9ad317b844c0e7451bc4c48e1a845dbf.ppt
- Количество слайдов: 35
 WIPO’s Patent services for external users Lutz Mailänder Head, Patent Information Section Global IP Infrastructure Sector Geneva 18 June 2013
WIPO’s Patent services for external users Lutz Mailänder Head, Patent Information Section Global IP Infrastructure Sector Geneva 18 June 2013
 Overview Search reports for developing countries (WPIS) Search and examination reports for patent offices in developing countries (complementing the PCT; ICE) Capacity building for examiners in using examination results for members of the patent family Patent landscapes Platform for national patent registers Background information Links „I have a PCT application – how can I find out what the status is in country X ? “ Open Innovation platforms: WIPO Green, WIPO Re: Search
Overview Search reports for developing countries (WPIS) Search and examination reports for patent offices in developing countries (complementing the PCT; ICE) Capacity building for examiners in using examination results for members of the patent family Patent landscapes Platform for national patent registers Background information Links „I have a PCT application – how can I find out what the status is in country X ? “ Open Innovation platforms: WIPO Green, WIPO Re: Search
 WIPO's patent landscape project Dedicated website Links to published reports Links to groups/institutions active in the field General background/information
WIPO's patent landscape project Dedicated website Links to published reports Links to groups/institutions active in the field General background/information
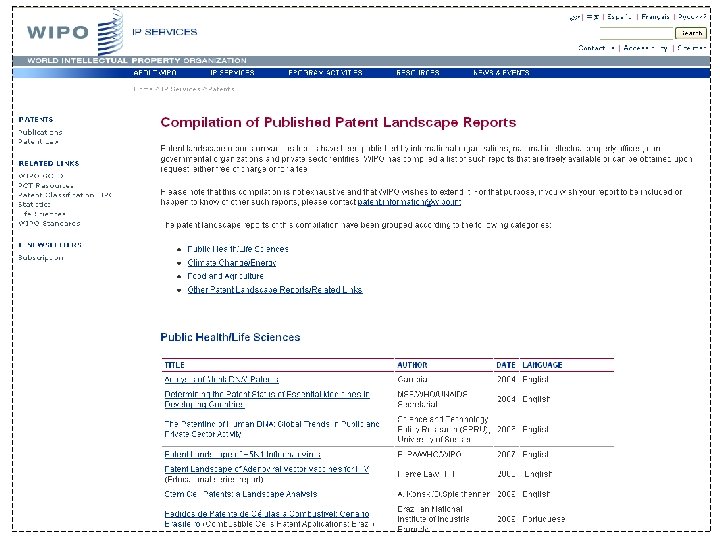
 Sample of individual report website Three standard components: Report body (. PDF) Database (. xls) Interactive visualization (Intellixir)
Sample of individual report website Three standard components: Report body (. PDF) Database (. xls) Interactive visualization (Intellixir)
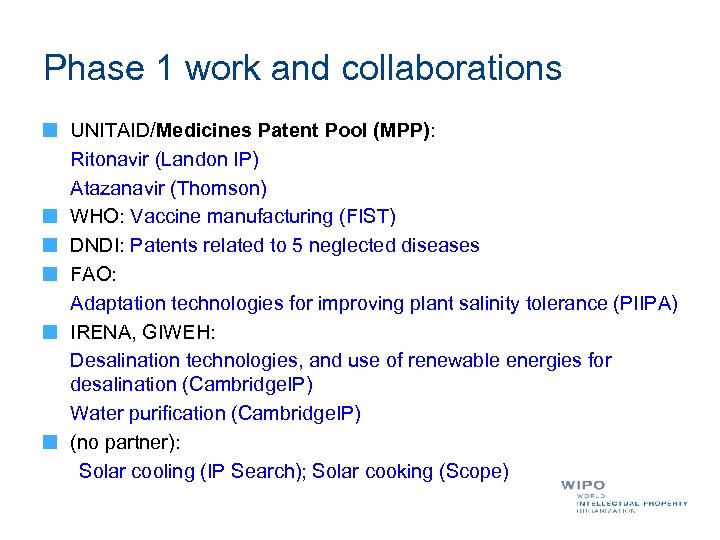 Phase 1 work and collaborations UNITAID/Medicines Patent Pool (MPP): Ritonavir (Landon IP) Atazanavir (Thomson) WHO: Vaccine manufacturing (FIST) DNDI: Patents related to 5 neglected diseases FAO: Adaptation technologies for improving plant salinity tolerance (PIIPA) IRENA, GIWEH: Desalination technologies, and use of renewable energies for desalination (Cambridge. IP) Water purification (Cambridge. IP) (no partner): Solar cooling (IP Search); Solar cooking (Scope)
Phase 1 work and collaborations UNITAID/Medicines Patent Pool (MPP): Ritonavir (Landon IP) Atazanavir (Thomson) WHO: Vaccine manufacturing (FIST) DNDI: Patents related to 5 neglected diseases FAO: Adaptation technologies for improving plant salinity tolerance (PIIPA) IRENA, GIWEH: Desalination technologies, and use of renewable energies for desalination (Cambridge. IP) Water purification (Cambridge. IP) (no partner): Solar cooling (IP Search); Solar cooking (Scope)
