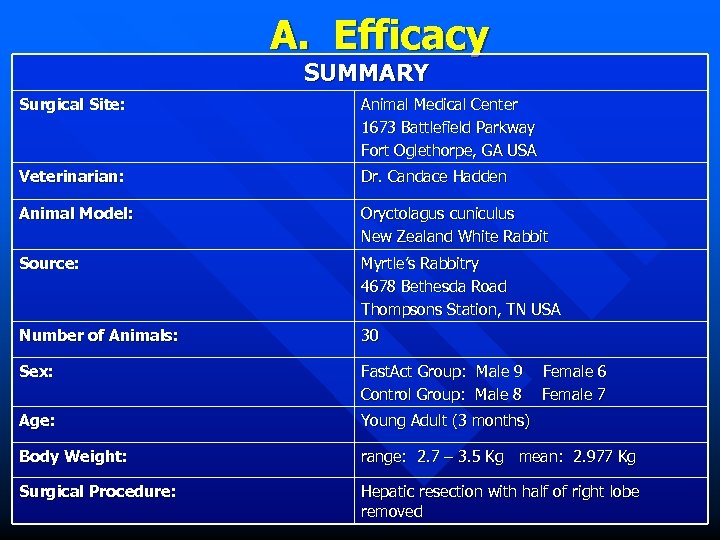
A. Efficacy SUMMARY Surgical Site: Animal Medical Center 1673 Battlefield Parkway Fort Oglethorpe, GA USA Veterinarian: Dr. Candace Hadden Animal Model: Oryctolagus cuniculus New Zealand White Rabbit Source: Myrtle’s Rabbitry 4678 Bethesda Road Thompsons Station, TN USA Number of Animals: 30 Sex: Fast. Act Group: Male 9 Control Group: Male 8 Age: Young Adult (3 months) Body Weight: range: 2. 7 – 3. 5 Kg mean: 2. 977 Kg Surgical Procedure: Hepatic resection with half of right lobe removed Female 6 Female 7
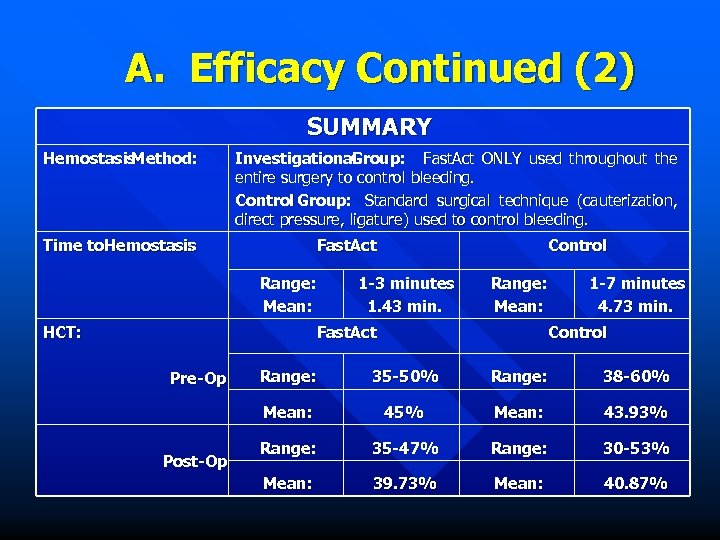
A. Efficacy Continued (2) SUMMARY Hemostasis Method: Investigational Group: Fast. Act ONLY used throughout the entire surgery to control bleeding. Control Group: Standard surgical technique (cauterization, direct pressure, ligature) used to control bleeding. Time to. Hemostasis : Fast. Act Range: Mean: HCT: Control 1 -3 minutes 1. 43 min. Range: Mean: Fast. Act 1 -7 minutes 4. 73 min. Control Post-Op Range: 35 -50% Range: 38 -60% Mean: Pre-Op 45% Mean: 43. 93% Range: 35 -47% Range: 30 -53% Mean: 39. 73% Mean: 40. 87%
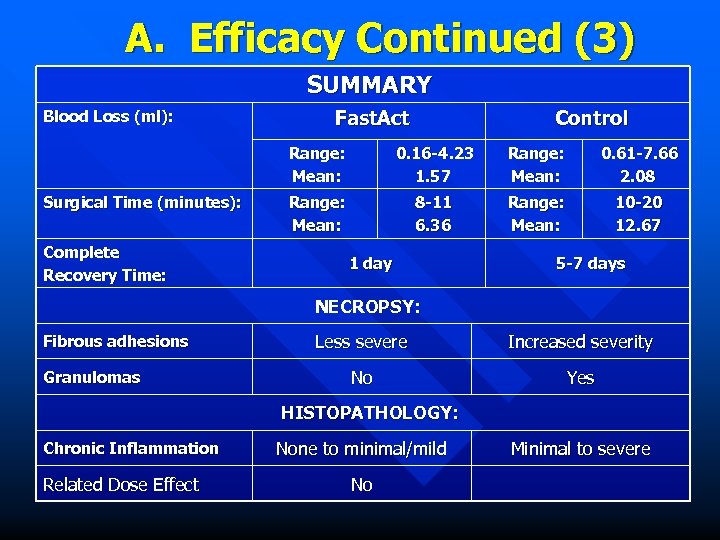
A. Efficacy Continued (3) SUMMARY Blood Loss (ml): Fast. Act Control Range: Mean: Surgical Time (minutes): Complete Recovery Time: 0. 16 -4. 23 1. 57 Range: Mean: 0. 61 -7. 66 2. 08 Range: Mean: 8 -11 6. 36 Range: Mean: 10 -20 12. 67 1 day 5 -7 days NECROPSY: Fibrous adhesions Granulomas Less severe Increased severity No Yes HISTOPATHOLOGY: Chronic Inflammation Related Dose Effect None to minimal/mild No Minimal to severe
