98c10c6e9fd41f49477bfcf39a33d192.ppt
- Количество слайдов: 33
 Workshops on Pedagogy and Resources for Learning and Teaching of Sixth Form Chemistry Session 1 Introduction to Resources Group Discussion
Workshops on Pedagogy and Resources for Learning and Teaching of Sixth Form Chemistry Session 1 Introduction to Resources Group Discussion
 http: //resources. edb. gov. h k/~science/chem. htm
http: //resources. edb. gov. h k/~science/chem. htm
 Directions for Revision n Trimming of topics to allow rooms for students n n to develop scientific investigation skills and higher order thinking skills Articulating pedagogies recommended in S 4 -5 Chemistry Curriculum Suggest appropriate learning activities so that students may have opportunities to develop their scientific investigation skills as well as higher order thinking skills Generic Skills: 3 C 1 P Updating the information of the curriculum content
Directions for Revision n Trimming of topics to allow rooms for students n n to develop scientific investigation skills and higher order thinking skills Articulating pedagogies recommended in S 4 -5 Chemistry Curriculum Suggest appropriate learning activities so that students may have opportunities to develop their scientific investigation skills as well as higher order thinking skills Generic Skills: 3 C 1 P Updating the information of the curriculum content
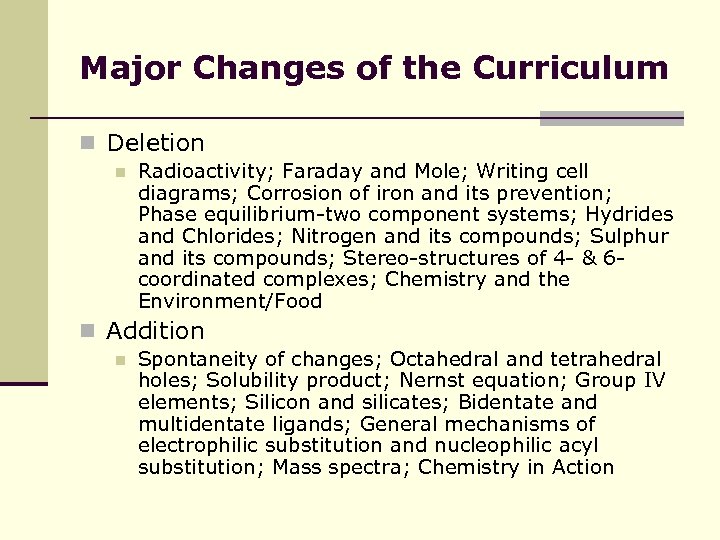 Major Changes of the Curriculum n Deletion n Radioactivity; Faraday and Mole; Writing cell diagrams; Corrosion of iron and its prevention; Phase equilibrium-two component systems; Hydrides and Chlorides; Nitrogen and its compounds; Sulphur and its compounds; Stereo-structures of 4 - & 6 coordinated complexes; Chemistry and the Environment/Food n Addition n Spontaneity of changes; Octahedral and tetrahedral holes; Solubility product; Nernst equation; Group IV elements; Silicon and silicates; Bidentate and multidentate ligands; General mechanisms of electrophilic substitution and nucleophilic acyl substitution; Mass spectra; Chemistry in Action
Major Changes of the Curriculum n Deletion n Radioactivity; Faraday and Mole; Writing cell diagrams; Corrosion of iron and its prevention; Phase equilibrium-two component systems; Hydrides and Chlorides; Nitrogen and its compounds; Sulphur and its compounds; Stereo-structures of 4 - & 6 coordinated complexes; Chemistry and the Environment/Food n Addition n Spontaneity of changes; Octahedral and tetrahedral holes; Solubility product; Nernst equation; Group IV elements; Silicon and silicates; Bidentate and multidentate ligands; General mechanisms of electrophilic substitution and nucleophilic acyl substitution; Mass spectra; Chemistry in Action