 Collaborations Collaboration partners contribute expertise in technical field WIPO contributes expertise in coordination PLRs and funding Each collaboration serves as vehicle for partners to familiarize themselves with patent information, analytics, patent system Collaboration in drafting TOR, delivery phase, evaluation of PLR
Collaborations Collaboration partners contribute expertise in technical field WIPO contributes expertise in coordination PLRs and funding Each collaboration serves as vehicle for partners to familiarize themselves with patent information, analytics, patent system Collaboration in drafting TOR, delivery phase, evaluation of PLR
 WIPO Patent Landscape project Evaluation after Phase II (2012 -13) Budget for 6 further PLRs Capacity building Manual for best practices Regional Workshop for exchange of best practices Refining standardized tools/procedures of Phase I and developing into future standard service
WIPO Patent Landscape project Evaluation after Phase II (2012 -13) Budget for 6 further PLRs Capacity building Manual for best practices Regional Workshop for exchange of best practices Refining standardized tools/procedures of Phase I and developing into future standard service
 Ritonavir patent landscape report Objectives of report: Identify all patents claiming an invention related to the active ingredient (synthesis, combinations, applications, . . ) Analysis of patenting activity Identify sample innovation tracks, subsequent generations of patents claiming subsequent improvements, new inventions (“evergreening”) Detailed description of search methodology for pharmaceuticals; suitable as training example
Ritonavir patent landscape report Objectives of report: Identify all patents claiming an invention related to the active ingredient (synthesis, combinations, applications, . . ) Analysis of patenting activity Identify sample innovation tracks, subsequent generations of patents claiming subsequent improvements, new inventions (“evergreening”) Detailed description of search methodology for pharmaceuticals; suitable as training example
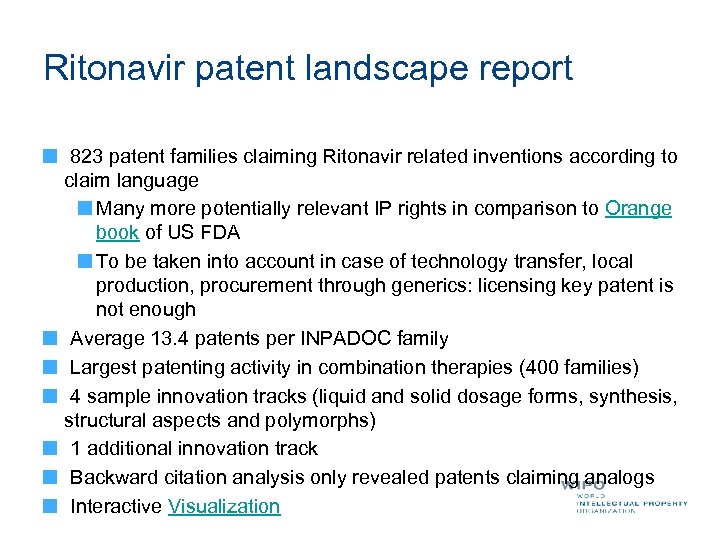 Ritonavir patent landscape report 823 patent families claiming Ritonavir related inventions according to claim language Many more potentially relevant IP rights in comparison to Orange book of US FDA To be taken into account in case of technology transfer, local production, procurement through generics: licensing key patent is not enough Average 13. 4 patents per INPADOC family Largest patenting activity in combination therapies (400 families) 4 sample innovation tracks (liquid and solid dosage forms, synthesis, structural aspects and polymorphs) 1 additional innovation track Backward citation analysis only revealed patents claiming analogs Interactive Visualization
