c6521f5a575c0842b06db1a1a8c76651.ppt
- Количество слайдов: 119
 Why do we need some perinatal mega -trials? William Tarnow-Mordi, Jonathan Morris NHMRC Clinical Trials Centre, Kolling Institute, Westmead International Network for Neonatal Education and Research (WINNER) Centre, NHMRC Clinical Trials Centre, University of Sydney
Why do we need some perinatal mega -trials? William Tarnow-Mordi, Jonathan Morris NHMRC Clinical Trials Centre, Kolling Institute, Westmead International Network for Neonatal Education and Research (WINNER) Centre, NHMRC Clinical Trials Centre, University of Sydney
 In every society, many babies die or survive with lifelong disabilities which could have been averted by effective treatment, before or after birth.
In every society, many babies die or survive with lifelong disabilities which could have been averted by effective treatment, before or after birth.
 " I shall give you a talisman. When faced with a dilemma as to what your next step should be, remember the most wretched and vulnerable human being you ever saw. The step you contemplate should help him ! "
" I shall give you a talisman. When faced with a dilemma as to what your next step should be, remember the most wretched and vulnerable human being you ever saw. The step you contemplate should help him ! "
 Quote by Mahatma Gandhi " What do I think of Western Civilisation? I think it would be a very good idea! "
Quote by Mahatma Gandhi " What do I think of Western Civilisation? I think it would be a very good idea! "
 • Large scale international collaboration to win healthy survival for the most disadvantaged in society is a mark of true civilisation.
• Large scale international collaboration to win healthy survival for the most disadvantaged in society is a mark of true civilisation.

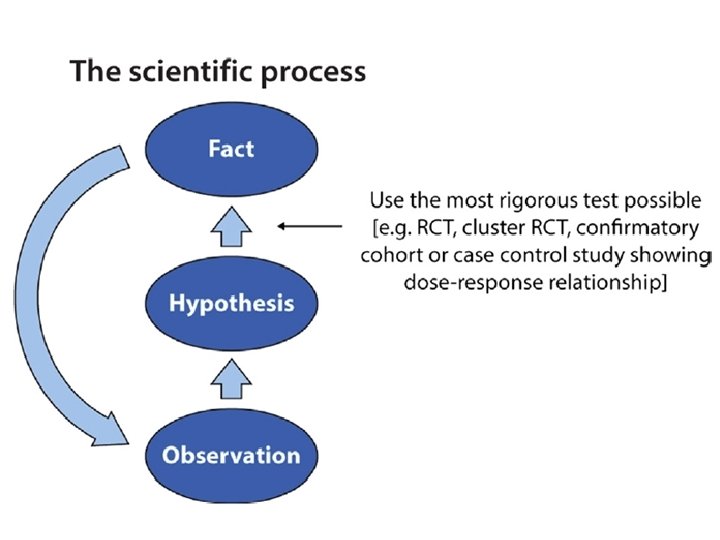
 How statistics saves lives (my first exposure to a mega-trial)
How statistics saves lives (my first exposure to a mega-trial)
 Alma Mater: Neonatal Clinical Fellow John Radcliffe Hospital 1988
Alma Mater: Neonatal Clinical Fellow John Radcliffe Hospital 1988