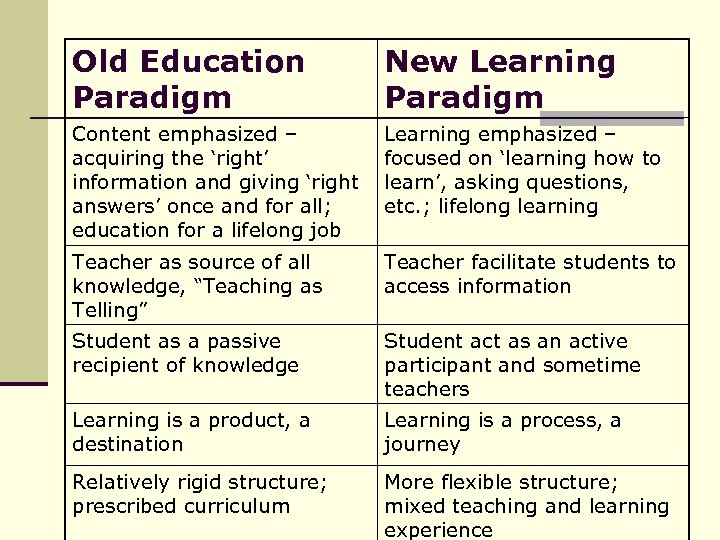 Old Education Paradigm New Learning Paradigm Content emphasized – acquiring the ‘right’ information and giving ‘right answers’ once and for all; education for a lifelong job Learning emphasized – focused on ‘learning how to learn’, asking questions, etc. ; lifelong learning Teacher as source of all knowledge, “Teaching as Telling” Teacher facilitate students to access information Student as a passive recipient of knowledge Student act as an active participant and sometime teachers Learning is a product, a destination Learning is a process, a journey Relatively rigid structure; prescribed curriculum More flexible structure; mixed teaching and learning experience
Old Education Paradigm New Learning Paradigm Content emphasized – acquiring the ‘right’ information and giving ‘right answers’ once and for all; education for a lifelong job Learning emphasized – focused on ‘learning how to learn’, asking questions, etc. ; lifelong learning Teacher as source of all knowledge, “Teaching as Telling” Teacher facilitate students to access information Student as a passive recipient of knowledge Student act as an active participant and sometime teachers Learning is a product, a destination Learning is a process, a journey Relatively rigid structure; prescribed curriculum More flexible structure; mixed teaching and learning experience
 Surface and Deep Approaches to Learning n Surface approach n Students reduce what is to be learnt to the status of unconnected facts to be memorised REGURGITATE !!! n Deep approach n Students attempt to make sense of what is to be learnt, which consists of ideas and concepts n This involves thinking, seeking integration between components and between tasks, and ‘playing’ with ideas Gibbs, G. (1992)
Surface and Deep Approaches to Learning n Surface approach n Students reduce what is to be learnt to the status of unconnected facts to be memorised REGURGITATE !!! n Deep approach n Students attempt to make sense of what is to be learnt, which consists of ideas and concepts n This involves thinking, seeking integration between components and between tasks, and ‘playing’ with ideas Gibbs, G. (1992)
 Key Elements of Good Teaching n Motivational Context n Deep learning is more likely when student experience a need to know something n Situated learning n Learner Activity n Student need to be active rather than passive n Interaction with others n Easier to negotiate meaning and to manipulate ideas with others than alone n A well Structured Knowledge Base n Link to students’ existing knowledge and experience n Content is taught in integrated wholes rather than in small separate pieces