Ritonavir patent landscape report 823 patent families claiming Ritonavir related inventions according to claim language Many more potentially relevant IP rights in comparison to Orange book of US FDA To be taken into account in case of technology transfer, local production, procurement through generics: licensing key patent is not enough Average 13. 4 patents per INPADOC family Largest patenting activity in combination therapies (400 families) 4 sample innovation tracks (liquid and solid dosage forms, synthesis, structural aspects and polymorphs) 1 additional innovation track Backward citation analysis only revealed patents claiming analogs Interactive Visualization
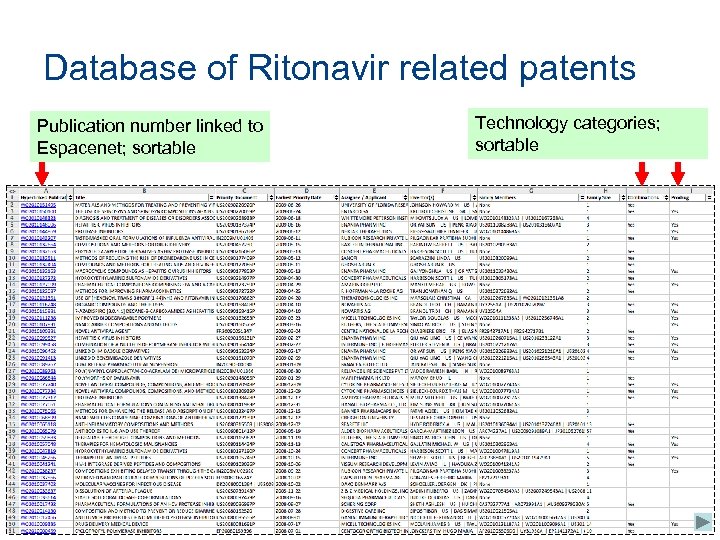 Database of Ritonavir related patents Publication number linked to Espacenet; sortable Technology categories; sortable
Database of Ritonavir related patents Publication number linked to Espacenet; sortable Technology categories; sortable
 Innovation tracks Sometimes key inventions take place, e. g. pharmaceutical substances Trigger series of further developments e. g. combinations/formulations, synthesis, . . Starting point of subsequent generations of related patents protecting the further innovation Such later patents perpetuate protection beyond the 20 years after the filing of the initial patent I. e. certain technologies using the initial invention may still be protected though protection of first invention may have expired
Innovation tracks Sometimes key inventions take place, e. g. pharmaceutical substances Trigger series of further developments e. g. combinations/formulations, synthesis, . . Starting point of subsequent generations of related patents protecting the further innovation Such later patents perpetuate protection beyond the 20 years after the filing of the initial patent I. e. certain technologies using the initial invention may still be protected though protection of first invention may have expired
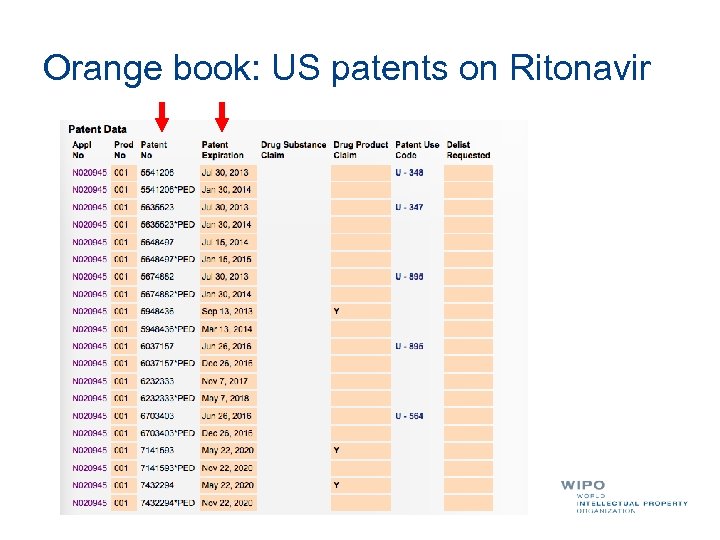 Orange book: US patents on Ritonavir
Orange book: US patents on Ritonavir
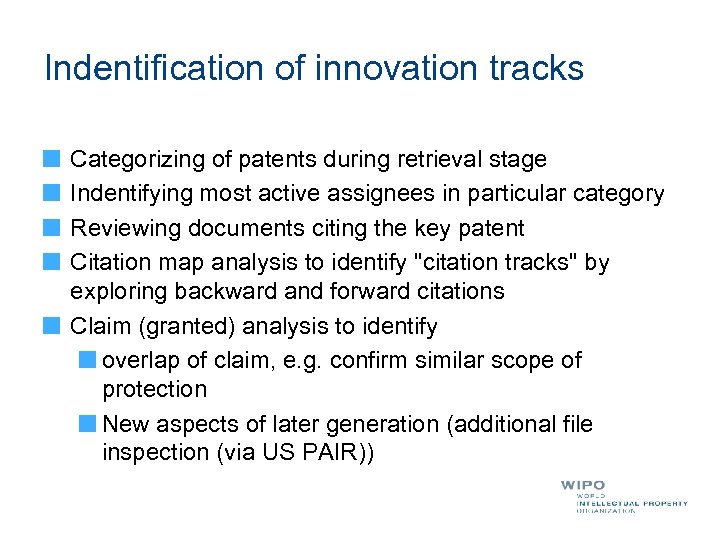 Indentification of innovation tracks Categorizing of patents during retrieval stage Indentifying most active assignees in particular category Reviewing documents citing the key patent Citation map analysis to identify "citation tracks" by exploring backward and forward citations Claim (granted) analysis to identify overlap of claim, e. g. confirm similar scope of protection New aspects of later generation (additional file inspection (via US PAIR))
Indentification of innovation tracks Categorizing of patents during retrieval stage Indentifying most active assignees in particular category Reviewing documents citing the key patent Citation map analysis to identify "citation tracks" by exploring backward and forward citations Claim (granted) analysis to identify overlap of claim, e. g. confirm similar scope of protection New aspects of later generation (additional file inspection (via US PAIR))
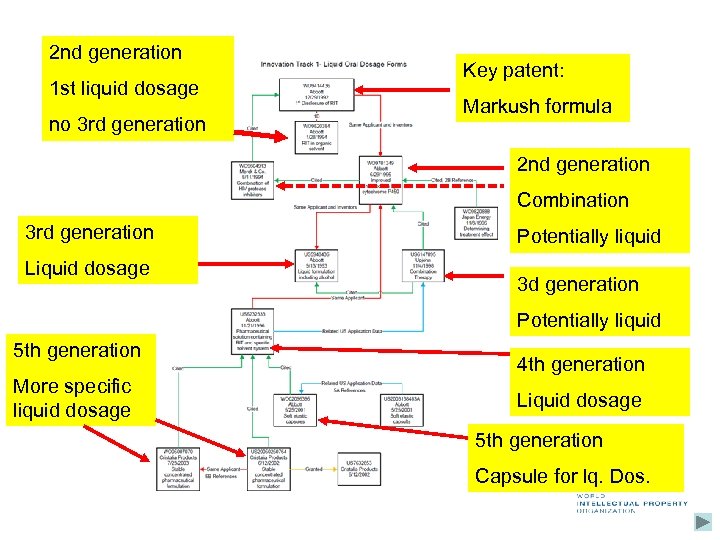 2 nd generation 1 st liquid dosage no 3 rd generation Key patent: Markush formula 2 nd generation Combination 3 rd generation Liquid dosage Potentially liquid 3 d generation Potentially liquid 5 th generation More specific liquid dosage 4 th generation Liquid dosage 5 th generation Capsule for lq. Dos.