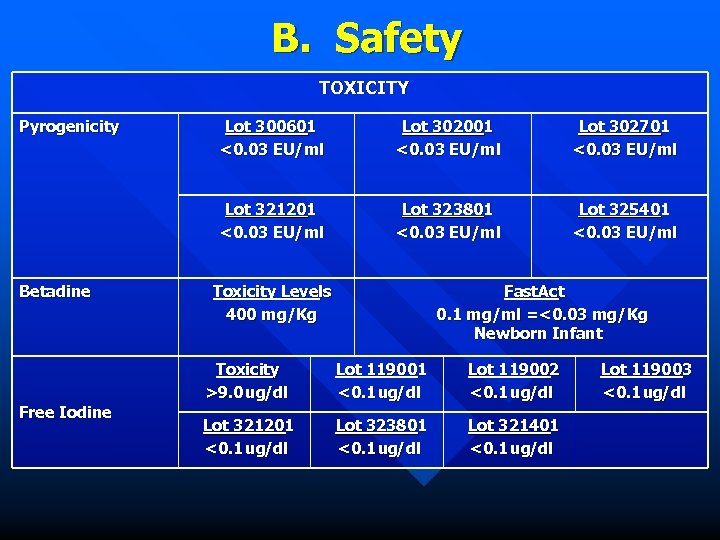
B. Safety TOXICITY Pyrogenicity Free Iodine Lot 302001 <0. 03 EU/ml Lot 302701 <0. 03 EU/ml Lot 321201 <0. 03 EU/ml Betadine Lot 300601 <0. 03 EU/ml Lot 323801 <0. 03 EU/ml Lot 325401 <0. 03 EU/ml Toxicity Levels 400 mg/Kg Fast. Act 0. 1 mg/ml =<0. 03 mg/Kg Newborn Infant Toxicity >9. 0 ug/dl Lot 119001 <0. 1 ug/dl Lot 119002 <0. 1 ug/dl Lot 321201 <0. 1 ug/dl Lot 323801 <0. 1 ug/dl Lot 321401 <0. 1 ug/dl Lot 119003 <0. 1 ug/dl
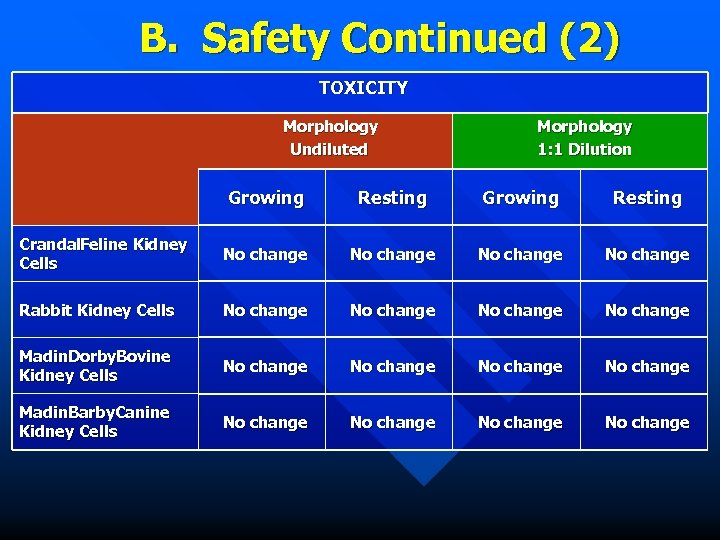
B. Safety Continued (2) TOXICITY Morphology Undiluted Morphology 1: 1 Dilution Growing Resting Crandal. Feline Kidney Cells No change Rabbit Kidney Cells No change Madin. Dorby. Bovine Kidney Cells No change Madin. Barby. Canine Kidney Cells No change
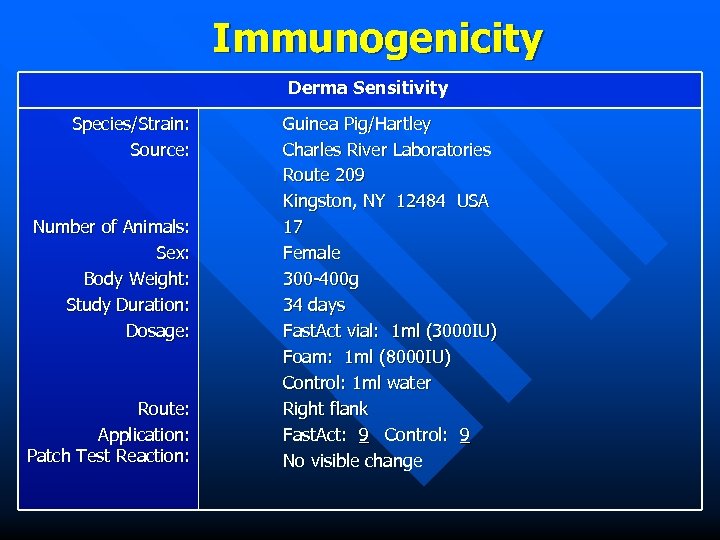
Immunogenicity Derma Sensitivity Species/Strain: Source: Number of Animals: Sex: Body Weight: Study Duration: Dosage: Route: Application: Patch Test Reaction: Guinea Pig/Hartley Charles River Laboratories Route 209 Kingston, NY 12484 USA 17 Female 300 -400 g 34 days Fast. Act vial: 1 ml (3000 IU) Foam: 1 ml (8000 IU) Control: 1 ml water Right flank Fast. Act: 9 Control: 9 No visible change