Key Elements of Good Teaching n Motivational Context n Deep learning is more likely when student experience a need to know something n Situated learning n Learner Activity n Student need to be active rather than passive n Interaction with others n Easier to negotiate meaning and to manipulate ideas with others than alone n A well Structured Knowledge Base n Link to students’ existing knowledge and experience n Content is taught in integrated wholes rather than in small separate pieces
 Exemplars of L/T Activities 1. IT for Interactive Learning 2. 3. 4. 5. 6. Activities Datalogging Experiments Microscale Chemistry Experiments Problem Solving Activities Inquiry-based Experiments Reading to Learn Activities Other Learning Activities 7. → Strategies for fostering a deep approach
Exemplars of L/T Activities 1. IT for Interactive Learning 2. 3. 4. 5. 6. Activities Datalogging Experiments Microscale Chemistry Experiments Problem Solving Activities Inquiry-based Experiments Reading to Learn Activities Other Learning Activities 7. → Strategies for fostering a deep approach
 Web. Quest Inquiry-based activity in which some or all of the information that learners interact with comes from resources on the internet (Bernie Dodge) n Involves real life activity where students are engaged in solving real-life problems n Acquire new information and make sense of it n Analyse a body of knowledge deeply and transform it in some way n n http: //www 3. fed. cuhk. edu. hk/community/webquest n http: //webquest. sdsu. edu n http: //www. jozie. net/JF/HS_Chem/Resources/webquest. htm n Chemicals in the House http: //educ. queensu. ca/~science/main/profdev/W eb. Quest. KSEK/index. htm
Web. Quest Inquiry-based activity in which some or all of the information that learners interact with comes from resources on the internet (Bernie Dodge) n Involves real life activity where students are engaged in solving real-life problems n Acquire new information and make sense of it n Analyse a body of knowledge deeply and transform it in some way n n http: //www 3. fed. cuhk. edu. hk/community/webquest n http: //webquest. sdsu. edu n http: //www. jozie. net/JF/HS_Chem/Resources/webquest. htm n Chemicals in the House http: //educ. queensu. ca/~science/main/profdev/W eb. Quest. KSEK/index. htm
 Simulations n Requires students to make decisions n n n to manipulate variables of a system in order to accomplish a goal Students can carry out a number of experiments quickly and discover the trends for themselves Allows students to take control of the organisation and content of their learning Students learn from mistakes without paying the price of real mistakes Dangerous, slow or costly experiments Revision of the topic at any time