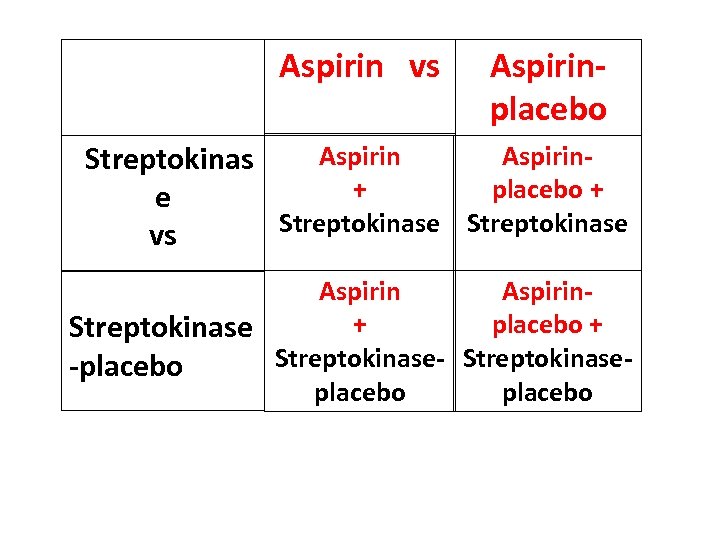 Aspirin vs Aspirinplacebo Aspirin. Streptokinas + placebo + e Streptokinase vs Aspirin+ placebo + Streptokinase-placebo
Aspirin vs Aspirinplacebo Aspirin. Streptokinas + placebo + e Streptokinase vs Aspirin+ placebo + Streptokinase-placebo
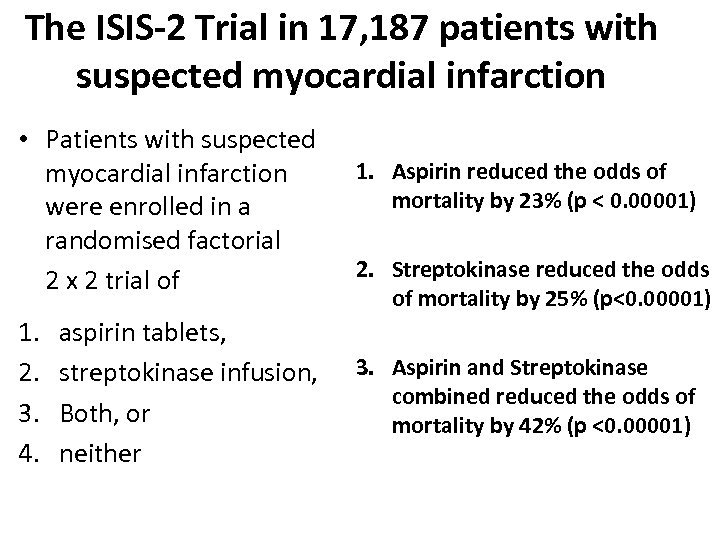
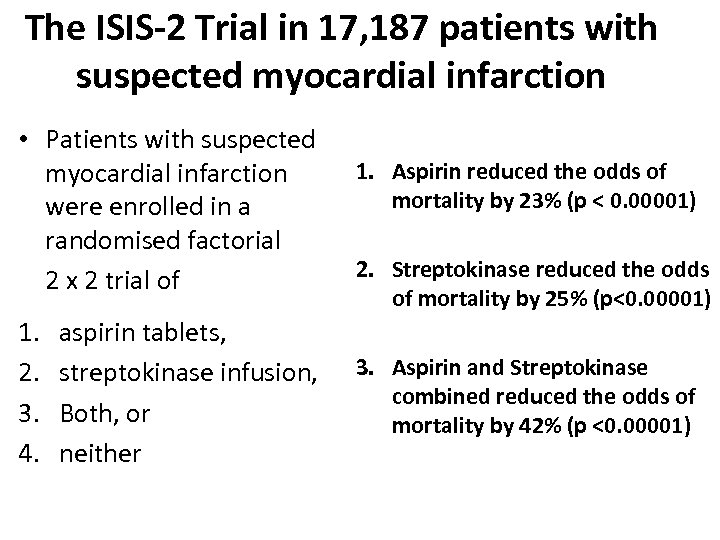 The ISIS-2 Trial in 17, 187 patients with suspected myocardial infarction • Patients with suspected myocardial infarction were enrolled in a randomised factorial 2 x 2 trial of 1. 2. 3. 4. aspirin tablets, streptokinase infusion, Both, or neither 1. Aspirin reduced the odds of mortality by 23% (p < 0. 00001) 2. Streptokinase reduced the odds of mortality by 25% (p<0. 00001) 3. Aspirin and Streptokinase combined reduced the odds of mortality by 42% (p <0. 00001)
The ISIS-2 Trial in 17, 187 patients with suspected myocardial infarction • Patients with suspected myocardial infarction were enrolled in a randomised factorial 2 x 2 trial of 1. 2. 3. 4. aspirin tablets, streptokinase infusion, Both, or neither 1. Aspirin reduced the odds of mortality by 23% (p < 0. 00001) 2. Streptokinase reduced the odds of mortality by 25% (p<0. 00001) 3. Aspirin and Streptokinase combined reduced the odds of mortality by 42% (p <0. 00001)
 ISIS-2 Randomised Factorial Trial • Within months of publication, worldwide practice had changed. • Aspirin and antifibrinolytic therapy became routinely used in suspected myocardial infarction
ISIS-2 Randomised Factorial Trial • Within months of publication, worldwide practice had changed. • Aspirin and antifibrinolytic therapy became routinely used in suspected myocardial infarction
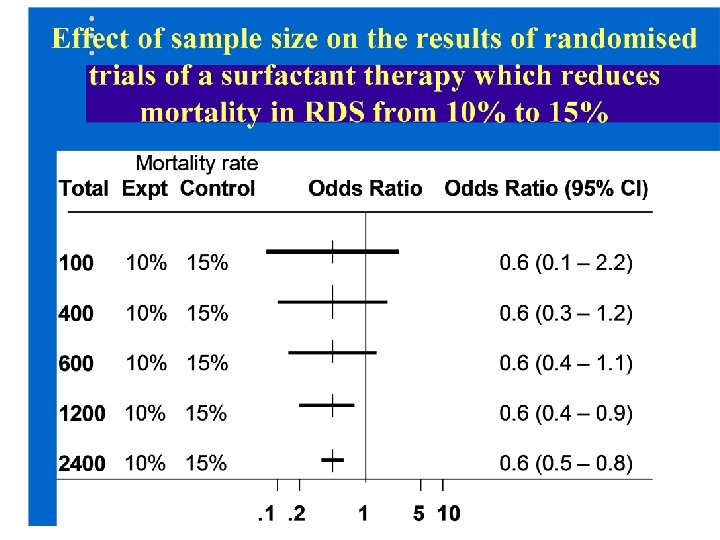
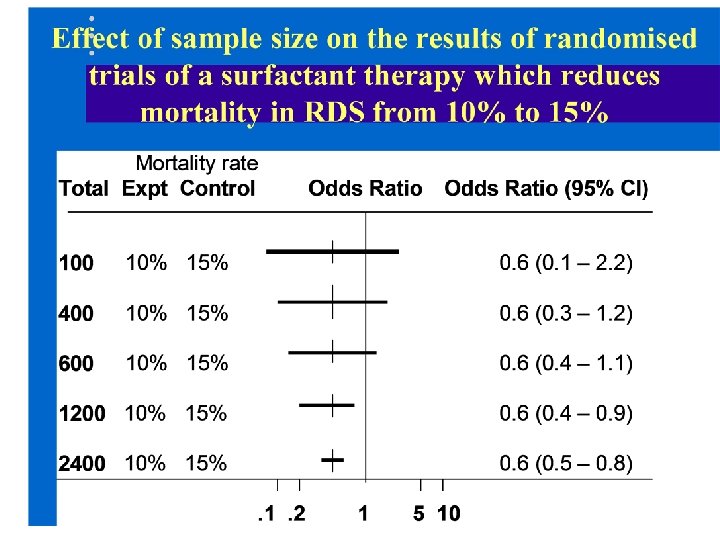
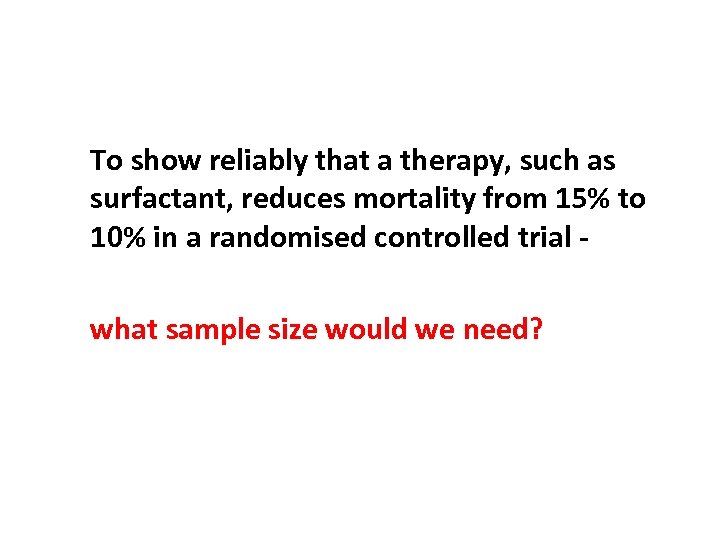 To show reliably that a therapy, such as surfactant, reduces mortality from 15% to 10% in a randomised controlled trial what sample size would we need?
To show reliably that a therapy, such as surfactant, reduces mortality from 15% to 10% in a randomised controlled trial what sample size would we need?
