a4c233c9926e203457fbfc2fda10a60d.ppt
- Количество слайдов: 24
 Women and Alport Syndrome Michelle Rheault, M. D. Assistant Professor Division of Pediatric Nephrology University of Minnesota, USA
Women and Alport Syndrome Michelle Rheault, M. D. Assistant Professor Division of Pediatric Nephrology University of Minnesota, USA
 Disclosures • None
Disclosures • None
 Historical Perspective • “The females have deafness and heamaturia and live to old age”- (Alport AC: Br Med J 1: 504 -506, 1927) • “Females usually remain well throughout life…and only rarely have women died of the disease. ”(Perkoff GT: Annu Rev Med 15: 115 -24, 1964)
Historical Perspective • “The females have deafness and heamaturia and live to old age”- (Alport AC: Br Med J 1: 504 -506, 1927) • “Females usually remain well throughout life…and only rarely have women died of the disease. ”(Perkoff GT: Annu Rev Med 15: 115 -24, 1964)
 Carrier Natural History Study • 195 families with known COL 4 A 5 mutations (349 women and girls) • Microscopic hematuria present in 95. 5% • Proteinuria present in 75% • Hearing loss present in 28% • By age 40, 12% of carriers had reached ESRD • By age 60, 30 -40% of carriers had reached ESRD Jais, et al. JASN. 14: 2603 -2610, 2003
Carrier Natural History Study • 195 families with known COL 4 A 5 mutations (349 women and girls) • Microscopic hematuria present in 95. 5% • Proteinuria present in 75% • Hearing loss present in 28% • By age 40, 12% of carriers had reached ESRD • By age 60, 30 -40% of carriers had reached ESRD Jais, et al. JASN. 14: 2603 -2610, 2003