2 nd generation 1 st liquid dosage no 3 rd generation Key patent: Markush formula 2 nd generation Combination 3 rd generation Liquid dosage Potentially liquid 3 d generation Potentially liquid 5 th generation More specific liquid dosage 4 th generation Liquid dosage 5 th generation Capsule for lq. Dos.
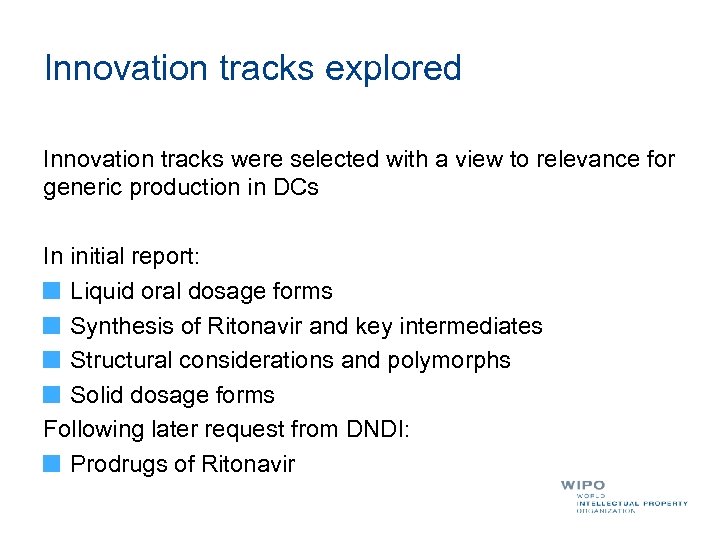 Innovation tracks explored Innovation tracks were selected with a view to relevance for generic production in DCs In initial report: Liquid oral dosage forms Synthesis of Ritonavir and key intermediates Structural considerations and polymorphs Solid dosage forms Following later request from DNDI: Prodrugs of Ritonavir
Innovation tracks explored Innovation tracks were selected with a view to relevance for generic production in DCs In initial report: Liquid oral dosage forms Synthesis of Ritonavir and key intermediates Structural considerations and polymorphs Solid dosage forms Following later request from DNDI: Prodrugs of Ritonavir
 Search methodology Report includes extensive description of name search strategies Names of pharmaceuticals change over phases of drug development E. g. "Atazanavir", the non proprietary generic name, was assigned at later clinical stage by WHO in 2001 (priority year of founder patent 1995) Various pre-clinical names Various chemical naming conventions CAS registry code CAS-198904313 -31 -3 Alternatively to Orange Book, founder family can be found by searching for SPCs in INPADOC using Atazanavir since SPC are granted at later stage when generic name is known 3 rd alternative to identify founder patent is backward citation analyis.
Search methodology Report includes extensive description of name search strategies Names of pharmaceuticals change over phases of drug development E. g. "Atazanavir", the non proprietary generic name, was assigned at later clinical stage by WHO in 2001 (priority year of founder patent 1995) Various pre-clinical names Various chemical naming conventions CAS registry code CAS-198904313 -31 -3 Alternatively to Orange Book, founder family can be found by searching for SPCs in INPADOC using Atazanavir since SPC are granted at later stage when generic name is known 3 rd alternative to identify founder patent is backward citation analyis.