Simulations n Requires students to make decisions n n n to manipulate variables of a system in order to accomplish a goal Students can carry out a number of experiments quickly and discover the trends for themselves Allows students to take control of the organisation and content of their learning Students learn from mistakes without paying the price of real mistakes Dangerous, slow or costly experiments Revision of the topic at any time
 Interactive 3 D Chemical Structures (1/8) n Provides multiple representations of molecules – wireframe, ball and stick, and space-filled modes n Interactive n n structure positioned by using the mouse measurement of bond distances and bond angles (right click → “Select” → “Mouse Click Action” → “Angle”) n Cost saving n Filename extensions: mol, pdb, xyz n Require web-browser plug-in: MDL Chime http: //www. mdl. com/products/framework/chime/index. jsp n Sources of 3 D structures n n http: //www. molecularmodels. ca/molecule. html http: //www. wellesley. edu/Chemistry/Flick/molecules/newlist. html
Interactive 3 D Chemical Structures (1/8) n Provides multiple representations of molecules – wireframe, ball and stick, and space-filled modes n Interactive n n structure positioned by using the mouse measurement of bond distances and bond angles (right click → “Select” → “Mouse Click Action” → “Angle”) n Cost saving n Filename extensions: mol, pdb, xyz n Require web-browser plug-in: MDL Chime http: //www. mdl. com/products/framework/chime/index. jsp n Sources of 3 D structures n n http: //www. molecularmodels. ca/molecule. html http: //www. wellesley. edu/Chemistry/Flick/molecules/newlist. html
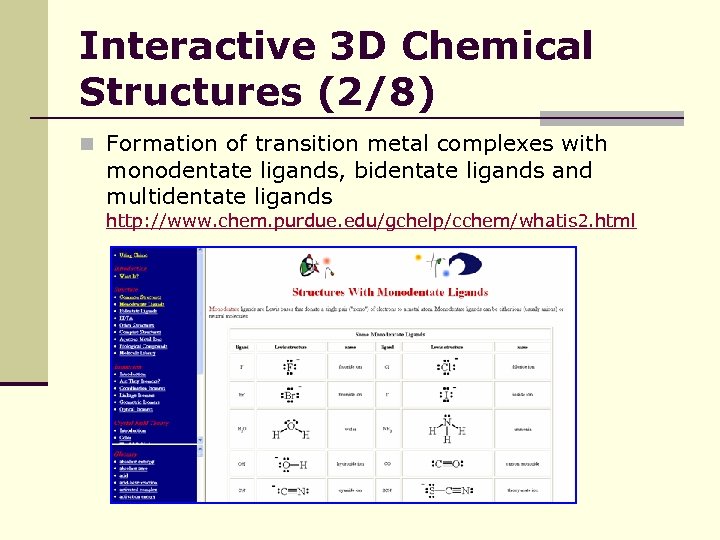 Interactive 3 D Chemical Structures (2/8) n Formation of transition metal complexes with monodentate ligands, bidentate ligands and multidentate ligands http: //www. chem. purdue. edu/gchelp/cchem/whatis 2. html
Interactive 3 D Chemical Structures (2/8) n Formation of transition metal complexes with monodentate ligands, bidentate ligands and multidentate ligands http: //www. chem. purdue. edu/gchelp/cchem/whatis 2. html
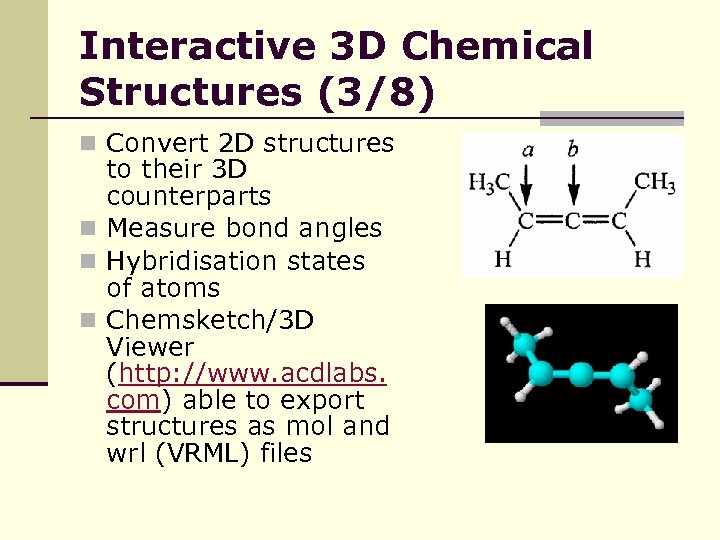 Interactive 3 D Chemical Structures (3/8) n Convert 2 D structures to their 3 D counterparts n Measure bond angles n Hybridisation states of atoms n Chemsketch/3 D Viewer (http: //www. acdlabs. com) able to export structures as mol and wrl (VRML) files
