dc979ac71534889cf1d717ac1f048cfe.ppt
- Количество слайдов: 43

Working with FDA: Biological Products and Clinical Development Chemistry, Manufacturing and Control Issues in Production of Therapeutic Biologic Protein Products Ingrid Markovic, Ph. D. , Biologist Laboratory of Biochemistry, Division of Therapeutic Proteins Office of Biotechnology Products, Office of Pharmaceutical Science Center for Drug Evaluation and Research FDA U. S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH
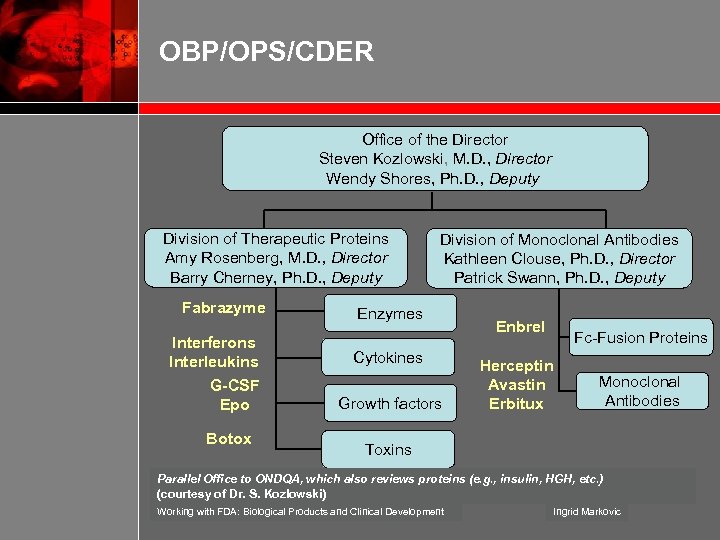
OBP/OPS/CDER Office of the Director Steven Kozlowski, M. D. , Director Wendy Shores, Ph. D. , Deputy Division of Therapeutic Proteins Amy Rosenberg, M. D. , Director Barry Cherney, Ph. D. , Deputy Fabrazyme Interferons Interleukins G-CSF Epo Botox Division of Monoclonal Antibodies Kathleen Clouse, Ph. D. , Director Patrick Swann, Ph. D. , Deputy Enzymes Cytokines Growth factors Enbrel Fc-Fusion Proteins Herceptin Avastin Erbitux Monoclonal Antibodies Toxins Parallel Office to ONDQA, which also reviews proteins (e. g. , insulin, HGH, etc. ) (courtesy of Dr. S. Kozlowski) Working with FDA: Biological Products and Clinical Development Ingrid Markovic
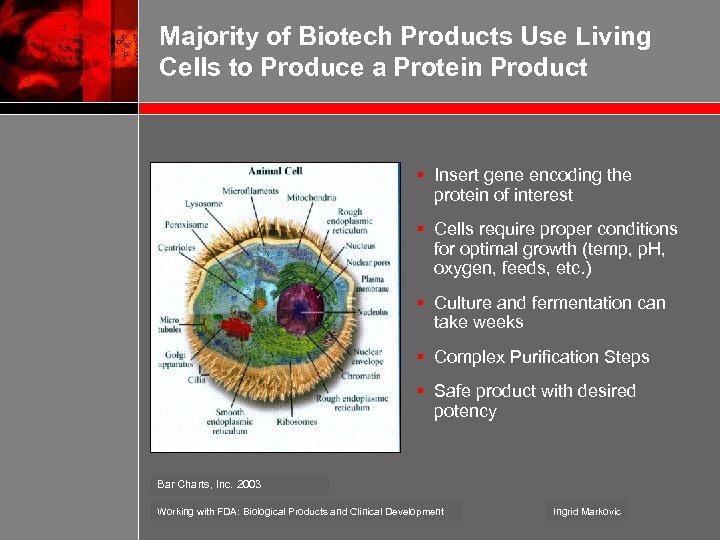
Majority of Biotech Products Use Living Cells to Produce a Protein Product § Insert gene encoding the protein of interest § Cells require proper conditions for optimal growth (temp, p. H, oxygen, feeds, etc. ) § Culture and fermentation can take weeks § Complex Purification Steps § Safe product with desired potency Bar Charts, Inc. 2003 Working with FDA: Biological Products and Clinical Development Ingrid Markovic
Protein Therapeutics § Protein Therapeutics are licensed through Biologics License Application (BLA) under provisions of both Public Health Service (PHS) and Food Drug & Cosmetic (FD&C) Acts § Protein therapeutics are also regulated through New Drug Application (NDA) under provisions of FD&C Act (e. g. , insulin & HGH) § BLA under PHS act lacks an abbreviated pathway for follow-on biologics or biosimilars Working with FDA: Biological Products and Clinical Development Ingrid Markovic