 Naming history Clinical names (usually language independent): Manufacturer name: CGP-73547, CGP-73355, CGP-75136, BM-75136 Generic name (USAN naming protocol), International Nonproprietary Name (INN): Atazanavir Also used for bioequivalents Brand name (product): Rayataz
Naming history Clinical names (usually language independent): Manufacturer name: CGP-73547, CGP-73355, CGP-75136, BM-75136 Generic name (USAN naming protocol), International Nonproprietary Name (INN): Atazanavir Also used for bioequivalents Brand name (product): Rayataz
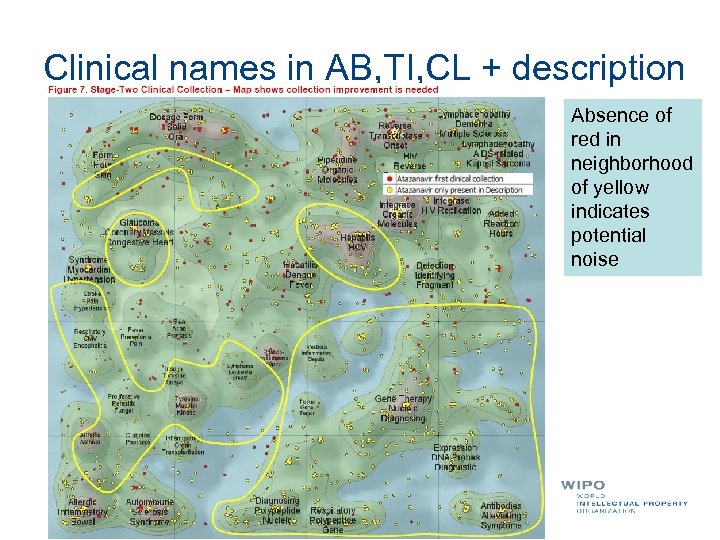 Clinical names in AB, TI, CL + description Absence of red in neighborhood of yellow indicates potential noise
Clinical names in AB, TI, CL + description Absence of red in neighborhood of yellow indicates potential noise