Interactive 3 D Chemical Structures (3/8) n Convert 2 D structures to their 3 D counterparts n Measure bond angles n Hybridisation states of atoms n Chemsketch/3 D Viewer (http: //www. acdlabs. com) able to export structures as mol and wrl (VRML) files
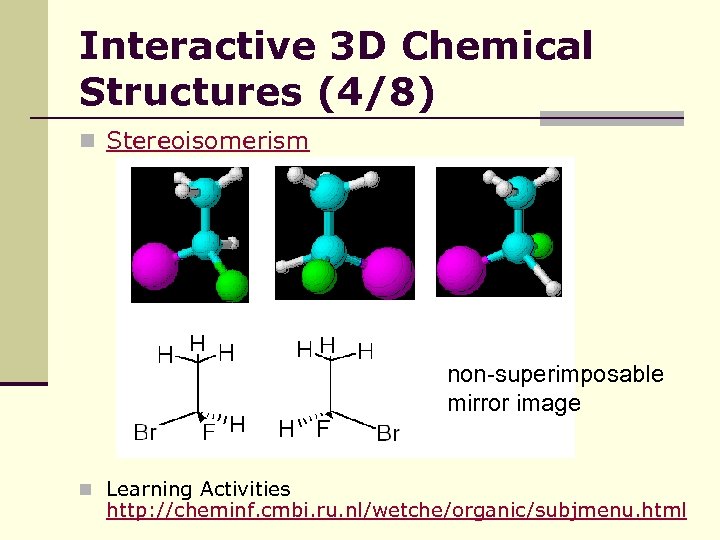 Interactive 3 D Chemical Structures (4/8) n Stereoisomerism non-superimposable mirror image n Learning Activities http: //cheminf. cmbi. ru. nl/wetche/organic/subjmenu. html
Interactive 3 D Chemical Structures (4/8) n Stereoisomerism non-superimposable mirror image n Learning Activities http: //cheminf. cmbi. ru. nl/wetche/organic/subjmenu. html
 Interactive 3 D Chemical Structures (5/8) n Embedding Interactive 3 D Images in Webpages n Using Dreamweaver or Frontpage “Insert Web Component” → “Advanced Controls” → “Plug-In” → “Plug-in Properties” n Using HTML codes
Interactive 3 D Chemical Structures (5/8) n Embedding Interactive 3 D Images in Webpages n Using Dreamweaver or Frontpage “Insert Web Component” → “Advanced Controls” → “Plug-In” → “Plug-in Properties” n Using HTML codes
 Interactive 3 D Chemical Structures (6/8) - Vibration n Vibrational Modes of Small Molecules http: //www. chem. purdue. edu/gchelp/vibs n Animations for Vibrational Mode of Molecules http: //www. nicol. ac. jp/~honma/mva/index. E. html n IR Interactive Visualizations http: //www. chem. umass. edu/~nermmw/Spectra/i rspectra/index. htm
Interactive 3 D Chemical Structures (6/8) - Vibration n Vibrational Modes of Small Molecules http: //www. chem. purdue. edu/gchelp/vibs n Animations for Vibrational Mode of Molecules http: //www. nicol. ac. jp/~honma/mva/index. E. html n IR Interactive Visualizations http: //www. chem. umass. edu/~nermmw/Spectra/i rspectra/index. htm
 Interactive 3 D Chemical Structures (7/8) - VRML n Virtual Reality Crystal Lattices, Dr Yeung, HKIEd http: //www. hkedcity. net/iclub_files/a/1/182/webpage/ vr_3 d/vrml/crystal/index. htm n Filename extension: wrl n VRML browser plug-in is needed e. g. Cortona & Cosmo
Interactive 3 D Chemical Structures (7/8) - VRML n Virtual Reality Crystal Lattices, Dr Yeung, HKIEd http: //www. hkedcity. net/iclub_files/a/1/182/webpage/ vr_3 d/vrml/crystal/index. htm n Filename extension: wrl n VRML browser plug-in is needed e. g. Cortona & Cosmo
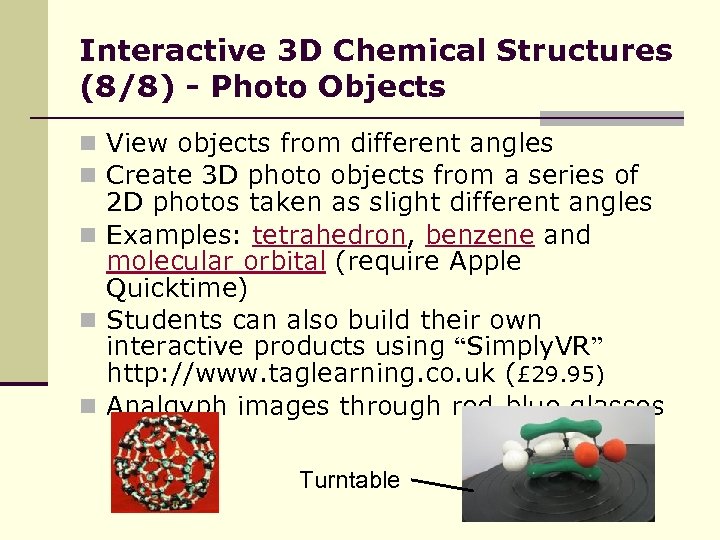 Interactive 3 D Chemical Structures (8/8) - Photo Objects n View objects from different angles n Create 3 D photo objects from a series of 2 D photos taken as slight different angles n Examples: tetrahedron, benzene and molecular orbital (require Apple Quicktime) n Students can also build their own interactive products using “Simply. VR” http: //www. taglearning. co. uk (£ 29. 95) n Analgyph images through red-blue glasses Turntable
