9c1c1cb6950969c4c7d7ad4e12356ed4.ppt
- Количество слайдов: 21

Workshop on Accreditation of Bodies Certifying Medical Devices 17 -18 November 2014, Kiev Requirements for the experts assessing technical competence of certification bodies for ISO 13485 Elita Skribnovska Standartization, Accreditation and Metrology Centre Republic of Latvia LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 1
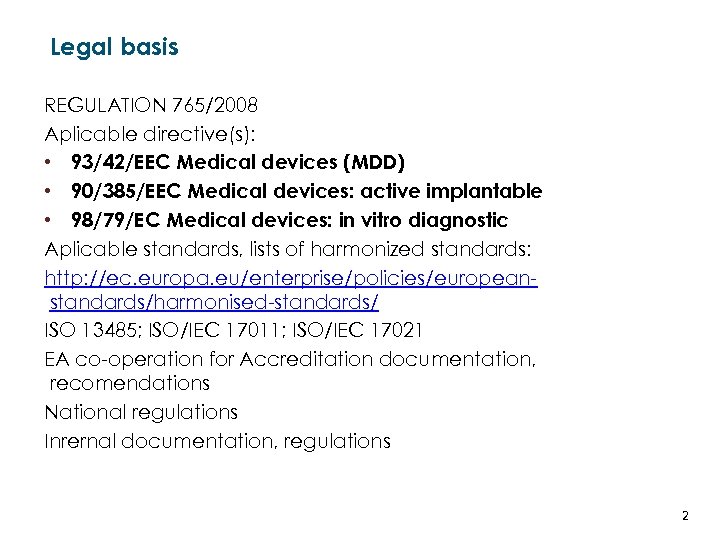
Legal basis REGULATION 765/2008 Aplicable directive(s): • 93/42/EEC Medical devices (MDD) • 90/385/EEC Medical devices: active implantable • 98/79/EC Medical devices: in vitro diagnostic Aplicable standards, lists of harmonized standards: http: //ec. europa. eu/enterprise/policies/europeanstandards/harmonised-standards/ ISO 13485; ISO/IEC 17011; ISO/IEC 17021 EA co-operation for Accreditation documentation, recomendations National regulations Inrernal documentation, regulations LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 2

REGULATION (EC) No 765/2008 Article 4 General principles • Each Member State shall ensure that its national accreditation body has the appropriate financial and personnel resources for the proper performance of its tasks, including the fulfilment of special tasks, such as activities for European and international accreditation cooperation and activities that are required to support public policy and which are not self-financing • The national accreditation body shall be a member of the body recognized under Article 14 • European accreditation infrastructure http: //www. european-accreditation. org/role LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 3

Requirements for national accreditation bodies Article 8 • it shall ensure that each decision relating to the attestation of competence is taken by competent persons different from those who carried out the assessment • it shall have a number of competent personnel at its disposal sufficient for the proper performance of its tasks • it shall document the duties, responsibilities and authorities of personnel who could affect the quality of the assessment and of the attestation of competence LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 4

ISO 13485 an International Organization for Standartization (ISO) standard, that represents the requirements for a comprehensive Quality management system for the design and manufacture of medical devices generally harmonized with ISO 0991. A principal difference, however, is that ISO 9001 requires the organization to demonstrate continual improvement, whereas ISO 13485 requires only that the certified organization demonstrate the quality system is effectively implemented and maintained diference is, the ISO 9001 requirements regarding customer satisfaction are absent from the medical device standard LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 5