How are Protein Therapeutics different from Small Molecule Drugs? § Contain intrinsic infectious agents § Aseptic techniques required during production (terminal heat or gamma sterilization rarely applied) § Usually have heterogeneous composition • Numerous process and product-related impurities • Change in the manufacturing process can cause change in product composition § Exact structure may be unknown (e. g. , all possible variants often not fully characterized) Working with FDA: Biological Products and Clinical Development Ingrid Markovic
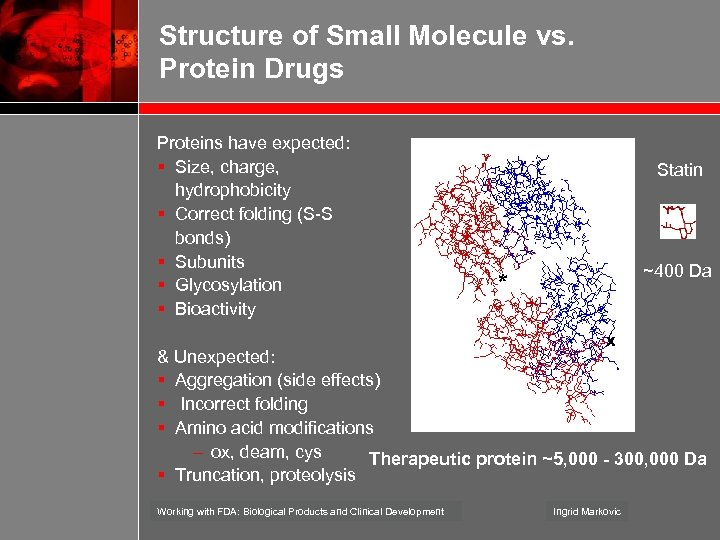
Structure of Small Molecule vs. Protein Drugs Proteins have expected: § Size, charge, hydrophobicity § Correct folding (S-S bonds) § Subunits § Glycosylation § Bioactivity Statin ~400 Da * x & Unexpected: § Aggregation (side effects) § Incorrect folding § Amino acid modifications – ox, deam, cys Therapeutic protein ~5, 000 - 300, 000 Da § Truncation, proteolysis Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Manufacturing Process Working with FDA: Biological Products and Clinical Development Ingrid Markovic
Components of the Manufacturing Process § Expression vector (plasmid) § Cell banking system • Master Cell Bank (MCB) • Working Cell Bank (WCB) • End of Production Cells (EOP) § Drug substance manufacturing and release § Drug product formulation and release Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Expression Vector and Cell Banking System Working with FDA: Biological Products and Clinical Development Ingrid Markovic
Source Materials Bacteria Mycoplasma Fungi Mice Humans Mammalian cell-culture Yeast Bacteria Viruses TSE agents Transgenics Working with FDA: Biological Products and Clinical Development Ingrid Markovic
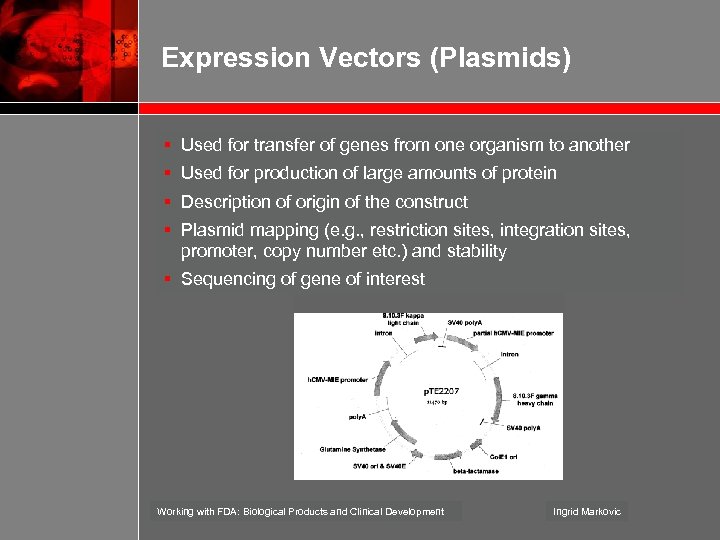
Expression Vectors (Plasmids) § Used for transfer of genes from one organism to another § Used for production of large amounts of protein § Description of origin of the construct § Plasmid mapping (e. g. , restriction sites, integration sites, promoter, copy number etc. ) and stability § Sequencing of gene of interest Working with FDA: Biological Products and Clinical Development Ingrid Markovic

MCB and WCB § A working cell bank (WCB) is derived from the master cell bank (MCB) and is used to initiate a production batch Working with FDA: Biological Products and Clinical Development Ingrid Markovic
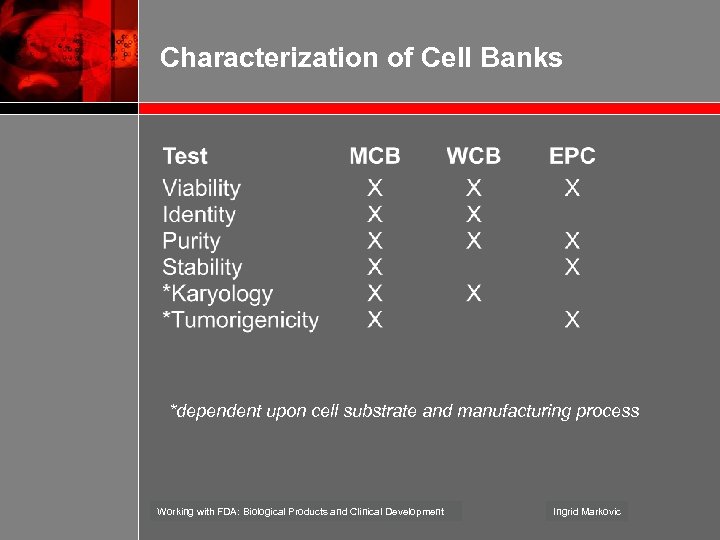