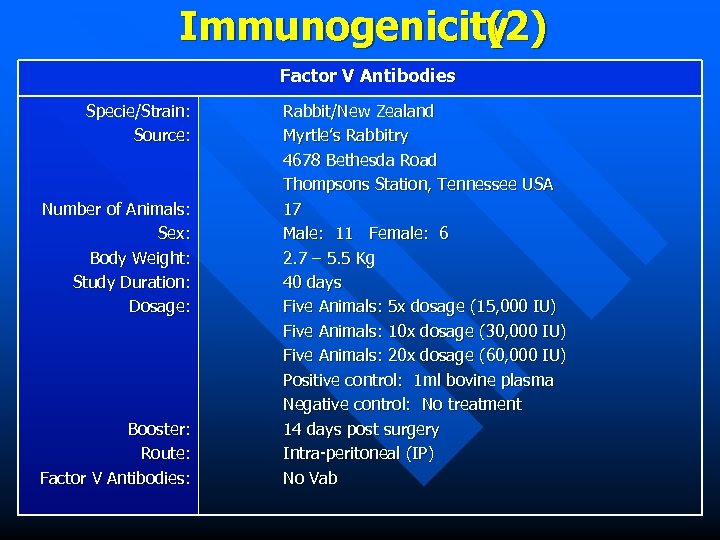
Immunogenicity (2) Factor V Antibodies Specie/Strain: Source: Number of Animals: Sex: Body Weight: Study Duration: Dosage: Booster: Route: Factor V Antibodies: Rabbit/New Zealand Myrtle’s Rabbitry 4678 Bethesda Road Thompsons Station, Tennessee USA 17 Male: 11 Female: 6 2. 7 – 5. 5 Kg 40 days Five Animals: 5 x dosage (15, 000 IU) Five Animals: 10 x dosage (30, 000 IU) Five Animals: 20 x dosage (60, 000 IU) Positive control: 1 ml bovine plasma Negative control: No treatment 14 days post surgery Intra-peritoneal (IP) No Vab
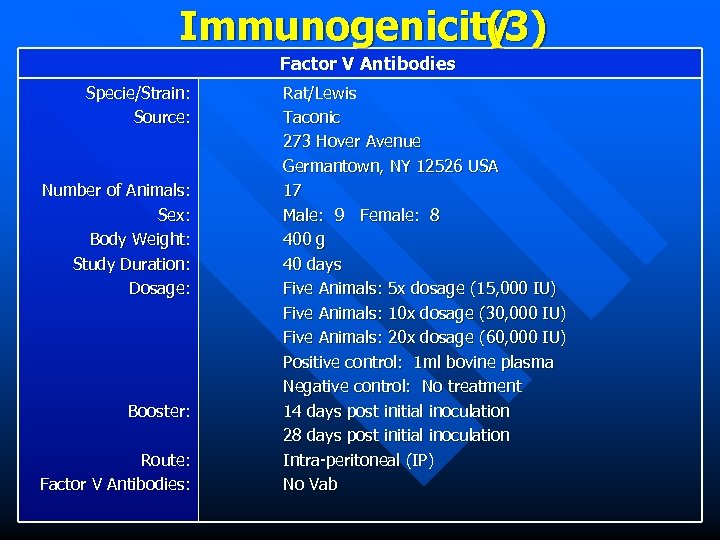
Immunogenicity (3) Factor V Antibodies Specie/Strain: Source: Number of Animals: Sex: Body Weight: Study Duration: Dosage: Booster: Route: Factor V Antibodies: Rat/Lewis Taconic 273 Hover Avenue Germantown, NY 12526 USA 17 Male: 9 Female: 8 400 g 40 days Five Animals: 5 x dosage (15, 000 IU) Five Animals: 10 x dosage (30, 000 IU) Five Animals: 20 x dosage (60, 000 IU) Positive control: 1 ml bovine plasma Negative control: No treatment 14 days post initial inoculation 28 days post initial inoculation Intra-peritoneal (IP) No Vab

Fast. Act Clinical Line Testing ® Phase I Clinical Trial
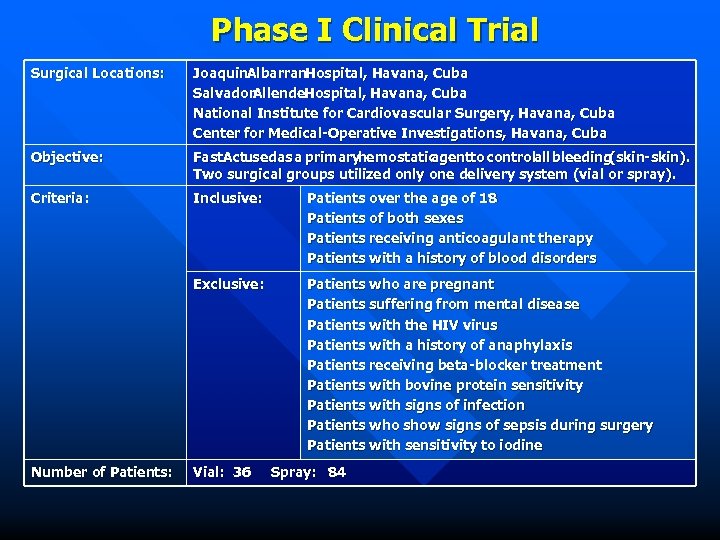