Other specific differences • the promotion and awareness of regulatory requirements as a management responsibility • controls in the work environment to ensure product safety • focus on risk management activities and designe control activities during product development • specific requirements for inspection and traceability for implantable devices • specific requirements for documentation and validation of processes for sterile medical devices • specific requirements for verification of the effectiveness of corrective and preventive actions LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 6
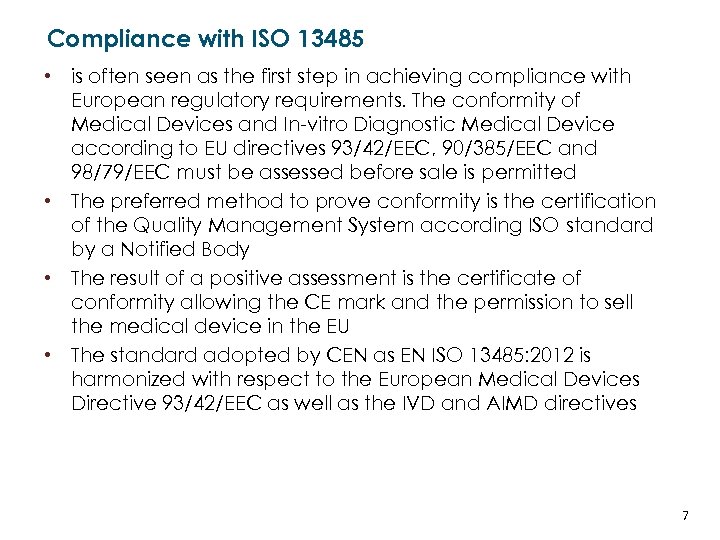
Compliance with ISO 13485 • is often seen as the first step in achieving compliance with European regulatory requirements. The conformity of Medical Devices and In-vitro Diagnostic Medical Device according to EU directives 93/42/EEC, 90/385/EEC and 98/79/EEC must be assessed before sale is permitted • The preferred method to prove conformity is the certification of the Quality Management System according ISO standard by a Notified Body • The result of a positive assessment is the certificate of conformity allowing the CE mark and the permission to sell the medical device in the EU • The standard adopted by CEN as EN ISO 13485: 2012 is harmonized with respect to the European Medical Devices Directive 93/42/EEC as well as the IVD and AIMD directives LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 7

Quality system for medical devices A quality management system (QMS) is a collection of business processes focused on achieving your quality policy and quality objectives — i. e. what your customer wants and needs • ISO 13485 is considered state of the art for medical device manufacturers QMS and related services. The standard is harmonised in the EU to the MD directives • Quality System requirements for medical devices have been internationally recognized as a way to assure product safety and efficacy and customer satisfaction • medical device manufacturers have the responsibility to use good judgment when developing their quality system and apply those sections LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 8

The essential elements that a quality system shall embody for design, production and distribution, without prescribing specific ways to establish these elements. These elements include: • inspection, measuring and test personnel training and qualification • controlling the product design • controlling documentation • controlling purchasing • product identification and traceability at all stages of production • controlling and defining production and process • defining and controequipment • validating processes • product acceptance • controlling nonconforming product • instituting corrective and preventive action when errors occur • labeling and packaging controls • handling, storage, distribution and installation • records • servicing • statistical techniques • all overseen by management and quality audits LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 9

QS regulation • The QS regulation covers a broad spectrum of devices and production processes, it allows some leeway in the details of quality system elements. It is left to manufacturers to determine the necessity for, or extent of, some quality elements and to develop and implement procedures tailored to their particular processes and devices. • For example, if it is impossible to mix up labels at a manufacturer because there is only one label to each product, then there is no necessity for the manufacturer to comply with all of the GMP requirements under device labeling. LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 10

Quality Management System processes include: • Order Processing • Production Planning • Measurement of product/service/process compliant with specified requirements including statistical techniques such as Statistical Process Control and Measurement systems Analysis • Callibration • Internal Audit • Corrective Action • Preventive Action • Identification, labeling and control of non conforming product to preclude its inadvertent use, delivery or processing. • Purchasing and related processes such as supplier selection and monitoring LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 11
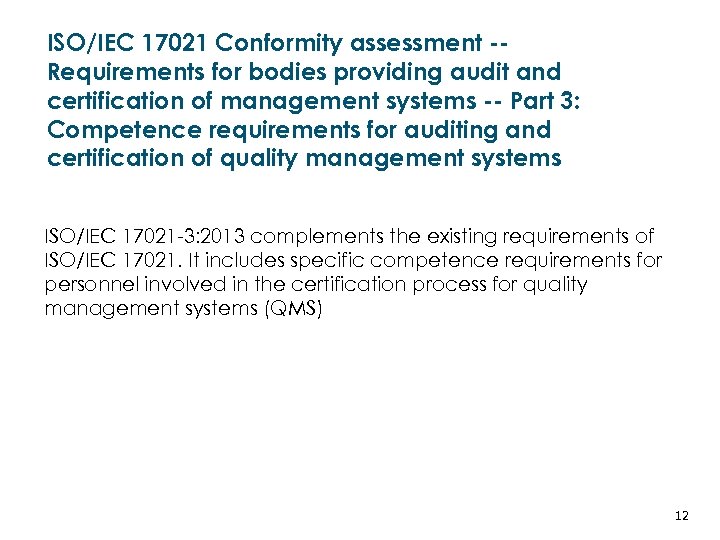
ISO/IEC 17021 Conformity assessment -Requirements for bodies providing audit and certification of management systems -- Part 3: Competence requirements for auditing and certification of quality management systems ISO/IEC 17021 -3: 2013 complements the existing requirements of ISO/IEC 17021. It includes specific competence requirements for personnel involved in the certification process for quality management systems (QMS) LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 12