Characterization of Cell Banks *dependent upon cell substrate and manufacturing process Working with FDA: Biological Products and Clinical Development Ingrid Markovic
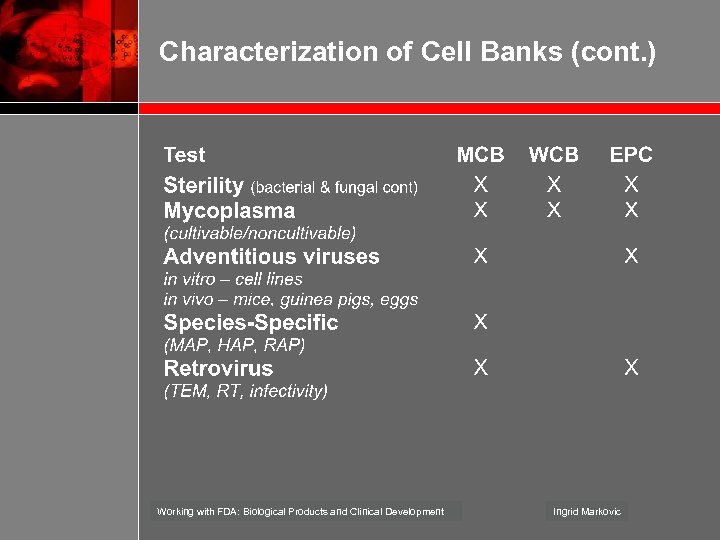
Characterization of Cell Banks (cont. ) Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Sources of Adventitious Agents § Cell Substrate • Endogenous viruses • Exogenous microbial contamination • Source material screening: – Human (HIV, HBV, HCV, CJD, etc. ) – Animal (TSE sources, species-specific viruses) § Raw Materials • Cell culture reagents (animal and non-animal derived) § Environment • Water • Air • Humans/technicians Working with FDA: Biological Products and Clinical Development Ingrid Markovic
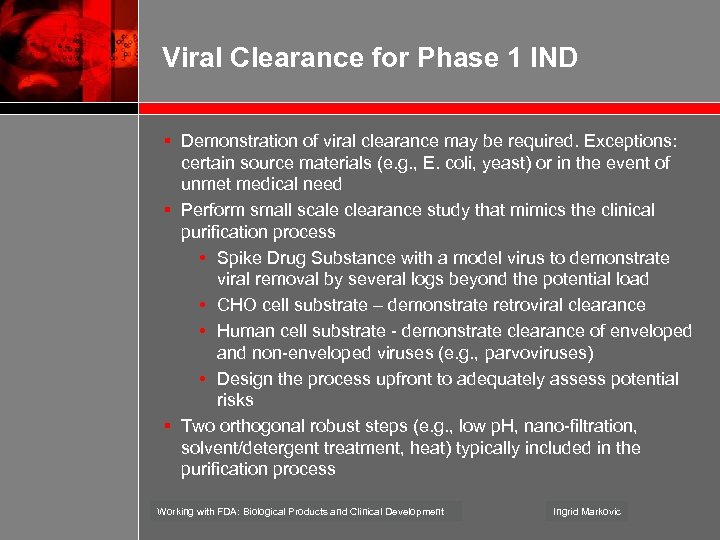
Viral Clearance for Phase 1 IND § Demonstration of viral clearance may be required. Exceptions: certain source materials (e. g. , E. coli, yeast) or in the event of unmet medical need § Perform small scale clearance study that mimics the clinical purification process • Spike Drug Substance with a model virus to demonstrate viral removal by several logs beyond the potential load • CHO cell substrate – demonstrate retroviral clearance • Human cell substrate - demonstrate clearance of enveloped and non-enveloped viruses (e. g. , parvoviruses) • Design the process upfront to adequately assess potential risks § Two orthogonal robust steps (e. g. , low p. H, nano-filtration, solvent/detergent treatment, heat) typically included in the purification process Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Production and Purification Working with FDA: Biological Products and Clinical Development Ingrid Markovic
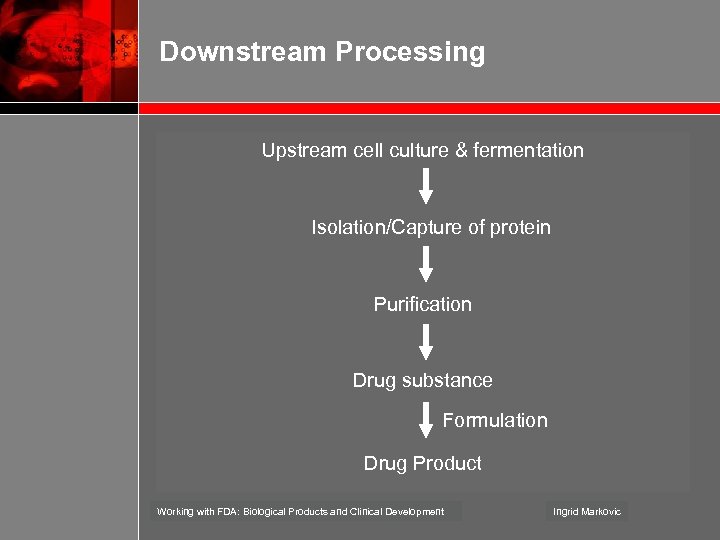
Downstream Processing Upstream cell culture & fermentation Isolation/Capture of protein Purification Drug substance Formulation Drug Product Working with FDA: Biological Products and Clinical Development Ingrid Markovic
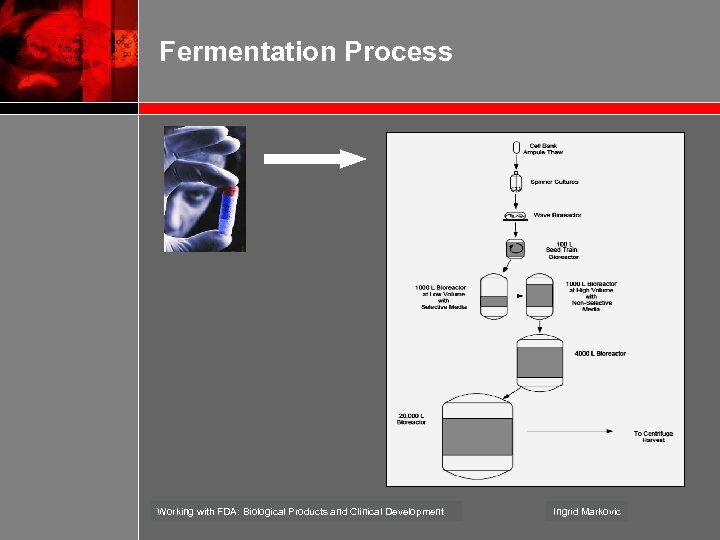
Fermentation Process Working with FDA: Biological Products and Clinical Development Ingrid Markovic
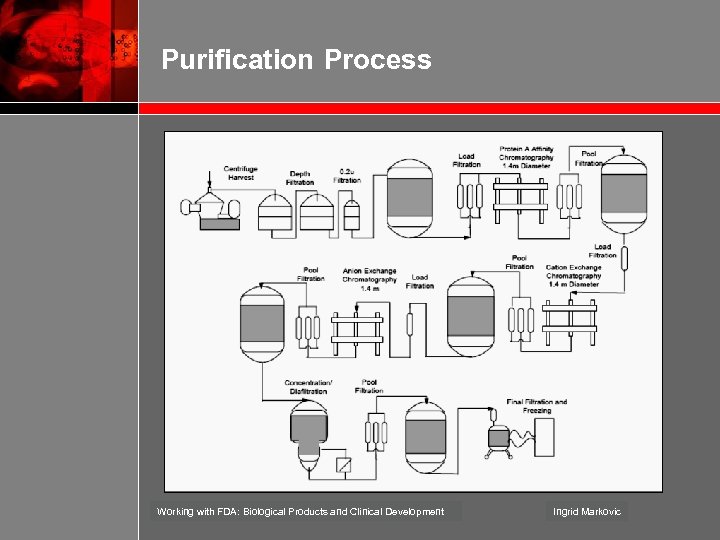
Purification Process Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Drug Substance and Drug Product Characterization Working with FDA: Biological Products and Clinical Development Ingrid Markovic
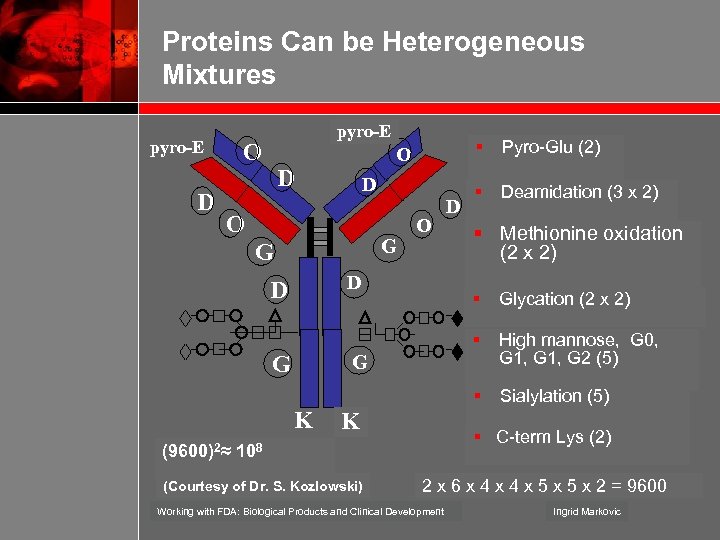