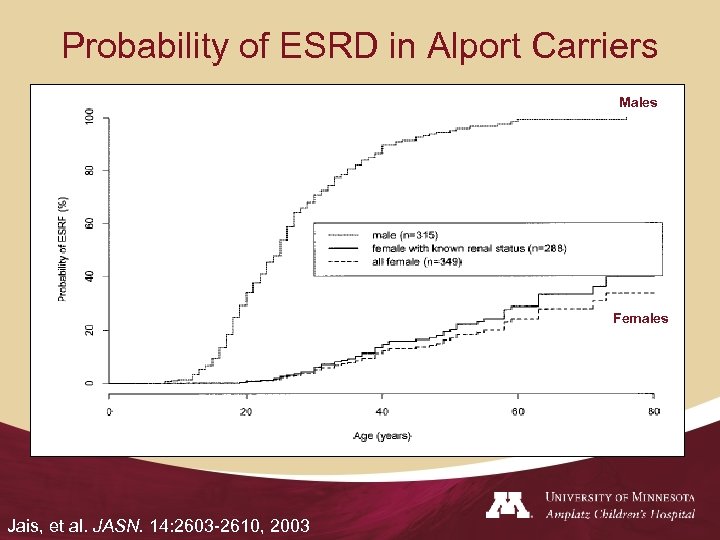 Probability of ESRD in Alport Carriers Males Females Jais, et al. JASN. 14: 2603 -2610, 2003
Probability of ESRD in Alport Carriers Males Females Jais, et al. JASN. 14: 2603 -2610, 2003
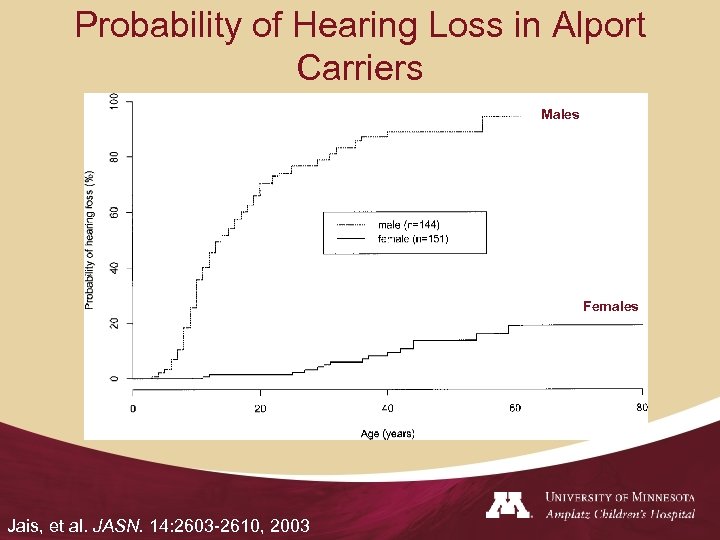 Probability of Hearing Loss in Alport Carriers Males Females Jais, et al. JASN. 14: 2603 -2610, 2003
Probability of Hearing Loss in Alport Carriers Males Females Jais, et al. JASN. 14: 2603 -2610, 2003
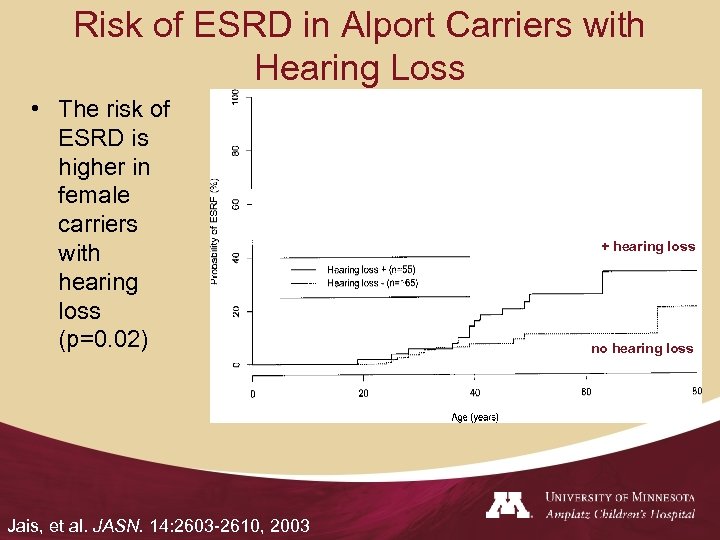 Risk of ESRD in Alport Carriers with Hearing Loss • The risk of ESRD is higher in female carriers with hearing loss (p=0. 02) Jais, et al. JASN. 14: 2603 -2610, 2003 + hearing loss no hearing loss
Risk of ESRD in Alport Carriers with Hearing Loss • The risk of ESRD is higher in female carriers with hearing loss (p=0. 02) Jais, et al. JASN. 14: 2603 -2610, 2003 + hearing loss no hearing loss
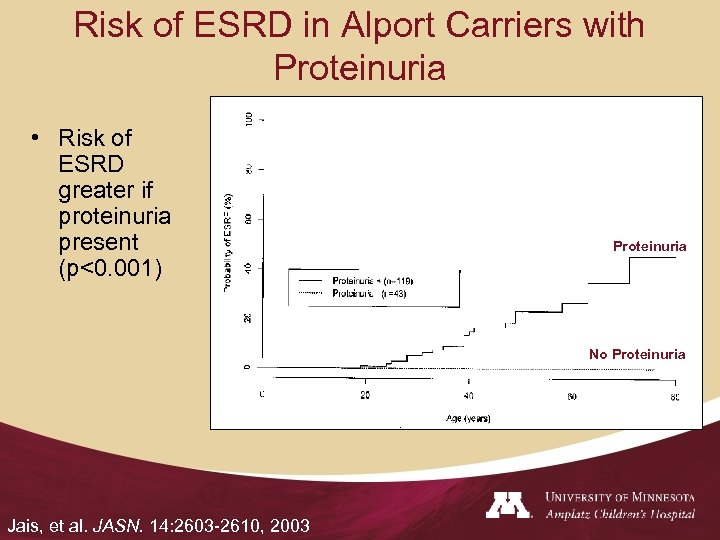 Risk of ESRD in Alport Carriers with Proteinuria • Risk of ESRD greater if proteinuria present (p<0. 001) Proteinuria No Proteinuria Jais, et al. JASN. 14: 2603 -2610, 2003
Risk of ESRD in Alport Carriers with Proteinuria • Risk of ESRD greater if proteinuria present (p<0. 001) Proteinuria No Proteinuria Jais, et al. JASN. 14: 2603 -2610, 2003
 What determines disease severity in Alport carriers? • Mutation – Unlike males, there is no correlation between type of mutation and rate of disease progression (Jais, et al. JASN. 14: 2603 -2610, 2003) – No correlation in disease severity between males and females within the same family • Modifier genes • X chromosome inactivation • ?