 Costeloe K, Hardy P, Juszczak E, et al. Lancet 2016 • 1310 infants – the largest ever single RCT of a neonatal probiotic. • concluded the sample was sufficiently large “to give clear answers” • This probiotic had no significant effect on risk ratios (RR) for – NEC (0· 93; 95% CI 0· 68– 1· 27) – sepsis (0· 97; 0· 73– 1· 29) – death (0· 93; 0· 67– 1· 30) • However, with wide confidence intervals of +/ 30%, these results are too imprecise to rule out substantial benefit or harm
Costeloe K, Hardy P, Juszczak E, et al. Lancet 2016 • 1310 infants – the largest ever single RCT of a neonatal probiotic. • concluded the sample was sufficiently large “to give clear answers” • This probiotic had no significant effect on risk ratios (RR) for – NEC (0· 93; 95% CI 0· 68– 1· 27) – sepsis (0· 97; 0· 73– 1· 29) – death (0· 93; 0· 67– 1· 30) • However, with wide confidence intervals of +/ 30%, these results are too imprecise to rule out substantial benefit or harm
 Norman J, Marlow N, et al. Lancet 2016 • Primary obstetric outcome: Fetal death or birth before 34 weeks gestation. – Adjusted Odds Ratio 0. 86 (95% CI 0. 61 – 1. 22) – But this does not rule out a 39% reduction or a 22% increase – Consistent with substantial benefit or harm
Norman J, Marlow N, et al. Lancet 2016 • Primary obstetric outcome: Fetal death or birth before 34 weeks gestation. – Adjusted Odds Ratio 0. 86 (95% CI 0. 61 – 1. 22) – But this does not rule out a 39% reduction or a 22% increase – Consistent with substantial benefit or harm
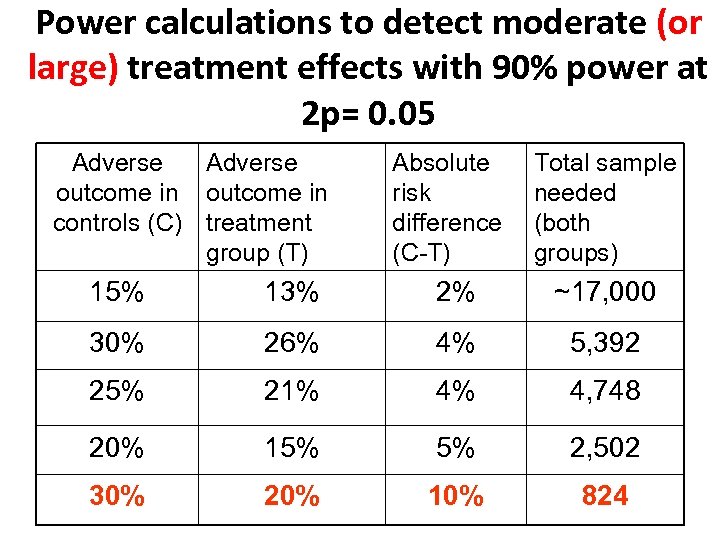 Power calculations to detect moderate (or large) treatment effects with 90% power at 2 p= 0. 05 Adverse outcome in controls (C) treatment group (T) Absolute risk difference (C-T) Total sample needed (both groups) 15% 13% 2% ~17, 000 30% 26% 4% 5, 392 25% 21% 4% 4, 748 20% 15% 5% 2, 502 30% 20% 10% 824
Power calculations to detect moderate (or large) treatment effects with 90% power at 2 p= 0. 05 Adverse outcome in controls (C) treatment group (T) Absolute risk difference (C-T) Total sample needed (both groups) 15% 13% 2% ~17, 000 30% 26% 4% 5, 392 25% 21% 4% 4, 748 20% 15% 5% 2, 502 30% 20% 10% 824
 Rationale - 1 • To show with 90% power if a treatment improves healthy survival by 4% say from 79% to 83% requires RCT evidence in over 5, 000 patients. • Few perinatal trials achieve this. • So we don’t know if most treatments for pregnant women or babies increase, decrease, or have no clinically important effect on survival or disability.
Rationale - 1 • To show with 90% power if a treatment improves healthy survival by 4% say from 79% to 83% requires RCT evidence in over 5, 000 patients. • Few perinatal trials achieve this. • So we don’t know if most treatments for pregnant women or babies increase, decrease, or have no clinically important effect on survival or disability.
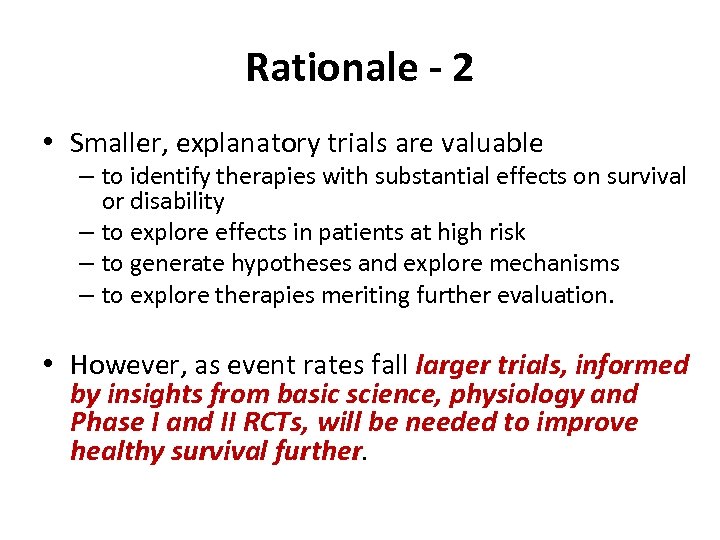 Rationale - 2 • Smaller, explanatory trials are valuable – to identify therapies with substantial effects on survival or disability – to explore effects in patients at high risk – to generate hypotheses and explore mechanisms – to explore therapies meriting further evaluation. • However, as event rates fall larger trials, informed by insights from basic science, physiology and Phase I and II RCTs, will be needed to improve healthy survival further.
Rationale - 2 • Smaller, explanatory trials are valuable – to identify therapies with substantial effects on survival or disability – to explore effects in patients at high risk – to generate hypotheses and explore mechanisms – to explore therapies meriting further evaluation. • However, as event rates fall larger trials, informed by insights from basic science, physiology and Phase I and II RCTs, will be needed to improve healthy survival further.