Proteins Can be Heterogeneous Mixtures pyro-E D pyro-E O § O D D O G G O D D D Pyro-Glu (2) § Deamidation (3 x 2) § Methionine oxidation (2 x 2) High mannose, G 0, G 1, G 2 (5) § Sialylation (5) G K (9600)2≈ Glycation (2 x 2) § G § K § C-term Lys (2) 108 (Courtesy of Dr. S. Kozlowski) 2 x 6 x 4 x 5 x 2 = 9600 Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Drug Substance Characterization § Drug Substance should be positive for identity and have specified criteria for purity, potency and microbial contamination § Acceptance criteria for release and stability attributes should be established • Often broader early in the development and subject to revisions (e. g. , narrowed down) as manufacturing process develops § Results from release and stability testing should be provided in the IND § Raw data supporting Drug Substance characterization should be provided in the IND Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Drug Substance Characterization (cont. ) § Safety • Ensured by the specified limits for bioburden and endotoxin, misc. process-related contaminants § Purity & Characterization • Assesses capability of purification process to remove processrelated impurities (e. g. , endogenous viruses, host-cell proteins, DNA, leachables, anti-foam, antibiotics, toxins, solvents, heavy metals, etc. ) • Product-related impurities (e. g. , aggregates, breakdown products, product variants due to: oxidation, deamidation, denaturation, loss of C-term Lys in MAbs etc. ) • Product substances (product variants that are active) § Identity • Unique for protein of interest, especially relevant for closely related proteins manufactured in the same facility Working with FDA: Biological Products and Clinical Development Ingrid Markovic
Drug Substance Characterization (cont. ) § Potency • Required to assess biological activity of the product • Assay should be relevant for protein mechanism of action • For MAb or Fc fusion proteins - a binding assay may be sufficient for early development, but a functional assay relevant for the mechanism of action should be developed • If mechanism of action unknown - multiple bioactivities plus elucidating higher order structure may be required § Strength • Protein content § Stability • Drug Substance stability should be demonstrated with appropriate stability-indicating assays Working with FDA: Biological Products and Clinical Development Ingrid Markovic