Phase I Clinical Trial Surgical Locations: Joaquin. Albarran. Hospital, Havana, Cuba Salvador. Allende. Hospital, Havana, Cuba National Institute for Cardiovascular Surgery, Havana, Cuba Center for Medical-Operative Investigations, Havana, Cuba Objective: Fast. Actusedas a primaryhemostatic agentto controlall bleeding (skin-skin). Two surgical groups utilized only one delivery system (vial or spray). Criteria: Inclusive: Patients over the age of 18 Patients of both sexes Patients receiving anticoagulant therapy Patients with a history of blood disorders Exclusive: Patients who are pregnant Patients suffering from mental disease Patients with the HIV virus Patients with a history of anaphylaxis Patients receiving beta-blocker treatment Patients with bovine protein sensitivity Patients with signs of infection Patients who show signs of sepsis during surgery Patients with sensitivity to iodine Number of Patients: Vial: 36 Spray: 84
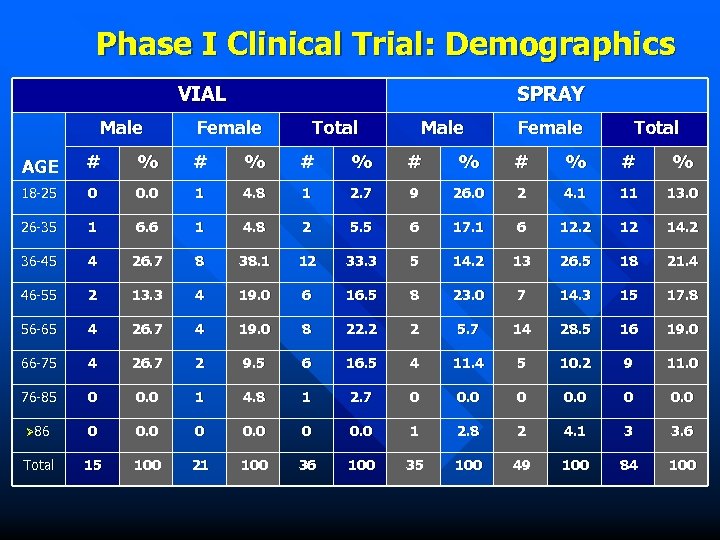
Phase I Clinical Trial: Demographics VIAL Male SPRAY Female Total Male Female Total AGE # % # % # % 18 -25 0 0. 0 1 4. 8 1 2. 7 9 26. 0 2 4. 1 11 13. 0 26 -35 1 6. 6 1 4. 8 2 5. 5 6 17. 1 6 12. 2 12 14. 2 36 -45 4 26. 7 8 38. 1 12 33. 3 5 14. 2 13 26. 5 18 21. 4 46 -55 2 13. 3 4 19. 0 6 16. 5 8 23. 0 7 14. 3 15 17. 8 56 -65 4 26. 7 4 19. 0 8 22. 2 2 5. 7 14 28. 5 16 19. 0 66 -75 4 26. 7 2 9. 5 6 16. 5 4 11. 4 5 10. 2 9 11. 0 76 -85 0 0. 0 1 4. 8 1 2. 7 0 0. 0 Ø 86 0 0. 0 1 2. 8 2 4. 1 3 3. 6 Total 15 100 21 100 36 100 35 100 49 100 84 100