What determines disease severity in Alport carriers? • Mutation – Unlike males, there is no correlation between type of mutation and rate of disease progression (Jais, et al. JASN. 14: 2603 -2610, 2003) – No correlation in disease severity between males and females within the same family • Modifier genes • X chromosome inactivation • ?
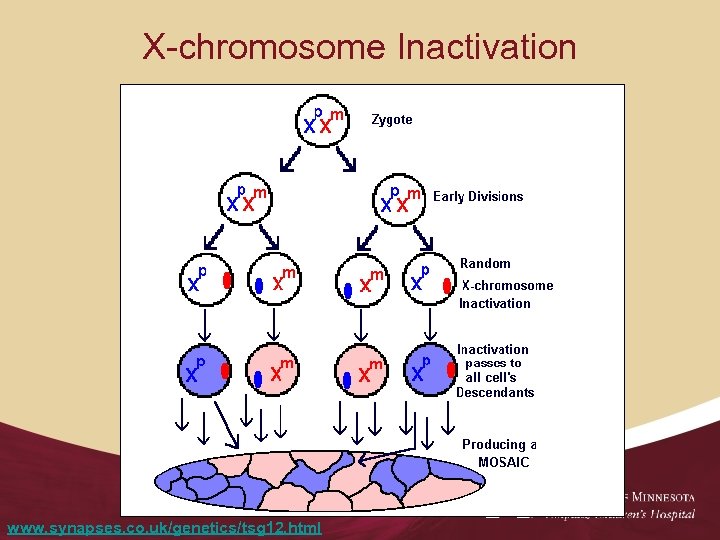 X-chromosome Inactivation www. synapses. co. uk/genetics/tsg 12. html
X-chromosome Inactivation www. synapses. co. uk/genetics/tsg 12. html
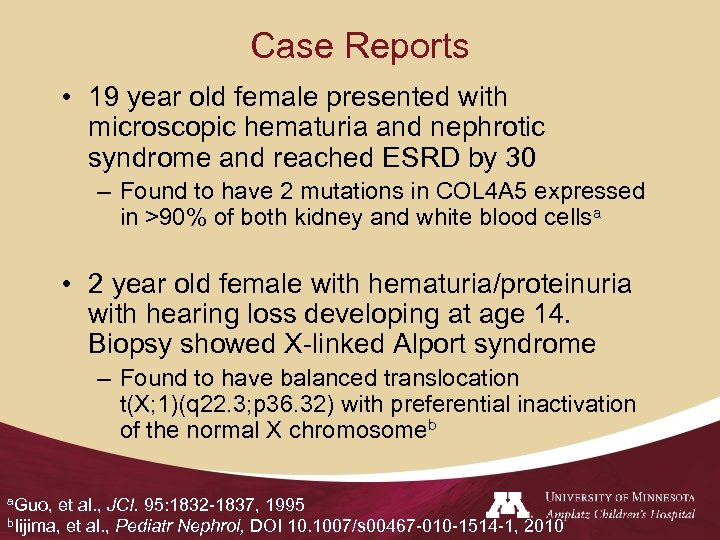 Case Reports • 19 year old female presented with microscopic hematuria and nephrotic syndrome and reached ESRD by 30 – Found to have 2 mutations in COL 4 A 5 expressed in >90% of both kidney and white blood cellsa • 2 year old female with hematuria/proteinuria with hearing loss developing at age 14. Biopsy showed X-linked Alport syndrome – Found to have balanced translocation t(X; 1)(q 22. 3; p 36. 32) with preferential inactivation of the normal X chromosomeb a. Guo, et al. , JCI. 95: 1832 -1837, 1995 b. Iijima, et al. , Pediatr Nephrol, DOI 10. 1007/s 00467 -010 -1514 -1, 2010
Case Reports • 19 year old female presented with microscopic hematuria and nephrotic syndrome and reached ESRD by 30 – Found to have 2 mutations in COL 4 A 5 expressed in >90% of both kidney and white blood cellsa • 2 year old female with hematuria/proteinuria with hearing loss developing at age 14. Biopsy showed X-linked Alport syndrome – Found to have balanced translocation t(X; 1)(q 22. 3; p 36. 32) with preferential inactivation of the normal X chromosomeb a. Guo, et al. , JCI. 95: 1832 -1837, 1995 b. Iijima, et al. , Pediatr Nephrol, DOI 10. 1007/s 00467 -010 -1514 -1, 2010