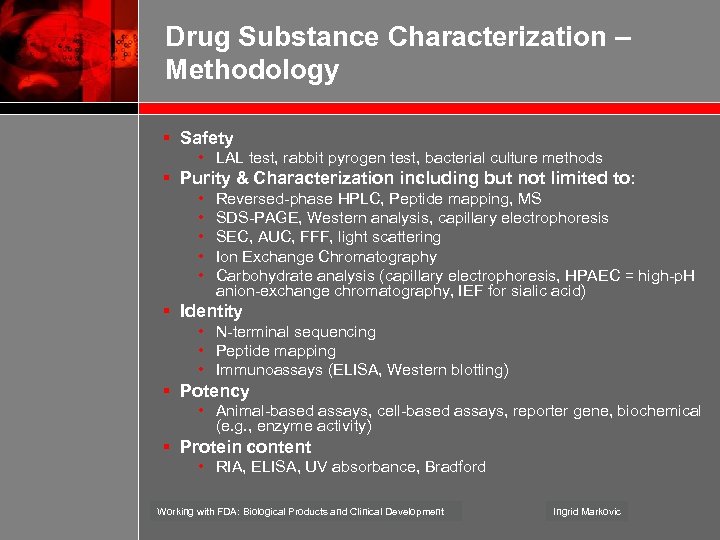
Drug Substance Characterization – Methodology § Safety • LAL test, rabbit pyrogen test, bacterial culture methods § Purity & Characterization including but not limited to: • • • Reversed-phase HPLC, Peptide mapping, MS SDS-PAGE, Western analysis, capillary electrophoresis SEC, AUC, FFF, light scattering Ion Exchange Chromatography Carbohydrate analysis (capillary electrophoresis, HPAEC = high-p. H anion-exchange chromatography, IEF for sialic acid) § Identity • N-terminal sequencing • Peptide mapping • Immunoassays (ELISA, Western blotting) § Potency • Animal-based assays, cell-based assays, reporter gene, biochemical (e. g. , enzyme activity) § Protein content • RIA, ELISA, UV absorbance, Bradford Working with FDA: Biological Products and Clinical Development Ingrid Markovic
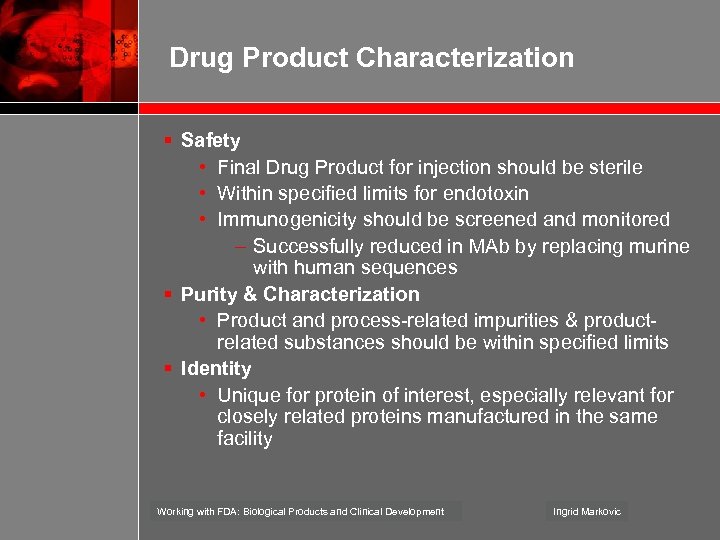
Drug Product Characterization § Safety • Final Drug Product for injection should be sterile • Within specified limits for endotoxin • Immunogenicity should be screened and monitored – Successfully reduced in MAb by replacing murine with human sequences § Purity & Characterization • Product and process-related impurities & productrelated substances should be within specified limits § Identity • Unique for protein of interest, especially relevant for closely related proteins manufactured in the same facility Working with FDA: Biological Products and Clinical Development Ingrid Markovic
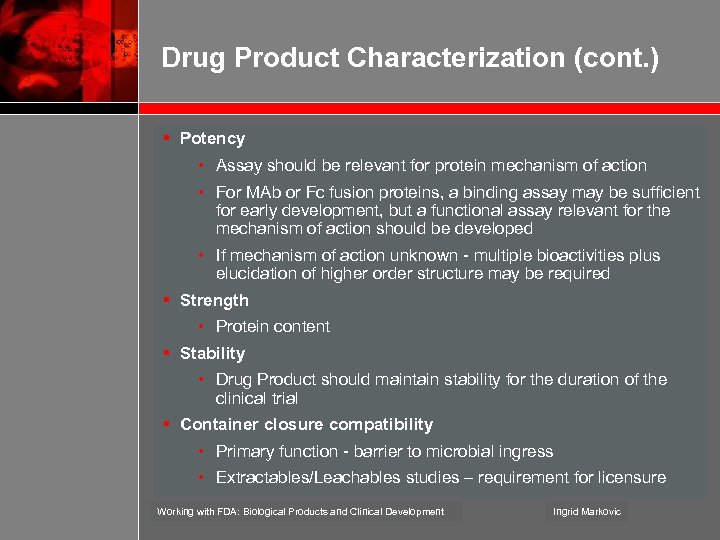
Drug Product Characterization (cont. ) § Potency • Assay should be relevant for protein mechanism of action • For MAb or Fc fusion proteins, a binding assay may be sufficient for early development, but a functional assay relevant for the mechanism of action should be developed • If mechanism of action unknown - multiple bioactivities plus elucidation of higher order structure may be required § Strength • Protein content § Stability • Drug Product should maintain stability for the duration of the clinical trial § Container closure compatibility • Primary function - barrier to microbial ingress • Extractables/Leachables studies – requirement for licensure Working with FDA: Biological Products and Clinical Development Ingrid Markovic