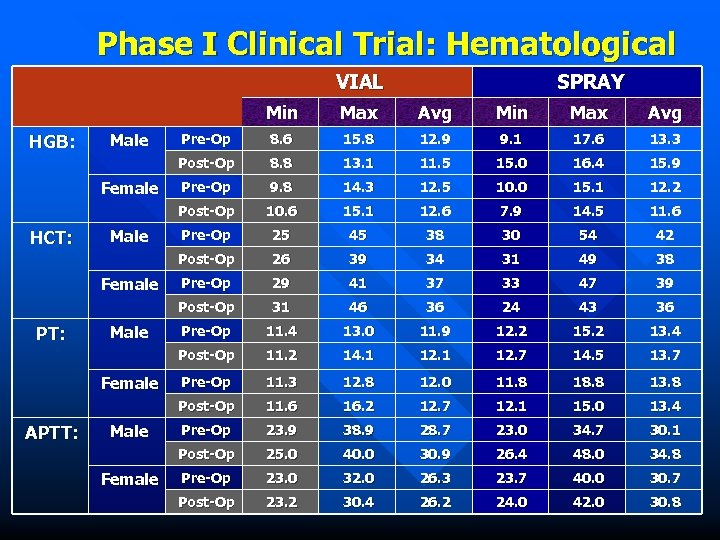
Phase I Clinical Trial: Hematological VIAL SPRAY Min PT: Female APTT: Male Female 15. 8 12. 9 9. 1 17. 6 13. 3 8. 8 13. 1 11. 5 15. 0 16. 4 15. 9 Pre-Op 9. 8 14. 3 12. 5 10. 0 15. 1 12. 2 10. 6 15. 1 12. 6 7. 9 14. 5 11. 6 Pre-Op 25 45 38 30 54 42 26 39 34 31 49 38 Pre-Op 29 41 37 33 47 39 31 46 36 24 43 36 Pre-Op 11. 4 13. 0 11. 9 12. 2 15. 2 13. 4 11. 2 14. 1 12. 7 14. 5 13. 7 Pre-Op 11. 3 12. 8 12. 0 11. 8 18. 8 13. 8 11. 6 16. 2 12. 7 12. 1 15. 0 13. 4 Pre-Op 23. 9 38. 9 28. 7 23. 0 34. 7 30. 1 Post-Op Male 8. 6 Post-Op Female Pre-Op Post-Op Male Avg Post-Op HCT: Max Post-Op Female Min Post-Op Male Avg Post-Op HGB: Max 25. 0 40. 0 30. 9 26. 4 48. 0 34. 8 Pre-Op 23. 0 32. 0 26. 3 23. 7 40. 0 30. 7 Post-Op 23. 2 30. 4 26. 2 24. 0 42. 0 30. 8
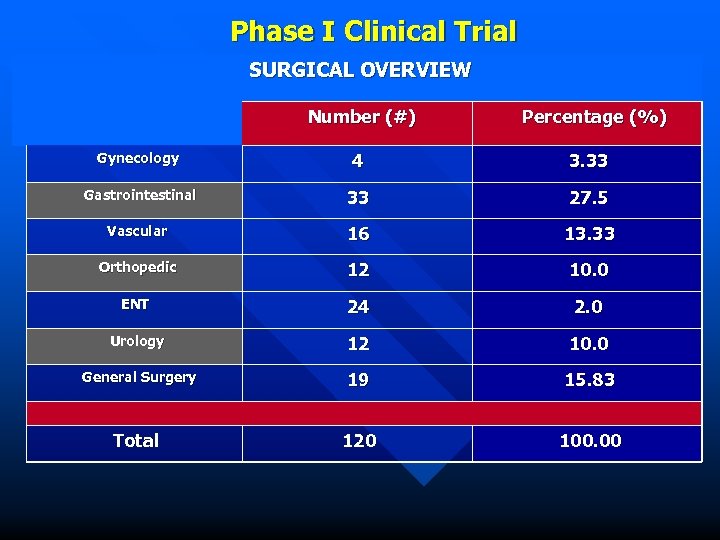
Phase I Clinical Trial SURGICAL OVERVIEW Number (#) Percentage (%) Gynecology 4 3. 33 Gastrointestinal 33 27. 5 Vascular 16 13. 33 Orthopedic 12 10. 0 ENT 24 2. 0 Urology 12 10. 0 General Surgery 19 15. 83 Total 120 100. 00
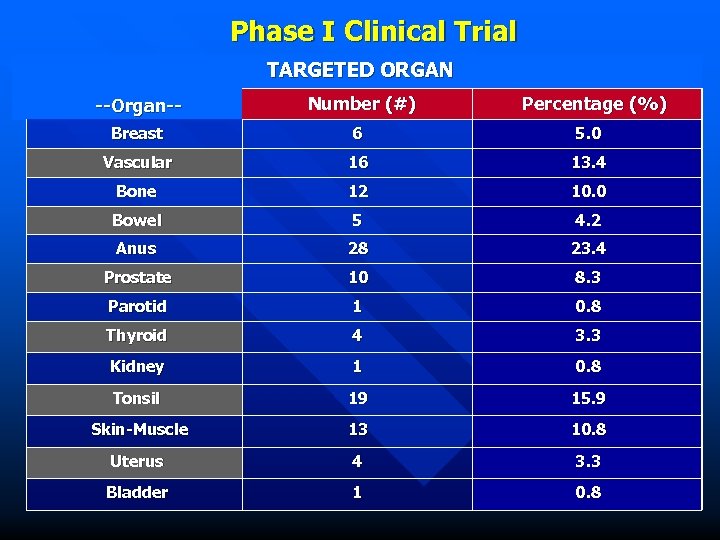
Phase I Clinical Trial TARGETED ORGAN --Organ-- Number (#) Percentage (%) Breast 6 5. 0 Vascular 16 13. 4 Bone 12 10. 0 Bowel 5 4. 2 Anus 28 23. 4 Prostate 10 8. 3 Parotid 1 0. 8 Thyroid 4 3. 3 Kidney 1 0. 8 Tonsil 19 15. 9 Skin-Muscle 13 10. 8 Uterus 4 3. 3 Bladder 1 0. 8