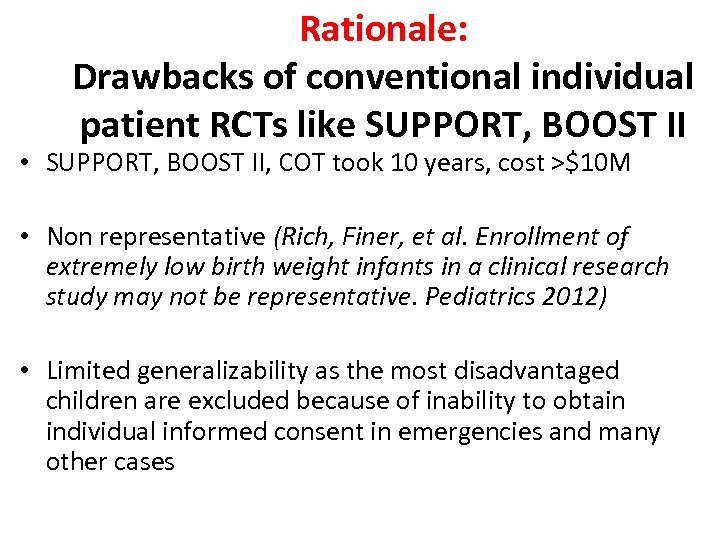 Rationale: Drawbacks of conventional individual patient RCTs like SUPPORT, BOOST II • SUPPORT, BOOST II, COT took 10 years, cost >$10 M • Non representative (Rich, Finer, et al. Enrollment of extremely low birth weight infants in a clinical research study may not be representative. Pediatrics 2012) • Limited generalizability as the most disadvantaged children are excluded because of inability to obtain individual informed consent in emergencies and many other cases
Rationale: Drawbacks of conventional individual patient RCTs like SUPPORT, BOOST II • SUPPORT, BOOST II, COT took 10 years, cost >$10 M • Non representative (Rich, Finer, et al. Enrollment of extremely low birth weight infants in a clinical research study may not be representative. Pediatrics 2012) • Limited generalizability as the most disadvantaged children are excluded because of inability to obtain individual informed consent in emergencies and many other cases
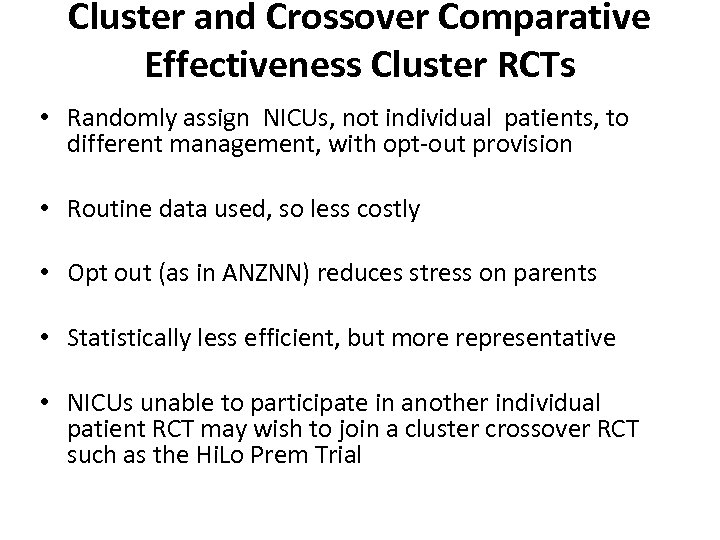 Cluster and Crossover Comparative Effectiveness Cluster RCTs • Randomly assign NICUs, not individual patients, to different management, with opt out provision • Routine data used, so less costly • Opt out (as in ANZNN) reduces stress on parents • Statistically less efficient, but more representative • NICUs unable to participate in another individual patient RCT may wish to join a cluster crossover RCT such as the Hi. Lo Prem Trial
Cluster and Crossover Comparative Effectiveness Cluster RCTs • Randomly assign NICUs, not individual patients, to different management, with opt out provision • Routine data used, so less costly • Opt out (as in ANZNN) reduces stress on parents • Statistically less efficient, but more representative • NICUs unable to participate in another individual patient RCT may wish to join a cluster crossover RCT such as the Hi. Lo Prem Trial
 The Hi-Lo Prem Trial www. hiloprem. org SHOULD WE USE (A) LOWER OR (B) HIGHER OXYGENATION TO RESUSCITATE PRETERM INFANTS. FIRST EVER PERINATAL CLUSTER CROSSOVER (CXO) MEGA TRIAL, TO RUN IN >11, 000 AUSTRALIAN, NEW ZEALAND, SE ASIAN, AMERICAN AND EUROPEAN NEONATAL UNITS, ALONGSIDE THE INDIVIDUAL PATIENT NHMRC TORPIDO 2
The Hi-Lo Prem Trial www. hiloprem. org SHOULD WE USE (A) LOWER OR (B) HIGHER OXYGENATION TO RESUSCITATE PRETERM INFANTS. FIRST EVER PERINATAL CLUSTER CROSSOVER (CXO) MEGA TRIAL, TO RUN IN >11, 000 AUSTRALIAN, NEW ZEALAND, SE ASIAN, AMERICAN AND EUROPEAN NEONATAL UNITS, ALONGSIDE THE INDIVIDUAL PATIENT NHMRC TORPIDO 2
 Team Capability The Hi-Lo Prem Trial www. hiloprem. org NEIL FINER, ROGER SOLL, PRAKESH SHAH, JU LEE OEI, KEI LUI, OLA SAUGSTAD, MAX VENTO, GEORG SCHMOELZER, ANNIE JANVIER, JOHN LANTOS, WILLIAM TARNOW MORDI FOR VERMONT OXFORD NETWORK, SHARP MARY BIRCH NEONATAL RESEARCH GROUP, NHMRC CLINICAL TRIALS CENTRE, TORPIDO 2 AND US PRESOX STUDY GROUPS AND HOLISTIC
Team Capability The Hi-Lo Prem Trial www. hiloprem. org NEIL FINER, ROGER SOLL, PRAKESH SHAH, JU LEE OEI, KEI LUI, OLA SAUGSTAD, MAX VENTO, GEORG SCHMOELZER, ANNIE JANVIER, JOHN LANTOS, WILLIAM TARNOW MORDI FOR VERMONT OXFORD NETWORK, SHARP MARY BIRCH NEONATAL RESEARCH GROUP, NHMRC CLINICAL TRIALS CENTRE, TORPIDO 2 AND US PRESOX STUDY GROUPS AND HOLISTIC
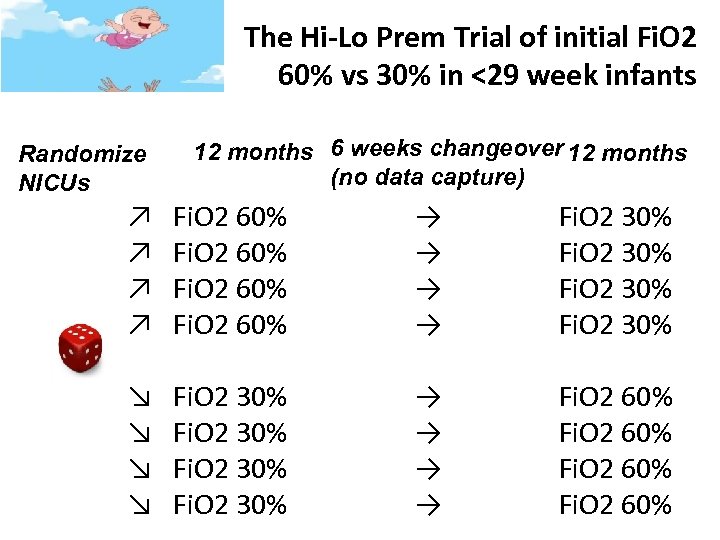 The Hi-Lo Prem Trial of initial Fi. O 2 60% vs 30% in <29 week infants Randomize NICUs 12 months 6 weeks changeover 12 months (no data capture) ↗ Fi. O 2 60% → → Fi. O 2 30% ↘ Fi. O 2 30% → → Fi. O 2 60%
The Hi-Lo Prem Trial of initial Fi. O 2 60% vs 30% in <29 week infants Randomize NICUs 12 months 6 weeks changeover 12 months (no data capture) ↗ Fi. O 2 60% → → Fi. O 2 30% ↘ Fi. O 2 30% → → Fi. O 2 60%
 ALPHA Collaboration for perinatal mega-trials Advancing Large Perinatal Trials in Health Outcomes Assessment The ALPHA Collaboration will work closely with colleagues, existing organisations such as IMPACT, Go. NET, PSANZ, ANZNN, Vermont Oxford Network, Canadian Neonatal Network, UK and other networks worldwide to promote perinatal trials that enrol 5, 000 -50, 000 patients or more, making them more efficient ,
ALPHA Collaboration for perinatal mega-trials Advancing Large Perinatal Trials in Health Outcomes Assessment The ALPHA Collaboration will work closely with colleagues, existing organisations such as IMPACT, Go. NET, PSANZ, ANZNN, Vermont Oxford Network, Canadian Neonatal Network, UK and other networks worldwide to promote perinatal trials that enrol 5, 000 -50, 000 patients or more, making them more efficient ,
 Agree among stakeholders • Seek substantial philanthropic support through Sydney University • Agree a process for selecting and developing perinatal mega trials through prioritization and peer review • and submission for competitive funding, • in collaboration with the PSANZ/ IMPACT Network for Improving Outcomes for Mothers and Babies and other national and international professional associations.