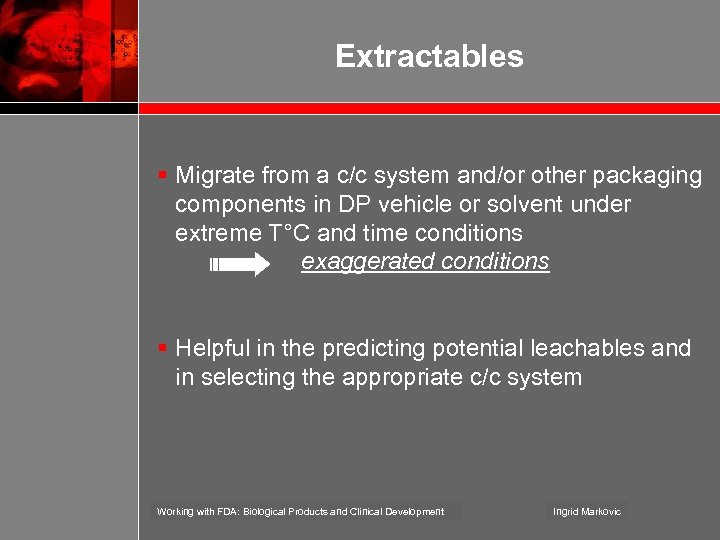
Extractables § Migrate from a c/c system and/or other packaging components in DP vehicle or solvent under extreme T°C and time conditions exaggerated conditions § Helpful in the predicting potential leachables and in selecting the appropriate c/c system Working with FDA: Biological Products and Clinical Development Ingrid Markovic
Leachables § Migrate spontaneously from a c/c system and/or other packaging components normal conditions of use and storage § Often a subset of extractables, or derived by their chemical modification Working with FDA: Biological Products and Clinical Development Ingrid Markovic
Sources of leachables in the product § Syringes/prefilled syringes, ampoules, vials, bottles § IV bags § Storage bags for product intermediates § Closures (screw caps, rubber stoppers) § Container liners (e. g. , tube liners) § Processing equipment: • stainless steel storage tanks/bioreactors • tubing • gaskets, valves, rings • filters • purification resins Working with FDA: Biological Products and Clinical Development Ingrid Markovic
Examples of leachables impacting on safety and product quality Example #1 – Impact of patient safety: § Change: from HSA formulation to a polysorbate § Unchanged container closure system (pre-filled syringes with the uncoated rubber stoppers) § Source: vulcanizing agents leached from rubber stopper over time § Outcome: • no detectable changes in product quality • safety: serious adverse event (PRCA) § Hypothesis: leachables acted as adjuvants triggering immunogenicity Example #2 - Impact on product quality: § Change from a lyophilized to a liquid formulation § Divalent cation leached from the rubber stopper § Caused activation of metalloprotease (a process-related impurity coeluted with the API) § Impact: product degradation at the N-terminal site (stability study) Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Stability Program § Drug Substance and Drug Product, real-time and accelerated stability data with several time points under upright and inverted conditions used to establish the expiration period § Stress studies (e. g. , UV, exaggerated light, temperature and p. H) useful to elucidate product degradation pathways and for defining acceptance criteria § Limited time stability studies may be acceptable if shortterm trial is anticipated § Stability data generated from engineering lots also acceptable § Failure to demonstrate product stability is a potential hold issue Working with FDA: Biological Products and Clinical Development Ingrid Markovic