Interactive 3 D Chemical Structures (8/8) - Photo Objects n View objects from different angles n Create 3 D photo objects from a series of 2 D photos taken as slight different angles n Examples: tetrahedron, benzene and molecular orbital (require Apple Quicktime) n Students can also build their own interactive products using “Simply. VR” http: //www. taglearning. co. uk (£ 29. 95) n Analgyph images through red-blue glasses Turntable
 Reading to Learn n Promote more independent n n learning capabilities Consolidate and widen students’ understanding of chemistry Historical and latest development in chemistry Reading materials: Internet, newspaper, magazines, journals, books Post-reading activities are essential to help students reflect on what they have learnt
Reading to Learn n Promote more independent n n learning capabilities Consolidate and widen students’ understanding of chemistry Historical and latest development in chemistry Reading materials: Internet, newspaper, magazines, journals, books Post-reading activities are essential to help students reflect on what they have learnt
 Problem Solving Activities “It represents the ultimate goal of chemistry education. Individuals who can address novel situations and arrive at a suitable course of action are valued in society” Routine vs Nonroutine; Well-defined vs Illdefined; Adversarial vs Nonadversarial “Essentially any activity that increase conceptual knowledge, encourage persistence, increase motivation, and helps students to see connections among ideas, to reflect on and check what was done, to consider alternative interpretations, and try different strategies is likely to improve problem solving. ” E. g. How much do you order?
Problem Solving Activities “It represents the ultimate goal of chemistry education. Individuals who can address novel situations and arrive at a suitable course of action are valued in society” Routine vs Nonroutine; Well-defined vs Illdefined; Adversarial vs Nonadversarial “Essentially any activity that increase conceptual knowledge, encourage persistence, increase motivation, and helps students to see connections among ideas, to reflect on and check what was done, to consider alternative interpretations, and try different strategies is likely to improve problem solving. ” E. g. How much do you order?