Agree among stakeholders • Seek substantial philanthropic support through Sydney University • Agree a process for selecting and developing perinatal mega trials through prioritization and peer review • and submission for competitive funding, • in collaboration with the PSANZ/ IMPACT Network for Improving Outcomes for Mothers and Babies and other national and international professional associations.
 Mission The ALPHA Collaboration will work closely with colleagues, existing organisations and networks worldwide to promote perinatal trials that enrol 5, 00020, 000 patients or more, making them more efficient , innovative and affordable, thus transforming perinatal care.
Mission The ALPHA Collaboration will work closely with colleagues, existing organisations and networks worldwide to promote perinatal trials that enrol 5, 00020, 000 patients or more, making them more efficient , innovative and affordable, thus transforming perinatal care.
 Alma Mater: Neonatal Clinical Fellow John Radcliffe Hospital 1988
Alma Mater: Neonatal Clinical Fellow John Radcliffe Hospital 1988

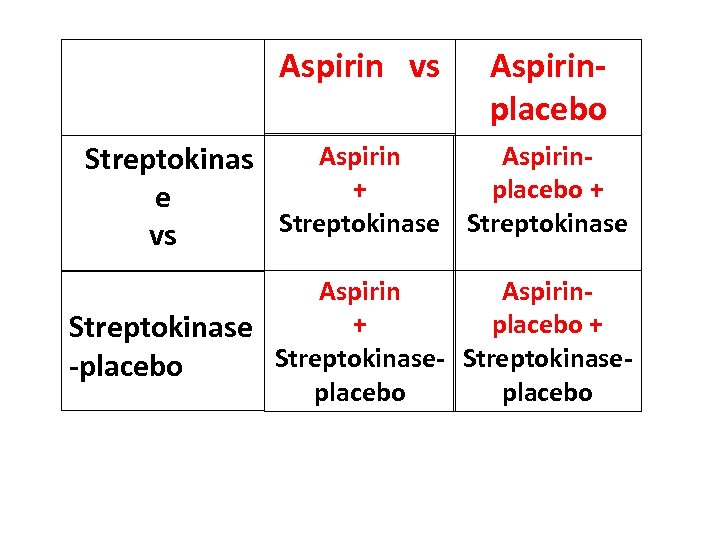 Aspirin vs Aspirinplacebo Aspirin. Streptokinas + placebo + e Streptokinase vs Aspirin+ placebo + Streptokinase-placebo
Aspirin vs Aspirinplacebo Aspirin. Streptokinas + placebo + e Streptokinase vs Aspirin+ placebo + Streptokinase-placebo
 2 x 2 Randomised Factorial Design Three questions for the price of one
2 x 2 Randomised Factorial Design Three questions for the price of one
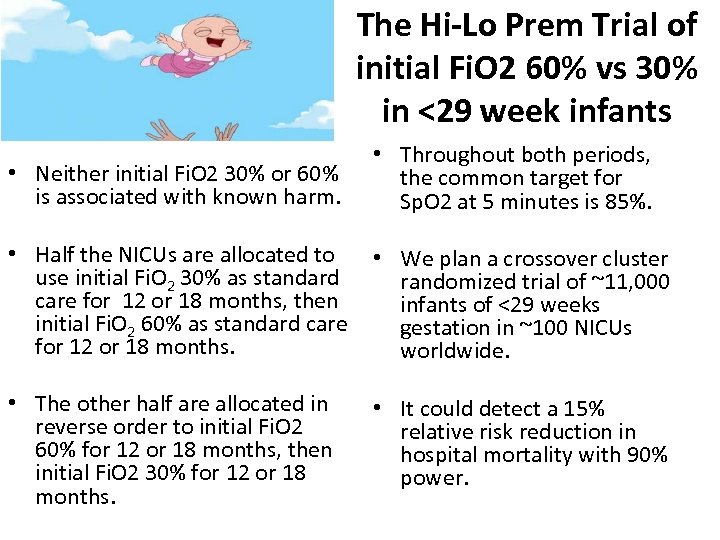 The Hi-Lo Prem Trial of initial Fi. O 2 60% vs 30% in <29 week infants • Neither initial Fi. O 2 30% or 60% is associated with known harm. • Throughout both periods, the common target for Sp. O 2 at 5 minutes is 85%. • Half the NICUs are allocated to • We plan a crossover cluster use initial Fi. O 2 30% as standard randomized trial of ~11, 000 care for 12 or 18 months, then infants of <29 weeks initial Fi. O 2 60% as standard care gestation in ~100 NICUs for 12 or 18 months. worldwide. • The other half are allocated in reverse order to initial Fi. O 2 60% for 12 or 18 months, then initial Fi. O 2 30% for 12 or 18 months. • It could detect a 15% relative risk reduction in hospital mortality with 90% power.