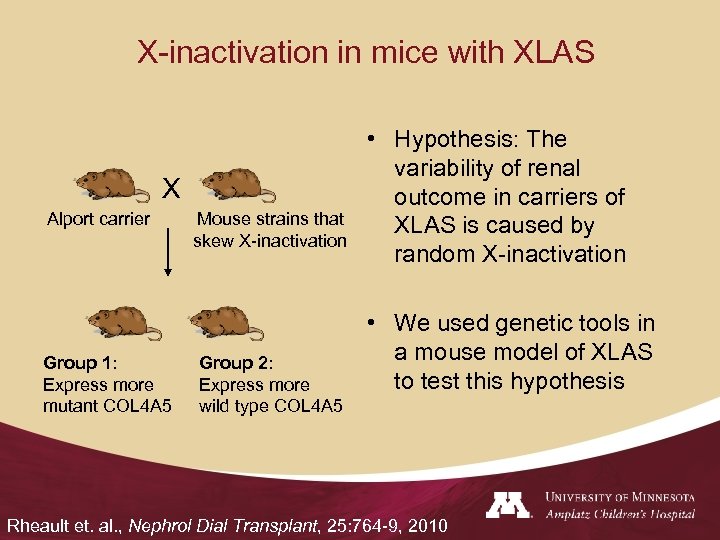 X-inactivation in mice with XLAS X Alport carrier Group 1: Express more mutant COL 4 A 5 Mouse strains that skew X-inactivation Group 2: Express more wild type COL 4 A 5 • Hypothesis: The variability of renal outcome in carriers of XLAS is caused by random X-inactivation • We used genetic tools in a mouse model of XLAS to test this hypothesis Rheault et. al. , Nephrol Dial Transplant, 25: 764 -9, 2010
X-inactivation in mice with XLAS X Alport carrier Group 1: Express more mutant COL 4 A 5 Mouse strains that skew X-inactivation Group 2: Express more wild type COL 4 A 5 • Hypothesis: The variability of renal outcome in carriers of XLAS is caused by random X-inactivation • We used genetic tools in a mouse model of XLAS to test this hypothesis Rheault et. al. , Nephrol Dial Transplant, 25: 764 -9, 2010
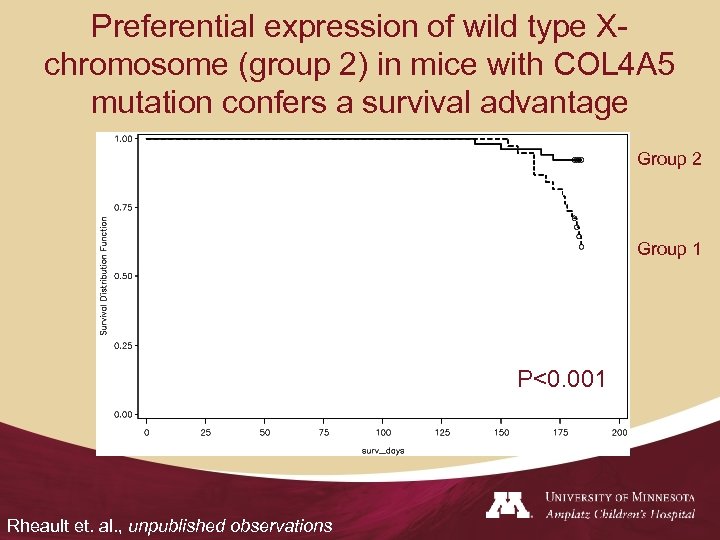 Preferential expression of wild type Xchromosome (group 2) in mice with COL 4 A 5 mutation confers a survival advantage Group 2 Group 1 P<0. 001 Rheault et. al. , unpublished observations
Preferential expression of wild type Xchromosome (group 2) in mice with COL 4 A 5 mutation confers a survival advantage Group 2 Group 1 P<0. 001 Rheault et. al. , unpublished observations
 X inactivation and Alport Syndrome • When tested directly in controlled genetic backgrounds, favorable X-inactivation increases survival and improves clinical parameters in female carriers of XLAS in mice • X inactivation is not the only factor that influences disease severity • Further research is needed
X inactivation and Alport Syndrome • When tested directly in controlled genetic backgrounds, favorable X-inactivation increases survival and improves clinical parameters in female carriers of XLAS in mice • X inactivation is not the only factor that influences disease severity • Further research is needed