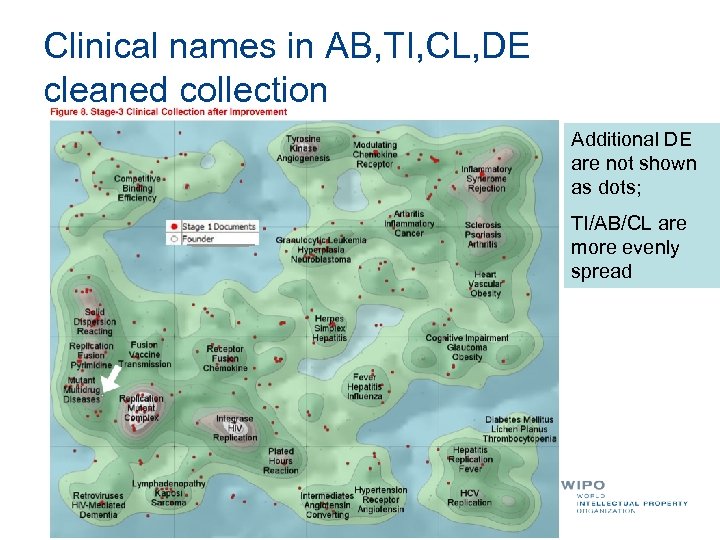 Clinical names in AB, TI, CL, DE cleaned collection Additional DE are not shown as dots; TI/AB/CL are more evenly spread
Clinical names in AB, TI, CL, DE cleaned collection Additional DE are not shown as dots; TI/AB/CL are more evenly spread
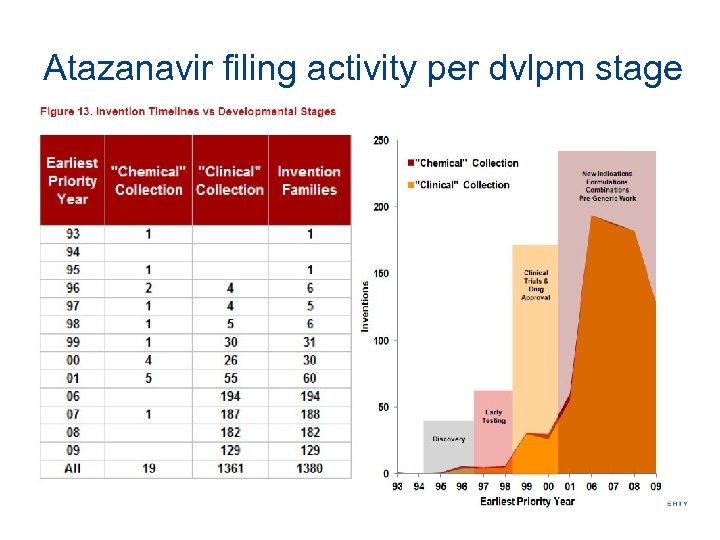 Atazanavir filing activity per dvlpm stage
Atazanavir filing activity per dvlpm stage
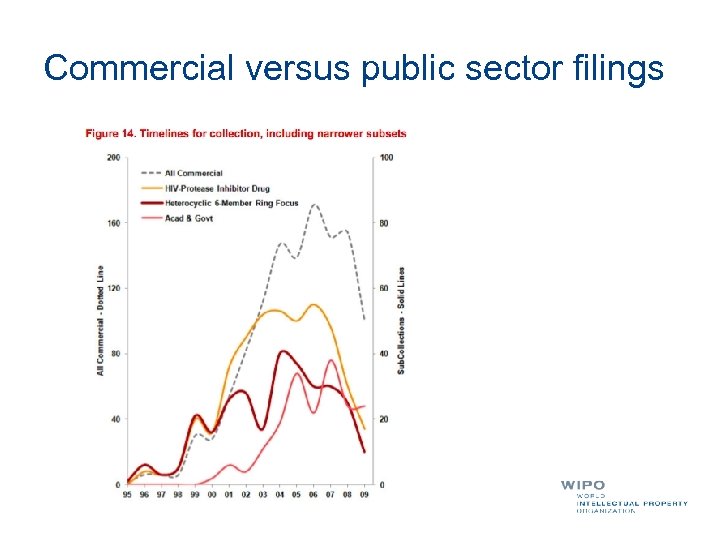 Commercial versus public sector filings
Commercial versus public sector filings
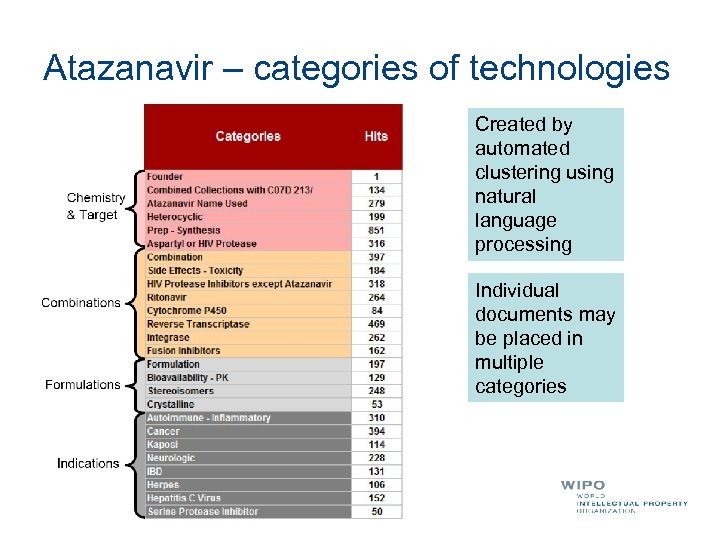 Atazanavir – categories of technologies Created by automated clustering using natural language processing Individual documents may be placed in multiple categories