The Hi-Lo Prem Trial of initial Fi. O 2 60% vs 30% in <29 week infants • Neither initial Fi. O 2 30% or 60% is associated with known harm. • Throughout both periods, the common target for Sp. O 2 at 5 minutes is 85%. • Half the NICUs are allocated to • We plan a crossover cluster use initial Fi. O 2 30% as standard randomized trial of ~11, 000 care for 12 or 18 months, then infants of <29 weeks initial Fi. O 2 60% as standard care gestation in ~100 NICUs for 12 or 18 months. worldwide. • The other half are allocated in reverse order to initial Fi. O 2 60% for 12 or 18 months, then initial Fi. O 2 30% for 12 or 18 months. • It could detect a 15% relative risk reduction in hospital mortality with 90% power.
 The ISIS-2 Trial in 17, 187 patients with suspected myocardial infarction • Patients with suspected myocardial infarction were enrolled in a randomised factorial 2 x 2 trial of 1. 2. 3. 4. aspirin tablets, streptokinase infusion, Both, or neither 1. Aspirin reduced the odds of mortality by 23% (p < 0. 00001) 2. Streptokinase reduced the odds of mortality by 25% (p<0. 00001) 3. Aspirin and Streptokinase combined reduced the odds of mortality by 42% (p <0. 00001)
The ISIS-2 Trial in 17, 187 patients with suspected myocardial infarction • Patients with suspected myocardial infarction were enrolled in a randomised factorial 2 x 2 trial of 1. 2. 3. 4. aspirin tablets, streptokinase infusion, Both, or neither 1. Aspirin reduced the odds of mortality by 23% (p < 0. 00001) 2. Streptokinase reduced the odds of mortality by 25% (p<0. 00001) 3. Aspirin and Streptokinase combined reduced the odds of mortality by 42% (p <0. 00001)
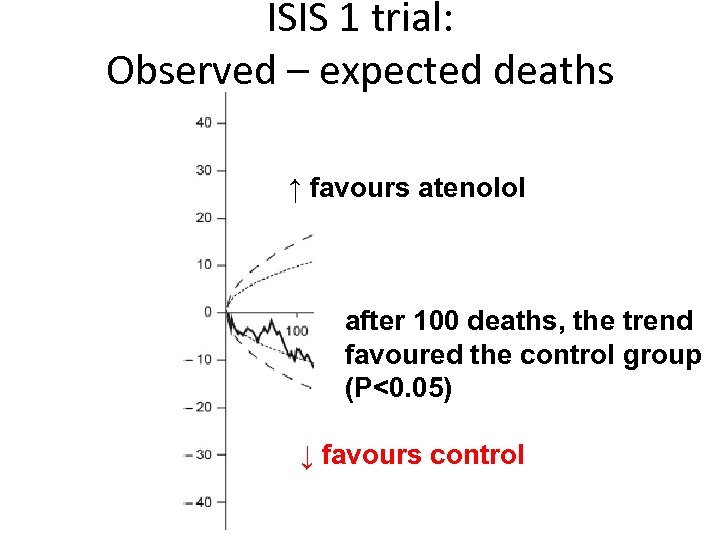 ISIS 1 trial: Observed – expected deaths ↑ favours atenolol after 100 deaths, the trend favoured the control group (P<0. 05) ↓ favours control
ISIS 1 trial: Observed – expected deaths ↑ favours atenolol after 100 deaths, the trend favoured the control group (P<0. 05) ↓ favours control
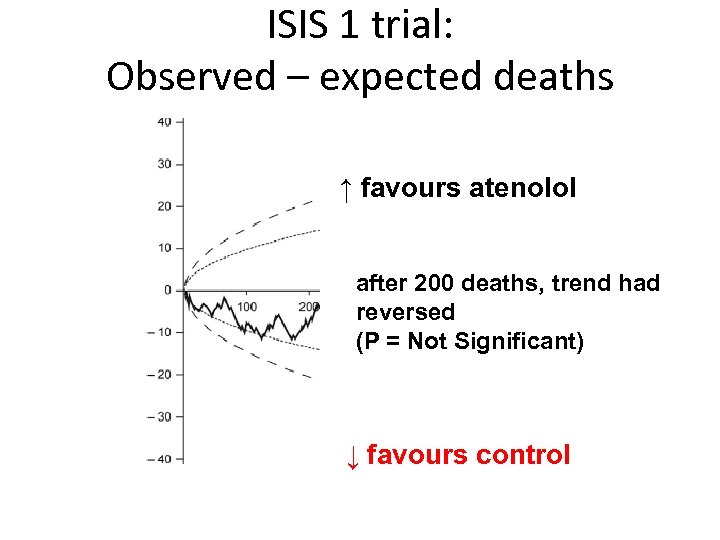 ISIS 1 trial: Observed – expected deaths ↑ favours atenolol after 200 deaths, trend had reversed (P = Not Significant) ↓ favours control
ISIS 1 trial: Observed – expected deaths ↑ favours atenolol after 200 deaths, trend had reversed (P = Not Significant) ↓ favours control
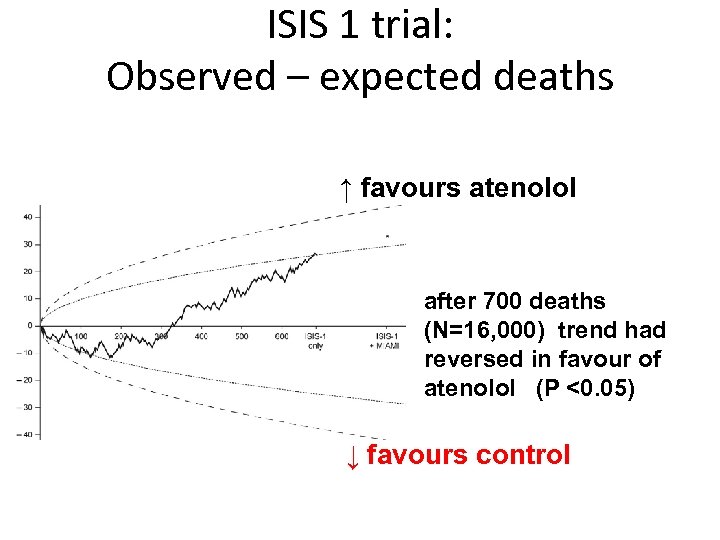 ISIS 1 trial: Observed – expected deaths ↑ favours atenolol after 700 deaths (N=16, 000) trend had reversed in favour of atenolol (P <0. 05) ↓ favours control
ISIS 1 trial: Observed – expected deaths ↑ favours atenolol after 700 deaths (N=16, 000) trend had reversed in favour of atenolol (P <0. 05) ↓ favours control

 Six reasons for large, simple trials
Six reasons for large, simple trials
 Why do we need some large, simple trials? 1. Common conditions are important: studies of common conditions can be large. 2. Treatments are likely to have more impact if they are simple and widely practicable, than if they are complex and restricted to specialist centres.