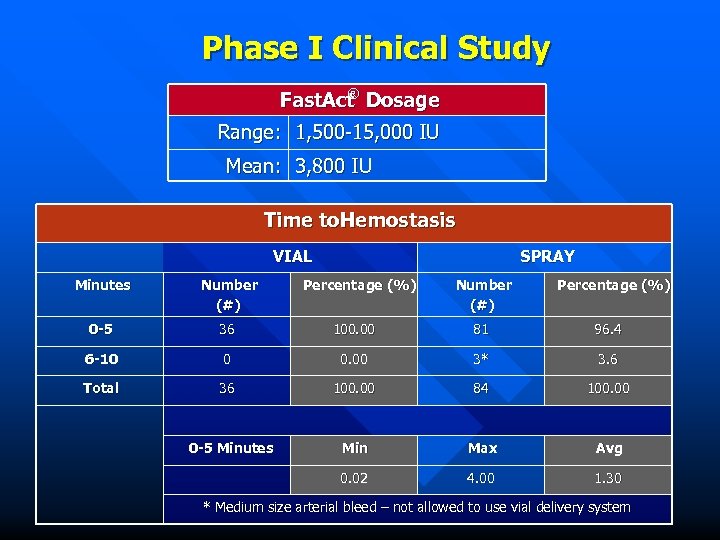
Phase I Clinical Study ® Fast. Act Dosage Range: 1, 500 -15, 000 IU Mean: 3, 800 IU Time to. Hemostasis VIAL SPRAY Minutes Number (#) 0 -5 36 100. 00 81 96. 4 6 -10 0 0. 00 3* 3. 6 Total 36 100. 00 84 100. 00 Min Max Avg 0. 02 4. 00 1. 30 0 -5 Minutes Percentage (%) Number (#) Percentage (%) * Medium size arterial bleed – not allowed to use vial delivery system
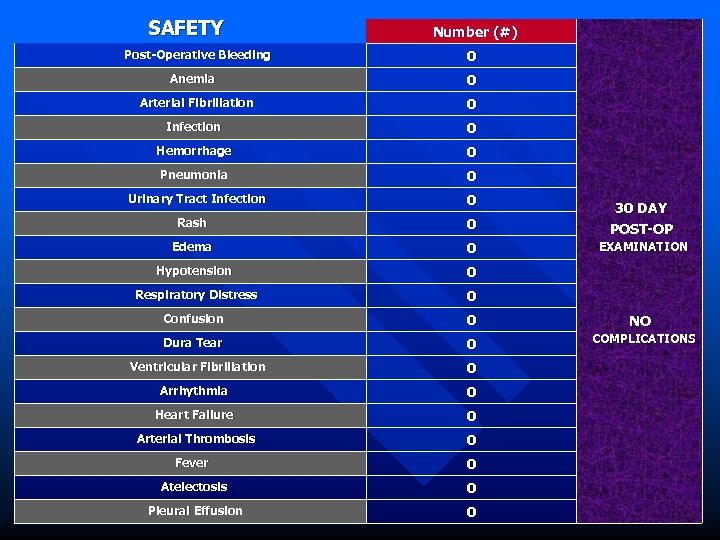
SAFETY Post-Operative Bleeding Anemia Number (#) 0 0 Arterial Fibrillation 0 Infection 0 Hemorrhage 0 Pneumonia 0 Urinary Tract Infection 0 Rash 0 30 DAY POST-OP Edema 0 EXAMINATION Hypotension 0 Respiratory Distress 0 Confusion 0 Dura Tear 0 Ventricular Fibrillation 0 Arrhythmia 0 Heart Failure 0 Arterial Thrombosis 0 Fever 0 Atelectosis 0 Pleural Effusion 0 NO COMPLICATIONS

Fast. Act Clinical Line Testing ® Phase II Clinical Trial

Phase II Clinical Trial Surgical Locations: Hospital. Edgardo. Rebagliati Martins, Lima, Peru Hospital. Nacional. Guillermo. Almentara Es Salud Lima, Peru 1. , Objective: Investigational group: Fast. Actused as sole means to controlall bleedingat the targeted organ. Criteria: Inclusive: All ages and both genders Wounds of a similar size, type, location and bleeding tendency. On anticoagulation therapy, with no dosage limitations. Diagnosed with a coagulation disorder. Participants must be able to participate for