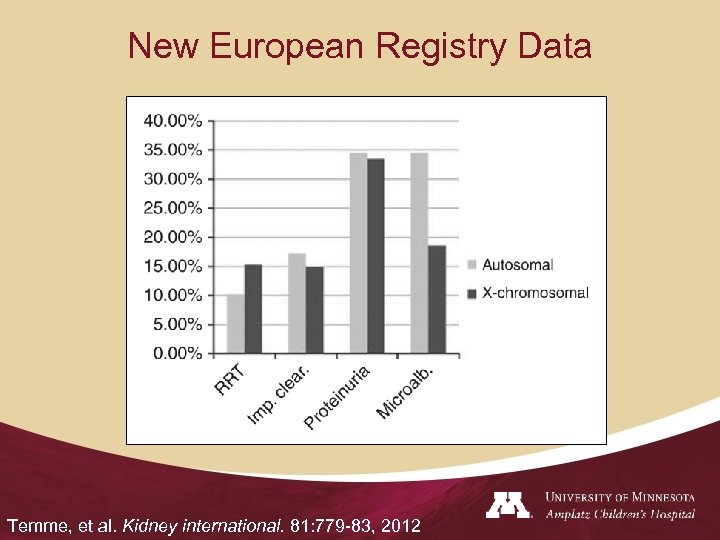 New European Registry Data Temme, et al. Kidney international. 81: 779 -83, 2012
New European Registry Data Temme, et al. Kidney international. 81: 779 -83, 2012
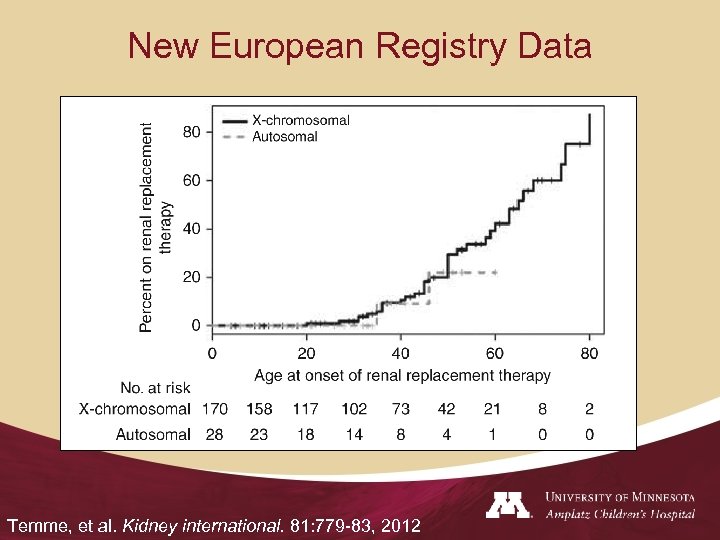 New European Registry Data Temme, et al. Kidney international. 81: 779 -83, 2012
New European Registry Data Temme, et al. Kidney international. 81: 779 -83, 2012
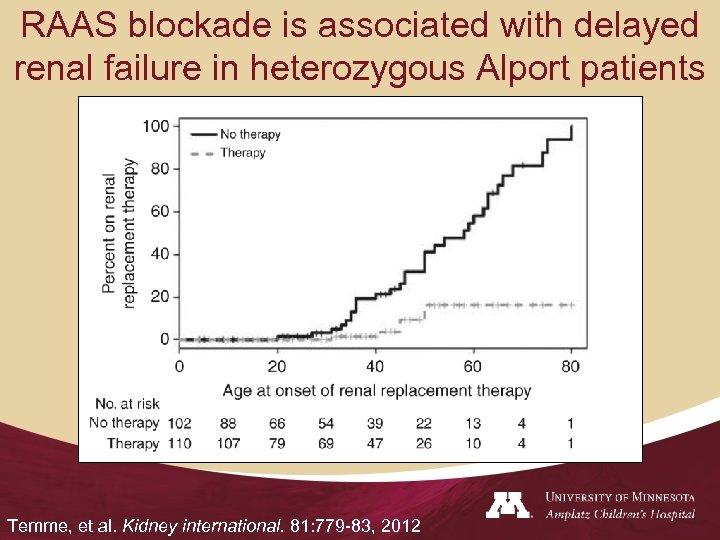 RAAS blockade is associated with delayed renal failure in heterozygous Alport patients Temme, et al. Kidney international. 81: 779 -83, 2012
RAAS blockade is associated with delayed renal failure in heterozygous Alport patients Temme, et al. Kidney international. 81: 779 -83, 2012
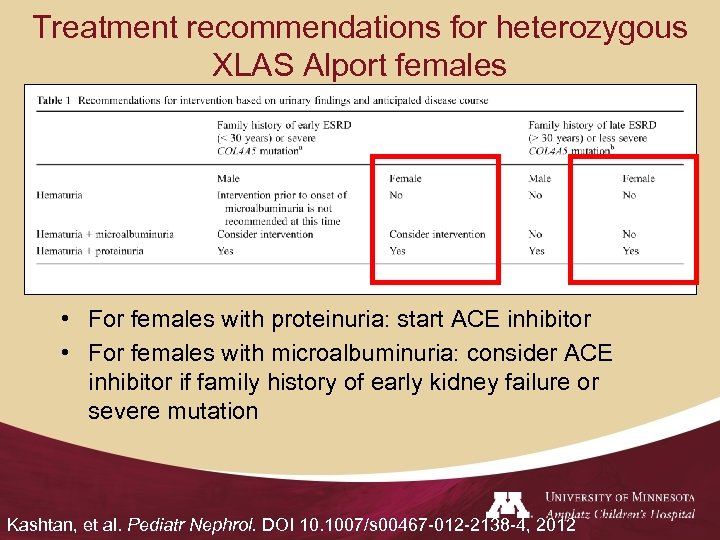 Treatment recommendations for heterozygous XLAS Alport females • For females with proteinuria: start ACE inhibitor • For females with microalbuminuria: consider ACE inhibitor if family history of early kidney failure or severe mutation Kashtan, et al. Pediatr Nephrol. DOI 10. 1007/s 00467 -012 -2138 -4, 2012
Treatment recommendations for heterozygous XLAS Alport females • For females with proteinuria: start ACE inhibitor • For females with microalbuminuria: consider ACE inhibitor if family history of early kidney failure or severe mutation Kashtan, et al. Pediatr Nephrol. DOI 10. 1007/s 00467 -012 -2138 -4, 2012
 Should Alport Carriers be Kidney Donors? • We can’t predict which carriers are going to progress to ESRD • Difficult balance between risk to donor and benefits for recipient • Little long term data about outcomes in carriers after donation
Should Alport Carriers be Kidney Donors? • We can’t predict which carriers are going to progress to ESRD • Difficult balance between risk to donor and benefits for recipient • Little long term data about outcomes in carriers after donation