Stability Program (cont. ) The following testing should be included at a minimum: § Safety • Bioburden/sterility § Purity • Product and process-related impurities & productrelated substances § Sialic acid - if appropriate § Potency § Protein content/strength § p. H § Appearance § Leachables (separate study, not part of routine stability testing) Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Good Manufacturing Practices Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Inspectional Activity § Three types: • Pre-licensed (PLI) - announced, generally required for approval • Pre-approval (PAI) – announced, could be waived • Surveillance (biennial post-licensure) – unannounced • No formal inspection requirement for sites manufacturing biologics under clinical investigation • Manufacturing and testing sites are subject to inspection § Inspection system undergoing revision for OBP products § Currently inspections of facilities manufacturing CDER BLA products: • PAI led by TFRB, Office of Compliance, with Product Reviewer(s) sometimes part of on-site team • Biennial post-licensure inspections led by Team Biologics with Product Reviewers which can be part of the on-site team involved in the inspection § NDA Products: • Pre-approval and post-licensure inspections led by district personnel Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Facilities and Practices § Closed systems whenever possible § Aseptic Processing § CIP/SIP § Disposable Systems § Environmental Monitoring § Water/HVAC § Good record-keeping and documentation (phase 1) Working with FDA: Biological Products and Clinical Development Ingrid Markovic
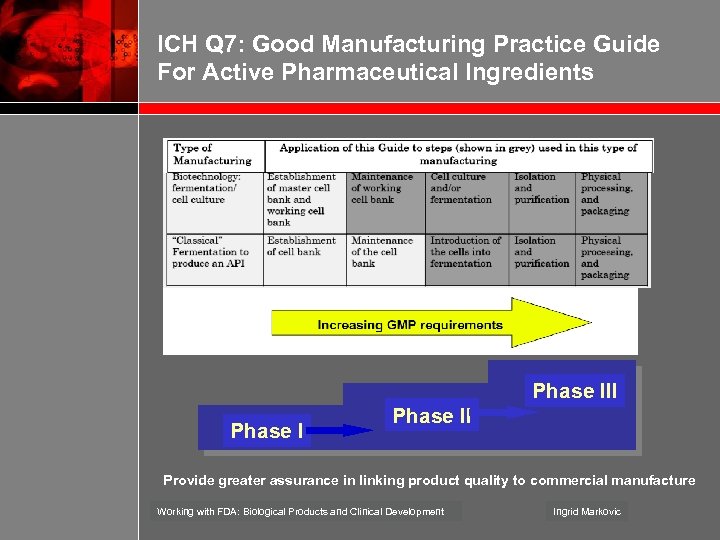
ICH Q 7: Good Manufacturing Practice Guide For Active Pharmaceutical Ingredients Phase III Phase II Provide greater assurance in linking product quality to commercial manufacture Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Potential Show Stoppers? Working with FDA: Biological Products and Clinical Development Ingrid Markovic