the 30 day duration of the study. Exclusive: Any clinically infected wound, draining pus, surrounding erythema or edema, or patients with systemic signs of infections. Subjects on antibiotic therapy prior to enrollment. Subjects with known allergy to bovine proteins, reactions, atopic history of anaphylaxis. Inability to give informed consent. Inability to return for a 30 day follow-up visit. Number of Patients: 127
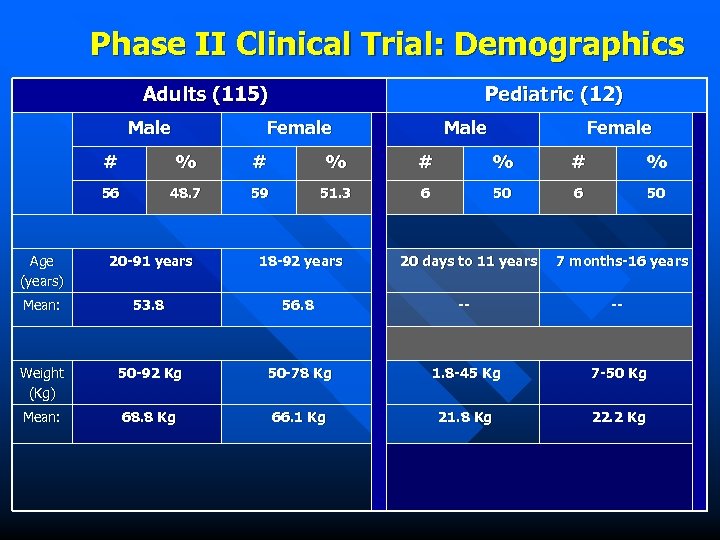
Phase II Clinical Trial: Demographics Adults (115) Male Pediatric (12) Female Male Female # % # % 56 48. 7 59 51. 3 6 50 Age (years) 20 -91 years 18 -92 years 20 days to 11 years 7 months-16 years Mean: 53. 8 56. 8 -- -- Weight (Kg) 50 -92 Kg 50 -78 Kg 1. 8 -45 Kg 7 -50 Kg Mean: 68. 8 Kg 66. 1 Kg 21. 8 Kg 22. 2 Kg
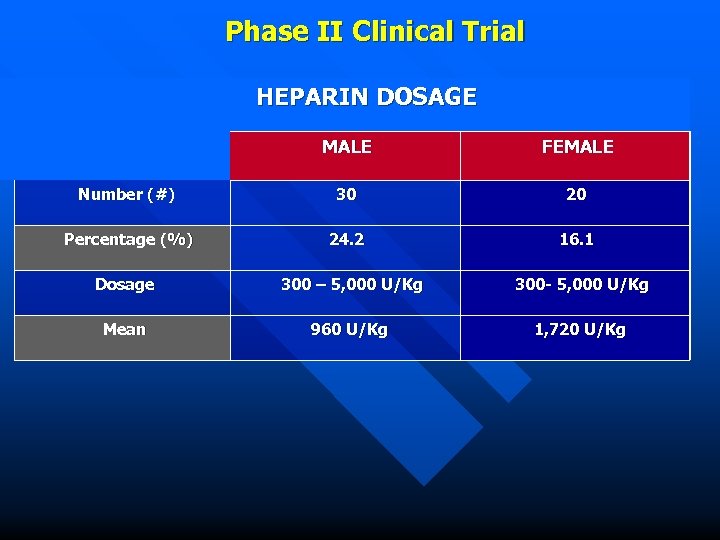
Phase II Clinical Trial HEPARIN DOSAGE MALE FEMALE Number (#) 30 20 Percentage (%) 24. 2 16. 1 Dosage 300 – 5, 000 U/Kg 300 - 5, 000 U/Kg Mean 960 U/Kg 1, 720 U/Kg
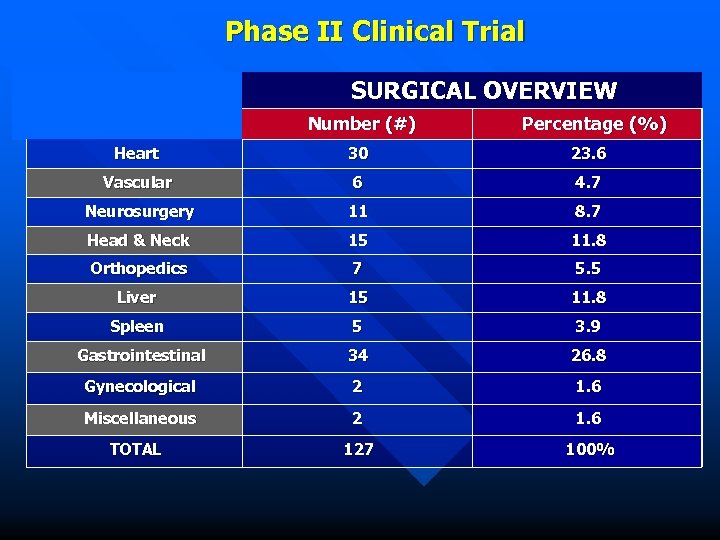
Phase II Clinical Trial SURGICAL OVERVIEW Number (#) Percentage (%) Heart 30 23. 6 Vascular 6 4. 7 Neurosurgery 11 8. 7 Head & Neck 15 11. 8 Orthopedics 7 5. 5 Liver 15 11. 8 Spleen 5 3. 9 Gastrointestinal 34 26. 8 Gynecological 2 1. 6 Miscellaneous 2 1. 6 TOTAL 127 100%
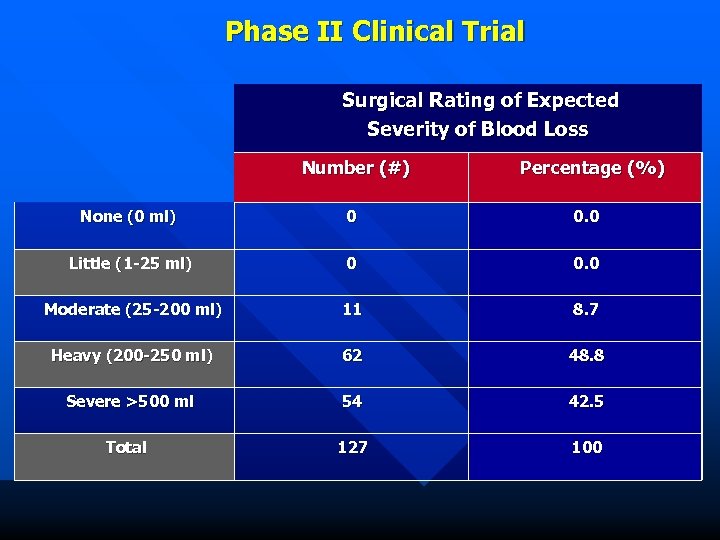