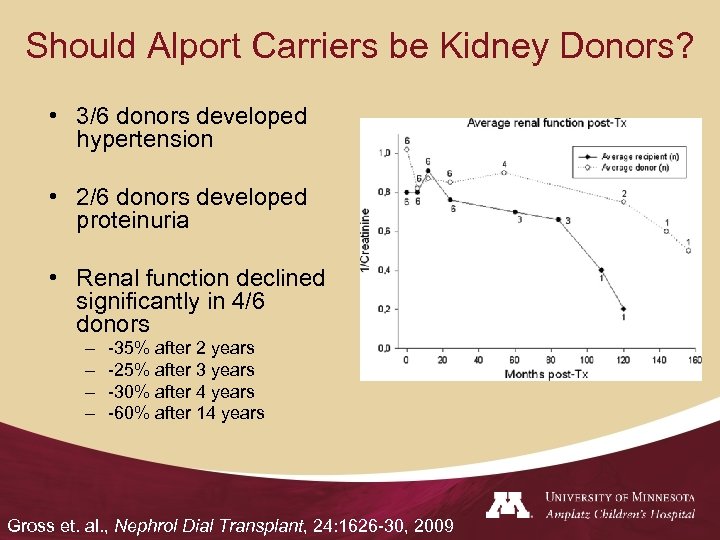 Should Alport Carriers be Kidney Donors? • 3/6 donors developed hypertension • 2/6 donors developed proteinuria • Renal function declined significantly in 4/6 donors – – -35% after 2 years -25% after 3 years -30% after 4 years -60% after 14 years Gross et. al. , Nephrol Dial Transplant, 24: 1626 -30, 2009
Should Alport Carriers be Kidney Donors? • 3/6 donors developed hypertension • 2/6 donors developed proteinuria • Renal function declined significantly in 4/6 donors – – -35% after 2 years -25% after 3 years -30% after 4 years -60% after 14 years Gross et. al. , Nephrol Dial Transplant, 24: 1626 -30, 2009
 Should Alport Carriers be Kidney Donors? • Alport carriers should be kidney donors of last resort • Alport carriers with proteinuria or hearing loss should be excluded as kidney donors • Alport carriers with only microscopic hematuria should be considered as donors only after careful counseling about risks and with close post-transplant monitoring • Renal protective strategies for donors are needed (ACE inhibitors? ) • Future collaborative studies are needed
Should Alport Carriers be Kidney Donors? • Alport carriers should be kidney donors of last resort • Alport carriers with proteinuria or hearing loss should be excluded as kidney donors • Alport carriers with only microscopic hematuria should be considered as donors only after careful counseling about risks and with close post-transplant monitoring • Renal protective strategies for donors are needed (ACE inhibitors? ) • Future collaborative studies are needed