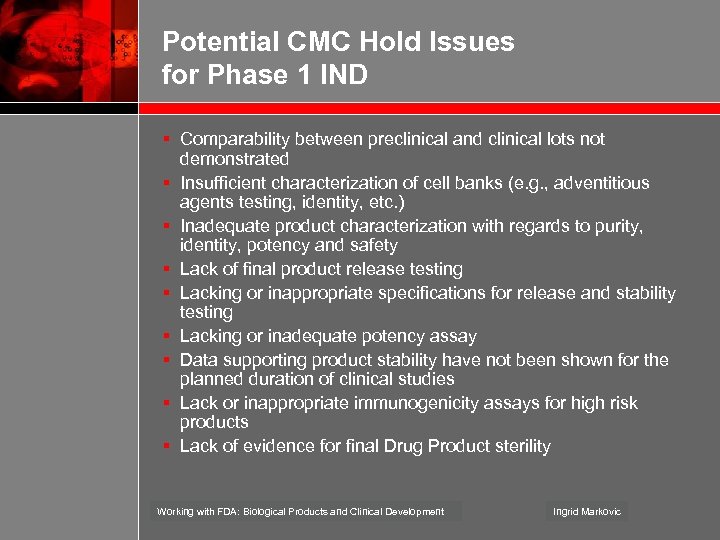
Potential CMC Hold Issues for Phase 1 IND § Comparability between preclinical and clinical lots not demonstrated § Insufficient characterization of cell banks (e. g. , adventitious agents testing, identity, etc. ) § Inadequate product characterization with regards to purity, identity, potency and safety § Lack of final product release testing § Lacking or inappropriate specifications for release and stability testing § Lacking or inadequate potency assay § Data supporting product stability have not been shown for the planned duration of clinical studies § Lack or inappropriate immunogenicity assays for high risk products § Lack of evidence for final Drug Product sterility Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Guidance Documents § Guidance for Industry: Content and Format of Investigational New Drug Applications for Phase I Studies of Drugs, including Well-Characterized, Therapeutic, Biotechnology derived Products (1995) § Guidance for Industry for the Submission of CMC Information for a Therapeutic Recombinant DNA-Derived Product or a Monoclonal Antibody Product for In Vivo Use (1996) § Guidance for Industry: IND for Phase 2 and 3 studies of Drugs, including Specified Therapeutic Biotechnology-Derived Products – CMC Content and Format (Draft, 1999) § FDA Guidance Concerning Demonstration of Comparability of Human Biological Products, including Therapeutic Biotechnology-derived Products (1996) § Guidance for Industry: INDs - Approaches to Complying with c. GMP's for Phase 1 Drugs (Draft, 2006) § Points to Consider in the Manufacture and Testing of Monoclonal Antibody Products for Human Use (1997) § Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals (1993) § International Conference on Harmonization (ICH) documents § 21 CFR 200’s, 600’s § PHS Act, FD&C Act Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Acknowledgments Emily Shacter Barry Cherney Steven Kozlowski Wendy Shores Susan Kirshner Emanuela Lacana Patricia Hughes Joe Kutza All of OBP Working with FDA: Biological Products and Clinical Development Ingrid Markovic

Questions? Comments? Working with FDA: Biological Products and Clinical Development Ingrid Markovic
dc979ac71534889cf1d717ac1f048cfe.ppt