Phase II Clinical Trial Surgical Rating of Expected Severity of Blood Loss Number (#) Percentage (%) None (0 ml) 0 0. 0 Little (1 -25 ml) 0 0. 0 Moderate (25 -200 ml) 11 8. 7 Heavy (200 -250 ml) 62 48. 8 Severe >500 ml 54 42. 5 Total 127 100
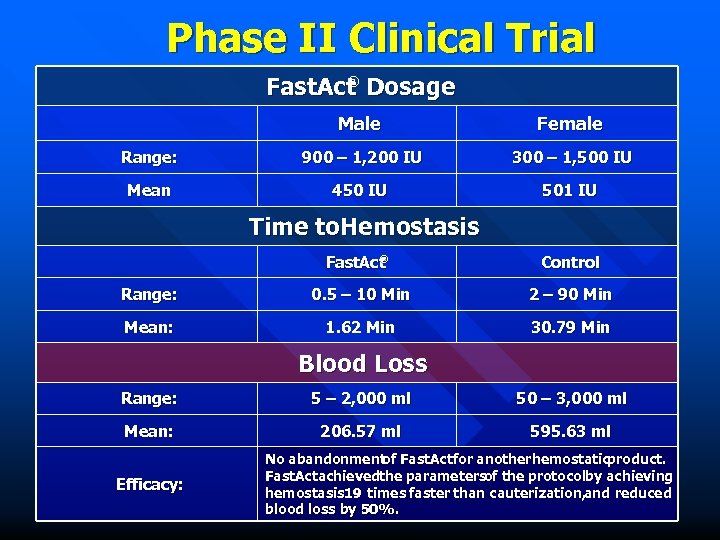
Phase II Clinical Trial ® Fast. Act Dosage Male Female Range: 900 – 1, 200 IU 300 – 1, 500 IU Mean 450 IU 501 IU Time to. Hemostasis ® Fast. Act Control Range: 0. 5 – 10 Min 2 – 90 Min Mean: 1. 62 Min 30. 79 Min Blood Loss Range: 5 – 2, 000 ml 50 – 3, 000 ml Mean: 206. 57 ml 595. 63 ml Efficacy: No abandonment Fast. Act for anotherhemostaticproduct. of Fast. Actachievedthe parametersof the protocolby achieving hemostasis 19 times faster than cauterization, and reduced blood loss by 50%.
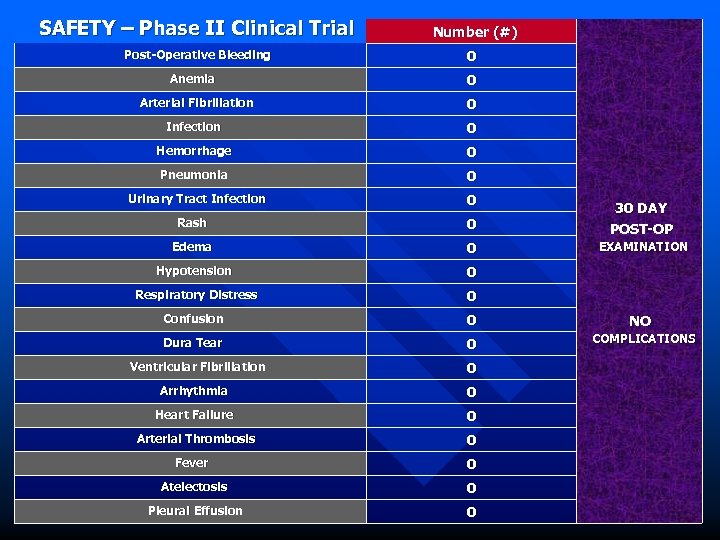
SAFETY – Phase II Clinical Trial Number (#) Post-Operative Bleeding 0 Anemia 0 Arterial Fibrillation 0 Infection 0 Hemorrhage 0 Pneumonia 0 Urinary Tract Infection 0 Rash 0 30 DAY POST-OP Edema 0 EXAMINATION Hypotension 0 Respiratory Distress 0 Confusion 0 Dura Tear 0 Ventricular Fibrillation 0 Arrhythmia 0 Heart Failure 0 Arterial Thrombosis 0 Fever 0 Atelectosis 0 Pleural Effusion 0 NO COMPLICATIONS

Presentation is Complete. Thank You Wortham Laboratories, Inc. www. worthamlabs. com © 2007 Wortham Laboratories, Inc. All Rights Reserved International Office: USA 1+423. 296. 0090
29e23a0cebe7a550b55c83e41b83c25d.ppt