 Alport syndrome and pregnancy • Case reports have been published suggesting increased risk of preterm delivery, decline in renal function, and increased proteinuria during pregnancy • No good data exists on renal outcomes in Alport carriers after pregnancy • Recommendation: Pregnant Alport carriers should have kidney function, blood pressure, and proteinuria monitored closely
Alport syndrome and pregnancy • Case reports have been published suggesting increased risk of preterm delivery, decline in renal function, and increased proteinuria during pregnancy • No good data exists on renal outcomes in Alport carriers after pregnancy • Recommendation: Pregnant Alport carriers should have kidney function, blood pressure, and proteinuria monitored closely
 Conclusions • Carriers of X-linked Alport syndrome are at risk for ESRD – Higher risk of ESRD if proteinuria or hearing loss present • In a mouse model of X-linked Alport Syndrome, favorable X-inactivation increases survival and improves clinical parameters in carriers • ACE inhibitors are associated with decreased risk of end stage kidney disease in Alport carriers
Conclusions • Carriers of X-linked Alport syndrome are at risk for ESRD – Higher risk of ESRD if proteinuria or hearing loss present • In a mouse model of X-linked Alport Syndrome, favorable X-inactivation increases survival and improves clinical parameters in carriers • ACE inhibitors are associated with decreased risk of end stage kidney disease in Alport carriers
 Acknowledgements • Alport Syndrome Foundation • University of Minnesota – – – – Yoav Segal Cliff Kashtan Stefan Kren Will Thomas Linda Hartich Melanie Wall Hector Mesa • Texas A & M University – George Lees • Pasteur Institute – Philip Avner
Acknowledgements • Alport Syndrome Foundation • University of Minnesota – – – – Yoav Segal Cliff Kashtan Stefan Kren Will Thomas Linda Hartich Melanie Wall Hector Mesa • Texas A & M University – George Lees • Pasteur Institute – Philip Avner