ISO/IEC 17011 • ISO/IEC 17011: 2004 specifies general requirements for accreditation bodies assessing and accrediting conformity assessment bodies (CABs). It is also appropriate as a requirements document for the peer evaluation process for mutual recognition arrangements between accreditation • Accreditation bodies operating in accordance with ISO/IEC 17011: 2004 do not have to offer accreditation to all types of CABs. • For the purposes of ISO/IEC 17011: 2004, CABs are organizations providing the following conformity assessment services: testing, inspection, management system certification, personnel certification, product certification and, in the context of this document, calibration. LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 13

Requirements for Accreditation Bodies • Accreditation serves as a quality-control mechanism to ensure the credibility of the work of certification bodies • Accreditation bodies independently evaluate the work of certification bodies and assess them to demonstrate their competence, impartiality and performance capability • Is required that national accreditation bodies to comply with ISO/IEC 17011: 2004 to ensure that they operate in a consistent, comparable and reliable manner worldwide • It is recomended that Accreditation body is a member of the International Accreditation Forum (IAF), the world association of accreditation bodies LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 14

QMS • Customers are increasingly becoming quality-conscious shoppers. They want to know up front that your business will meet their needs. A certified Quality Management System demonstrates your commitment to quality and customer satisfaction. Implementing a Quality Management System will help you enhance customer satisfaction, achieve consistency, and improve internal processes. It can minimize the risk that customer expectations are not met. LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 15

Quality Management Principles • • Customer focus Leadership Involvement of people Process approach System approach Continual Improvement Fact based decision making Mutually beneficial supplier relationship LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 16

EA's Mission • EA exists to coordinate and lead the European accreditation infrastructure to allow the results of conformity assessment services in one country to be accepted by Regulators and the market place in another country without further examination, for the benefit of the European community and the global economy http: //www. european-accreditation. org/home LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 17

European co-operation for Accreditation or EA • • an association of national accreditation bodies in Europe that are officially recognised by their national Governments to assess and verify—against international standards—organisations that carry out evaluation services such as certification, verification, inspection, testing and calibration (also known as conformity assessment services) there has been a growth in specified national and international standards for products, processes and services. When applied correctly, these can make life safer, healthier and easier for everyone and can enable communication and trade, while allowing resources to be used more efficiently organisations that check conformity and compliance against standards must have the technical competence and integrity to carry out these assessment services If a supplier is accredited by one of the members in the EA network, its customers can have confidence in the competence, independence and impartiality of its conformity assessment work LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 18

The EA members provide accreditation of • Laboratories Ø Testing (ISO/IEC 17025), Medical examinations (ISO 15189) Ø Calibration (ISO/IEC 17025) • Inspection bodies( ISO/IEC 17020) • Certification bodies: Ø Product Certification (EN 45011 - ISO/IEC 17065) Ø Certification of Persons (ISO/IEC 17024) Ø Management Systems Certification (ISO/IEC 17021) • Verification bodies according to the European Management and Audit Scheme (EMAS) or EU/ETS Regulations These are the standards used for accreditation by the EA members LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 19

The EA’s mission consists of: • Defining, harmonising and building consistency in accreditation in Europe, by ensuring common interpretation and application of the standards used by its members • Ensuring transparency of the operations (including assessments) performed and results provided by its members • Maintaining a multilateral agreement on mutual recognition between accreditation activities and reciprocal acceptance of accredited conformity assessment services and results • Managing a peer evaluation system consistent with international practices • Acting as a technical resource on matters related to the implementation and operation of the European policies on accreditation LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 20

Thank you! Web sites: • www. samc. lv • www. latak. lv e-mail: ESkribnovska@inbox. lv LATVIJAS REPUBLIKAS EKONOMIKAS MINISTRIJA MINISTRY OF ECONOMICS OF THE REPUBLIC OF LATVIA 21
9c1c1cb6950969c4c7d7ad4e12356ed4.ppt