Atazanavir – categories of technologies Created by automated clustering using natural language processing Individual documents may be placed in multiple categories
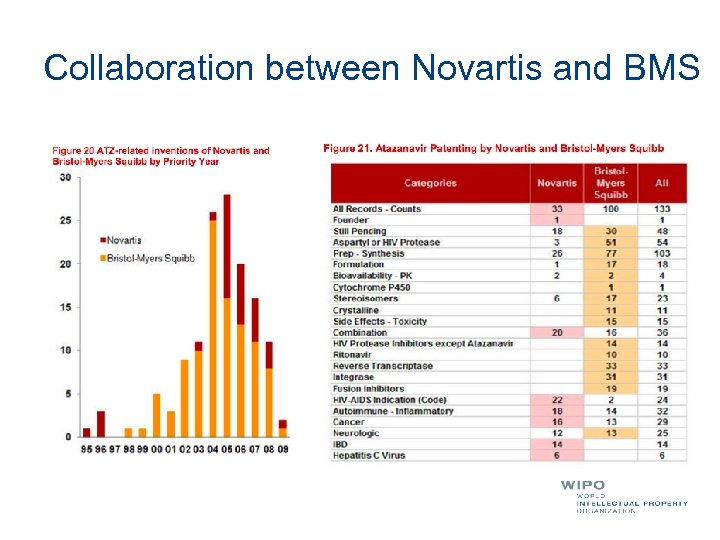 Collaboration between Novartis and BMS
Collaboration between Novartis and BMS
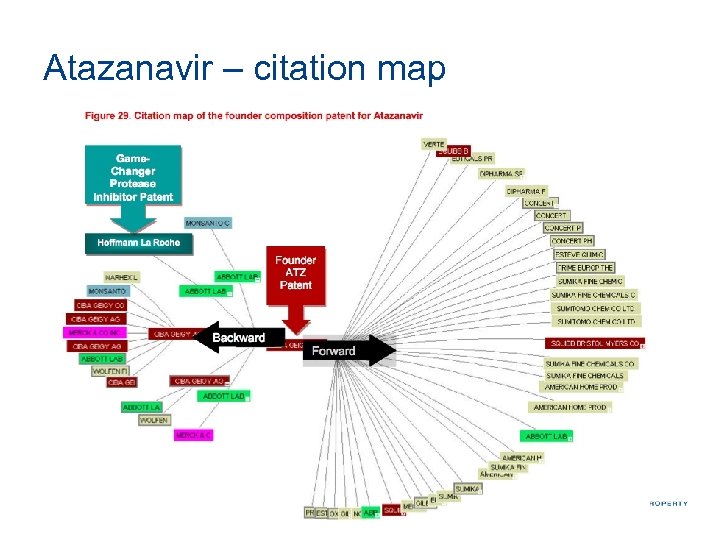 Atazanavir – citation map
Atazanavir – citation map
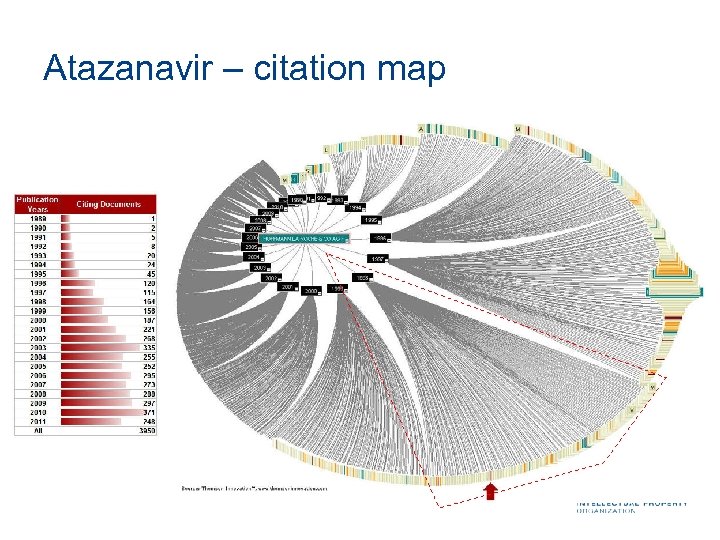 Atazanavir – citation map
Atazanavir – citation map
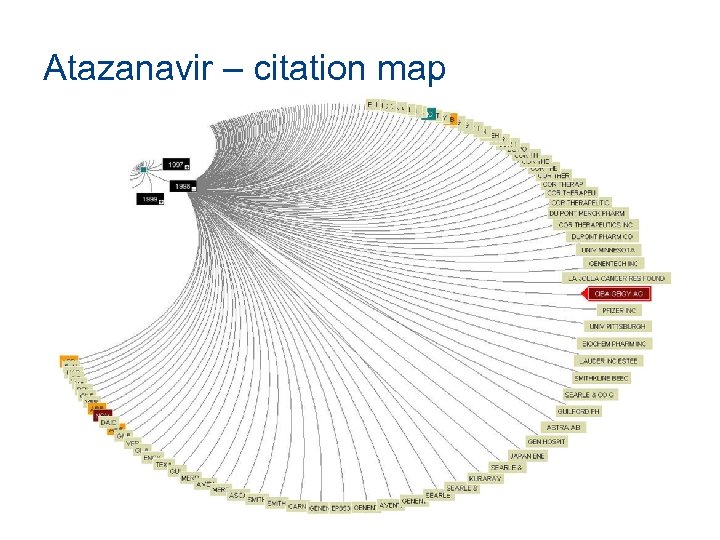 Atazanavir – citation map
Atazanavir – citation map
 PLR on vaccines Two parts of PLR Part 1: Vaccine related patents in general Part 2: Vaccines for selected diseases (Pneumonia) Special focus on Brazil, India, China