 Problem Solving Activities n “Student should spend more time on thinking than on doing, ‘more time interacting with ideas and less time interacting with apparatus’. ” n Free learners from some of the drudgery that goes with practical work in order to allow them to move on higher order skills e. g. predicting, observing, discussing, explaining, hypothesising, interpreting
Problem Solving Activities n “Student should spend more time on thinking than on doing, ‘more time interacting with ideas and less time interacting with apparatus’. ” n Free learners from some of the drudgery that goes with practical work in order to allow them to move on higher order skills e. g. predicting, observing, discussing, explaining, hypothesising, interpreting
 Problem Solving
Problem Solving
 Problem Solving
Problem Solving
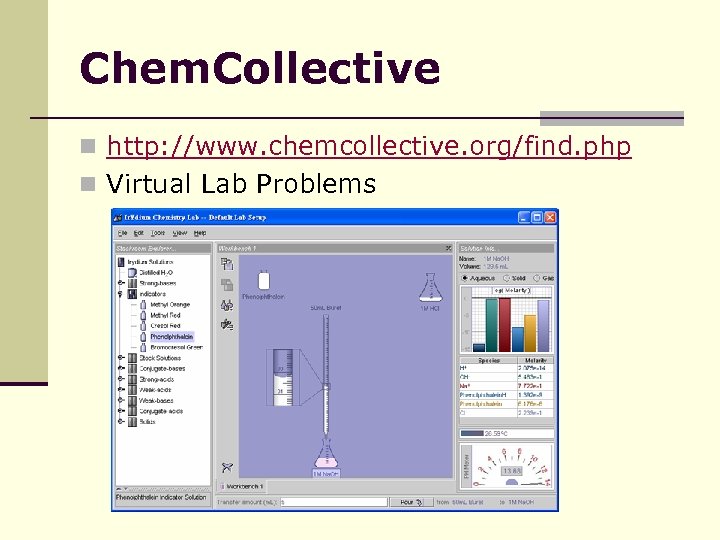 Chem. Collective n http: //www. chemcollective. org/find. php n Virtual Lab Problems
Chem. Collective n http: //www. chemcollective. org/find. php n Virtual Lab Problems
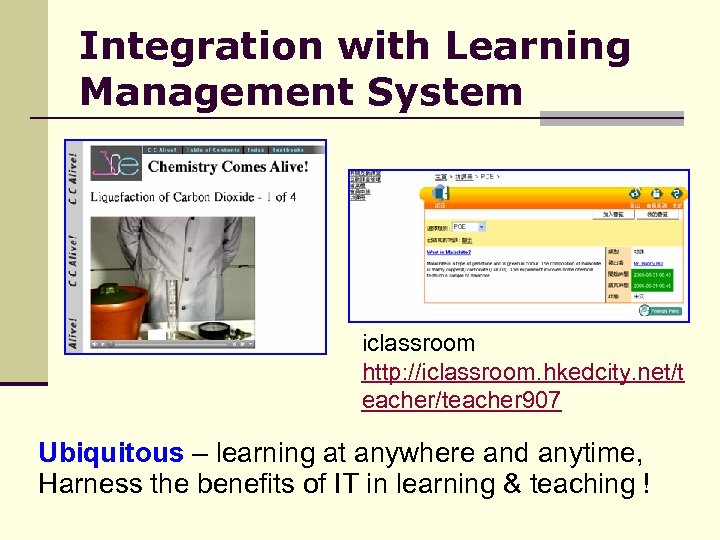 Integration with Learning Management System iclassroom http: //iclassroom. hkedcity. net/t eacher/teacher 907 Ubiquitous – learning at anywhere and anytime, Harness the benefits of IT in learning & teaching !
Integration with Learning Management System iclassroom http: //iclassroom. hkedcity. net/t eacher/teacher 907 Ubiquitous – learning at anywhere and anytime, Harness the benefits of IT in learning & teaching !
 Inquiry-based Chemistry n Scientific Inquiry - an active engaging n n n process that mimics the work done by actual scientists. Structured, Guided and Open Inquiry Increase the opportunities for students to think about the data they should collect and their presentation Require students to design some or all of the procedures (autonomy, ownership and motivation) and justify their decisions Authentic problems Become active participants and actually enjoyed science Lead to a deeper understanding of scientific concepts
Inquiry-based Chemistry n Scientific Inquiry - an active engaging n n n process that mimics the work done by actual scientists. Structured, Guided and Open Inquiry Increase the opportunities for students to think about the data they should collect and their presentation Require students to design some or all of the procedures (autonomy, ownership and motivation) and justify their decisions Authentic problems Become active participants and actually enjoyed science Lead to a deeper understanding of scientific concepts
 Discussion • You may also refer to the draft learning and teaching activities. • Results of discussion will be posted on the workshops webpages.
Discussion • You may also refer to the draft learning and teaching activities. • Results of discussion will be posted on the workshops webpages.