Why do we need some large, simple trials? 1. Common conditions are important: studies of common conditions can be large. 2. Treatments are likely to have more impact if they are simple and widely practicable, than if they are complex and restricted to specialist centres.
 Why do we need some large, simple trials? 3. Major outcomes, like death, are more important than intermediate outcomes, like CRP, PCR, sepsis. Outcomes like death can be simply and very accurately measured. 4. Detailed stratification by prognostic criteria add little power, so entry criteria can be simple. 5. The direction of any treatment effect is likely to be similar across subcategories of patients, so entry criteria can again be simple.
Why do we need some large, simple trials? 3. Major outcomes, like death, are more important than intermediate outcomes, like CRP, PCR, sepsis. Outcomes like death can be simply and very accurately measured. 4. Detailed stratification by prognostic criteria add little power, so entry criteria can be simple. 5. The direction of any treatment effect is likely to be similar across subcategories of patients, so entry criteria can again be simple.
 Why do we need some large, simple trials? 6. For a question to be important it must not yet have been answered reliably. If a widely practicable treatment had a big effect on a major outcome (like mortality) in a common disease it would probably already be known, so the true effect is likely to be either null or, at most, moderate, not large.
Why do we need some large, simple trials? 6. For a question to be important it must not yet have been answered reliably. If a widely practicable treatment had a big effect on a major outcome (like mortality) in a common disease it would probably already be known, so the true effect is likely to be either null or, at most, moderate, not large.
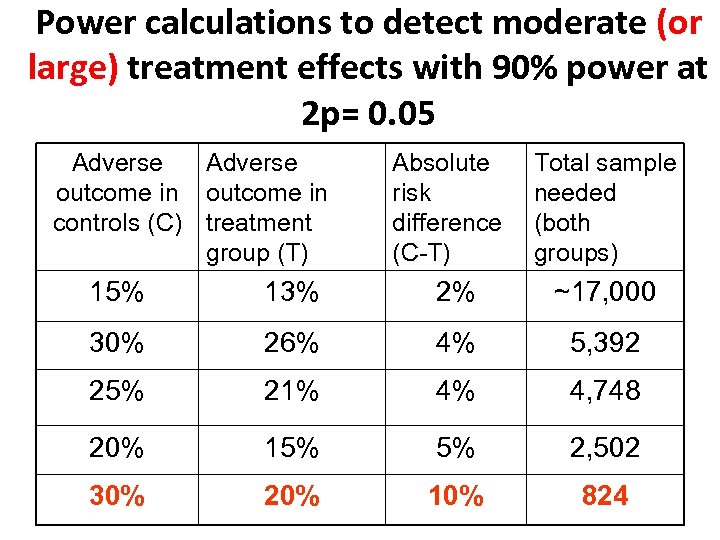 Power calculations to detect moderate (or large) treatment effects with 90% power at 2 p= 0. 05 Adverse outcome in controls (C) treatment group (T) Absolute risk difference (C-T) Total sample needed (both groups) 15% 13% 2% ~17, 000 30% 26% 4% 5, 392 25% 21% 4% 4, 748 20% 15% 5% 2, 502 30% 20% 10% 824
Power calculations to detect moderate (or large) treatment effects with 90% power at 2 p= 0. 05 Adverse outcome in controls (C) treatment group (T) Absolute risk difference (C-T) Total sample needed (both groups) 15% 13% 2% ~17, 000 30% 26% 4% 5, 392 25% 21% 4% 4, 748 20% 15% 5% 2, 502 30% 20% 10% 824
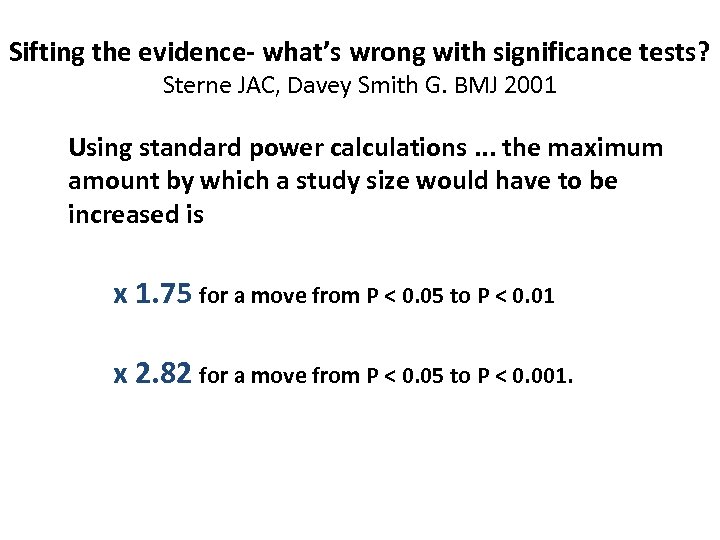 Sifting the evidence- what’s wrong with significance tests? Sterne JAC, Davey Smith G. BMJ 2001 Using standard power calculations. . . the maximum amount by which a study size would have to be increased is x 1. 75 for a move from P < 0. 05 to P < 0. 01 x 2. 82 for a move from P < 0. 05 to P < 0. 001.
Sifting the evidence- what’s wrong with significance tests? Sterne JAC, Davey Smith G. BMJ 2001 Using standard power calculations. . . the maximum amount by which a study size would have to be increased is x 1. 75 for a move from P < 0. 05 to P < 0. 01 x 2. 82 for a move from P < 0. 05 to P < 0. 001.

 The design of prospective epidemiological studies: more subjects or better measurements? Phillips AN, Davey Smith G. J Clin Epidemiol. 1993; 46: 1203 11 “It is also possible, and generally preferable, to increase power by decreasing measurement error rather than by increasing sample size. ” Simple outcomes, like death, can be measured very accurately.
The design of prospective epidemiological studies: more subjects or better measurements? Phillips AN, Davey Smith G. J Clin Epidemiol. 1993; 46: 1203 11 “It is also possible, and generally preferable, to increase power by decreasing measurement error rather than by increasing sample size. ” Simple outcomes, like death, can be measured very accurately.
 Need for international collaboration
Need for international collaboration
 • Few neonatal RCTs recruit >2, 000 patients. • The impact of most neonatal therapies on mortality or major morbidity is unknown.
• Few neonatal RCTs recruit >2, 000 patients. • The impact of most neonatal therapies on mortality or major morbidity is unknown.