PLR on vaccines Two parts of PLR Part 1: Vaccine related patents in general Part 2: Vaccines for selected diseases (Pneumonia) Special focus on Brazil, India, China
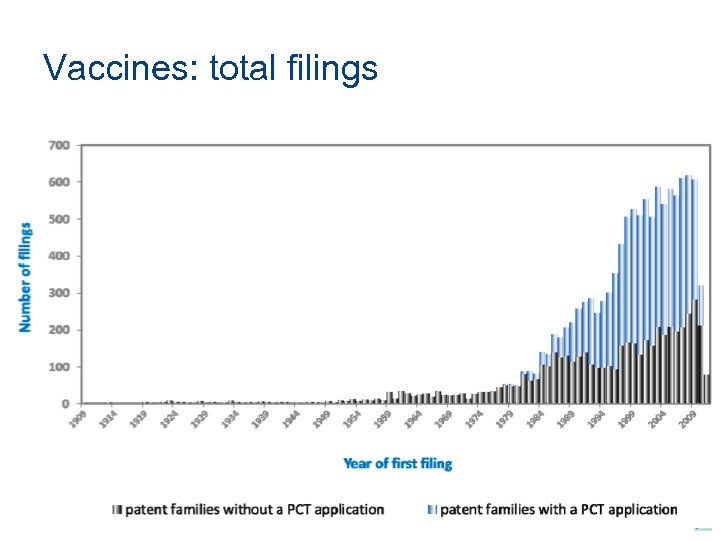 Vaccines: total filings
Vaccines: total filings
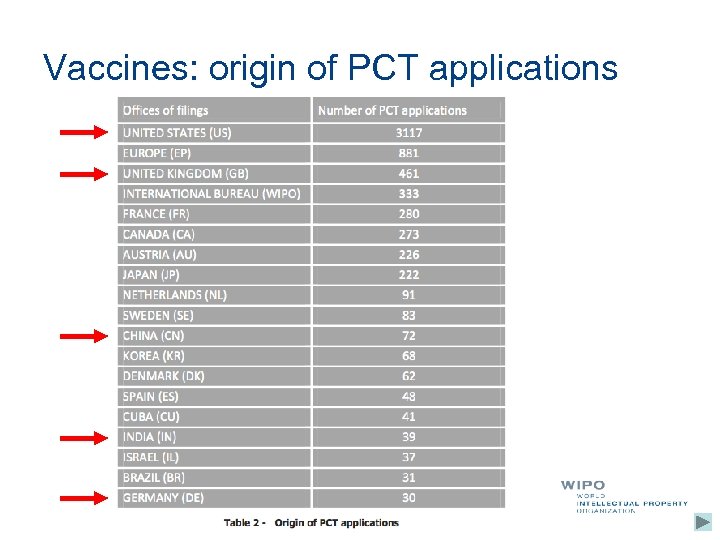 Vaccines: origin of PCT applications
Vaccines: origin of PCT applications
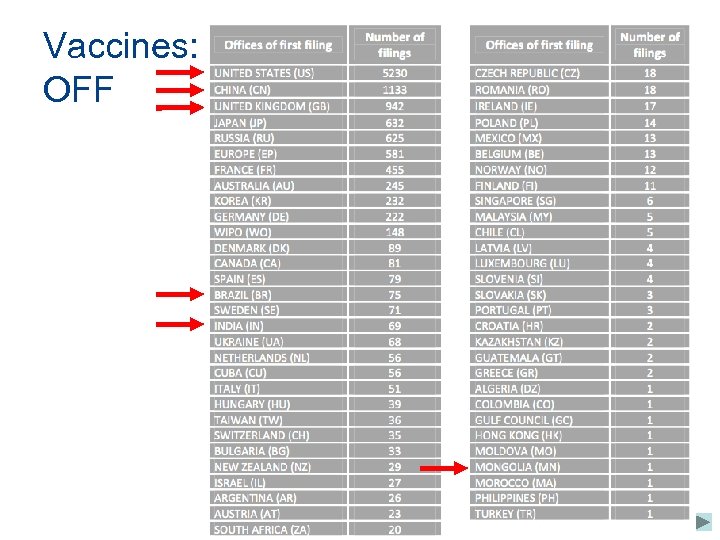 Vaccines: OFF
Vaccines: OFF
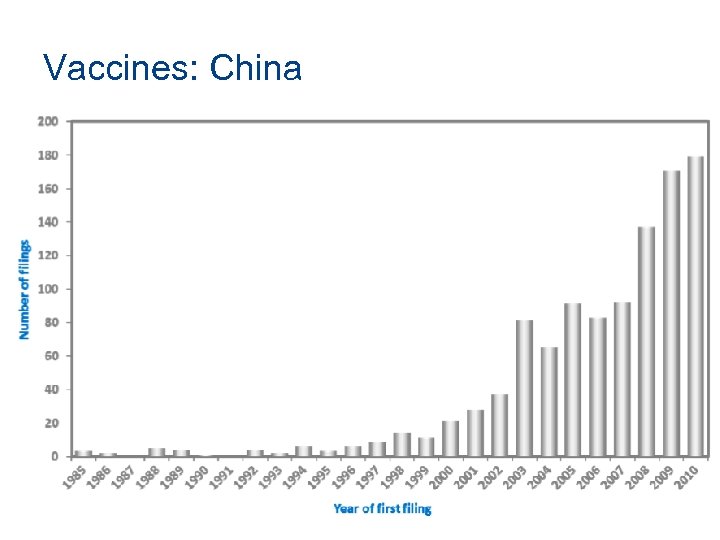 Vaccines: China
Vaccines: China
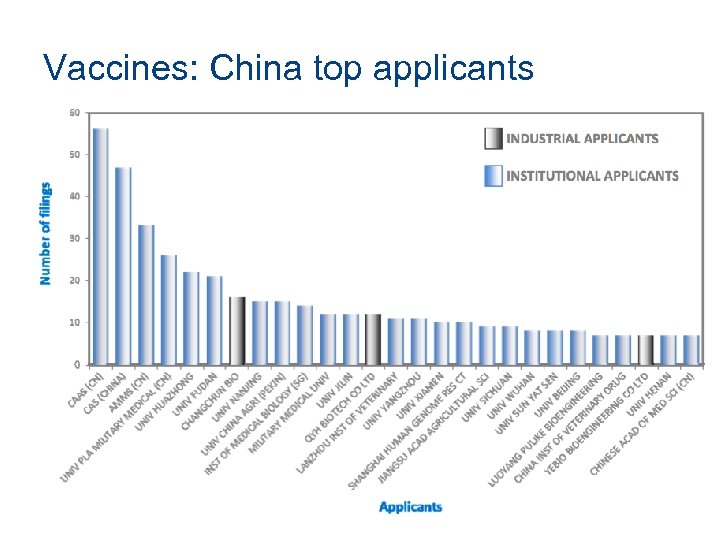 Vaccines: China top applicants
Vaccines: China top applicants
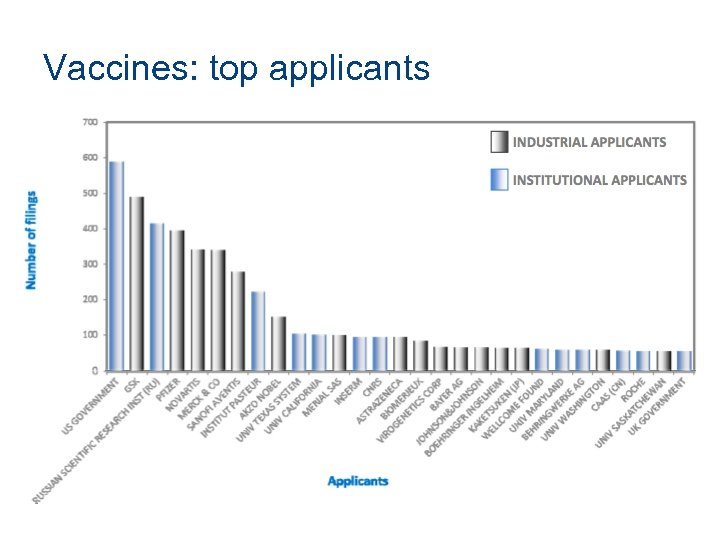 Vaccines: top applicants
Vaccines: top applicants
 Thank you lutz. mailander@wipo. int
Thank you lutz. mailander@wipo. int