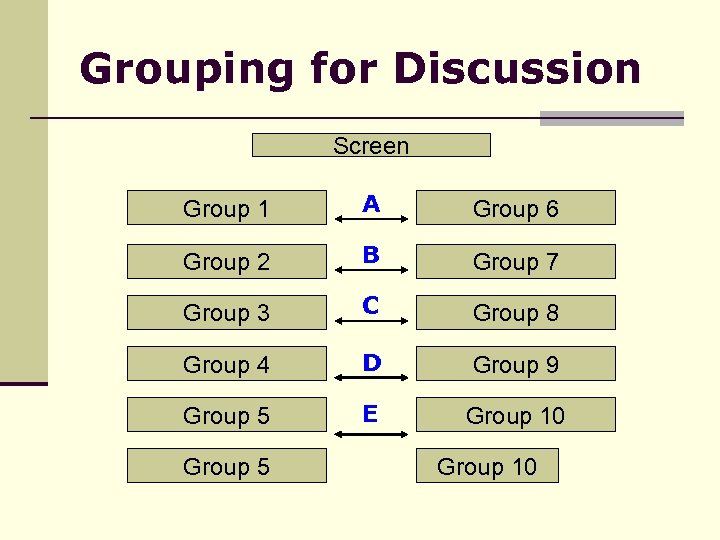 Grouping for Discussion Screen Group 1 A Group 6 Group 2 B Group 7 Group 3 C Group 8 Group 4 D Group 9 Group 5 E Group 10 Group 5 Group 10
Grouping for Discussion Screen Group 1 A Group 6 Group 2 B Group 7 Group 3 C Group 8 Group 4 D Group 9 Group 5 E Group 10 Group 5 Group 10
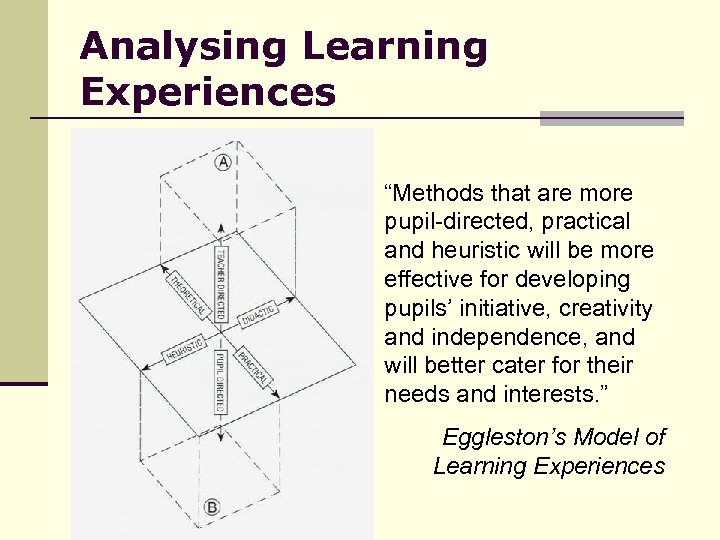 Analysing Learning Experiences “Methods that are more pupil-directed, practical and heuristic will be more effective for developing pupils’ initiative, creativity and independence, and will better cater for their needs and interests. ” Eggleston’s Model of Learning Experiences
Analysing Learning Experiences “Methods that are more pupil-directed, practical and heuristic will be more effective for developing pupils’ initiative, creativity and independence, and will better cater for their needs and interests. ” Eggleston’s Model of Learning Experiences
 Reflections n Different methods serve different goals. Successful teachers draw from a wealth of pedagogical strategies n Learning how to reflect upon the selection, planning and orchestration of science content and pedagogy that provide meaningful learning for students is the essence of pedagogical content knowledge n Developing students’ overall capacities for self-directed, life-long learning by embedding independent learning and generic skills into subjects
Reflections n Different methods serve different goals. Successful teachers draw from a wealth of pedagogical strategies n Learning how to reflect upon the selection, planning and orchestration of science content and pedagogy that provide meaningful learning for students is the essence of pedagogical content knowledge n Developing students’ overall capacities for self-directed, life-long learning by embedding independent learning and generic skills into subjects
 Developing student autonomy in learning The role of teachers is not simply to transmit knowledge but also to encourage students to take increasing responsibility for their own education and help them to find ways in which they can learn without the constant presence or supervision of a teacher. (David Boud, 1988)
Developing student autonomy in learning The role of teachers is not simply to transmit knowledge but also to encourage students to take increasing responsibility for their own education and help them to find ways in which they can learn without the constant presence or supervision of a teacher. (David Boud, 1988)
 Reference Books Details of the reference books provided at “chem. htm”.
Reference Books Details of the reference books provided at “chem. htm”.
 Thank you ! Mr W C HO cdosc 21@edb. gov. hk Tel 2712 8476
Thank you ! Mr W C HO cdosc 21@edb. gov. hk Tel 2712 8476