 INIS: International Neonatal Immunotherapy Study RCT of IVIG in suspected or proven sepsis • INIS has recruited 3, 493 newborns with suspected or proven sepsis in over 100 NICUs worldwide. • It is the largest ever individual patient RCT of adjunctive therapy in sepsis
INIS: International Neonatal Immunotherapy Study RCT of IVIG in suspected or proven sepsis • INIS has recruited 3, 493 newborns with suspected or proven sepsis in over 100 NICUs worldwide. • It is the largest ever individual patient RCT of adjunctive therapy in sepsis
 BOOST II: Second Benefits of Oxygen Saturation Targeting Trial • BOOST II has recruited over 1, 350 infants < 28 weeks gestation in Australia and New Zealand • It is part of the Neo. PROM collaboration of 5 similar RCTs worldwide, with a total of > 4, 250 infants • Neo. PROM is the first Prospective Meta Analysis of RCTs in neonatology
BOOST II: Second Benefits of Oxygen Saturation Targeting Trial • BOOST II has recruited over 1, 350 infants < 28 weeks gestation in Australia and New Zealand • It is part of the Neo. PROM collaboration of 5 similar RCTs worldwide, with a total of > 4, 250 infants • Neo. PROM is the first Prospective Meta Analysis of RCTs in neonatology
 The SUPPORT Oxygen Targeting Trial in infants < 28 weeks • High target 91 – 95% Sp. O 2 was associated with double the rate of severe retinopathy (p<0. 001). • Low target was associated with 15% increase in mortality (p = 0. 04). • Further studies are needed before firm recommendation for policy.
The SUPPORT Oxygen Targeting Trial in infants < 28 weeks • High target 91 – 95% Sp. O 2 was associated with double the rate of severe retinopathy (p<0. 001). • Low target was associated with 15% increase in mortality (p = 0. 04). • Further studies are needed before firm recommendation for policy.
 APTS: Australian Placental Tranfusion Study • This RCT will test if delayed cord clamping reduces mortality or major morbidity in 1440 infants < 30 weeks gestation. • Bill and Melinda Gates Foundation are considering funding a similar RCT led by Dr Jorge Toloda in < 32 weeks gestation in low income countries.
APTS: Australian Placental Tranfusion Study • This RCT will test if delayed cord clamping reduces mortality or major morbidity in 1440 infants < 30 weeks gestation. • Bill and Melinda Gates Foundation are considering funding a similar RCT led by Dr Jorge Toloda in < 32 weeks gestation in low income countries.
 How can we achieve more evidence, more rapidly and reliably in neonatal medicine? • Large simple individual patient RCTs using simple, important outcomes like mortality or major morbidity • Randomised factorial trials, e. g. 2 x 2 or 3 x 2, to answer more than one question simultaneously • Cluster randomised RCTs, where the unit randomised is the NICU, clinic or village, not the patient
How can we achieve more evidence, more rapidly and reliably in neonatal medicine? • Large simple individual patient RCTs using simple, important outcomes like mortality or major morbidity • Randomised factorial trials, e. g. 2 x 2 or 3 x 2, to answer more than one question simultaneously • Cluster randomised RCTs, where the unit randomised is the NICU, clinic or village, not the patient
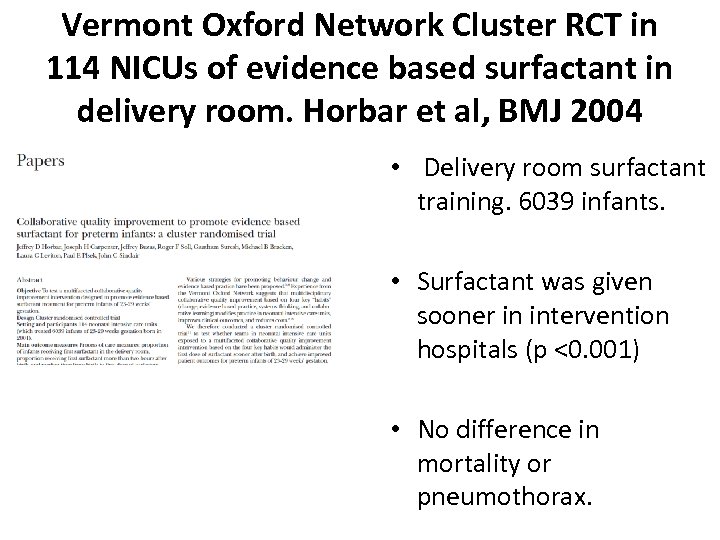 Vermont Oxford Network Cluster RCT in 114 NICUs of evidence based surfactant in delivery room. Horbar et al, BMJ 2004 • Delivery room surfactant training. 6039 infants. • Surfactant was given sooner in intervention hospitals (p <0. 001) • No difference in mortality or pneumothorax.
Vermont Oxford Network Cluster RCT in 114 NICUs of evidence based surfactant in delivery room. Horbar et al, BMJ 2004 • Delivery room surfactant training. 6039 infants. • Surfactant was given sooner in intervention hospitals (p <0. 001) • No difference in mortality or pneumothorax.
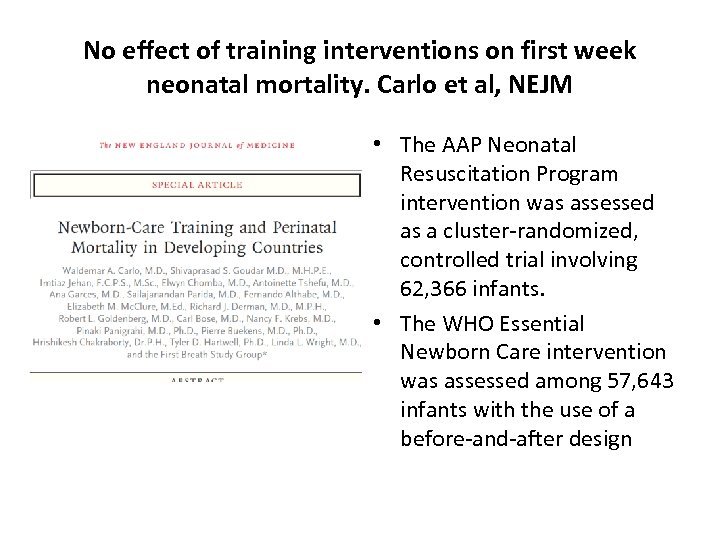 No effect of training interventions on first week neonatal mortality. Carlo et al, NEJM • The AAP Neonatal Resuscitation Program intervention was assessed as a cluster randomized, controlled trial involving 62, 366 infants. • The WHO Essential Newborn Care intervention was assessed among 57, 643 infants with the use of a before and after design
No effect of training interventions on first week neonatal mortality. Carlo et al, NEJM • The AAP Neonatal Resuscitation Program intervention was assessed as a cluster randomized, controlled trial involving 62, 366 infants. • The WHO Essential Newborn Care intervention was assessed among 57, 643 infants with the use of a before and after design
 Evidence for the impact of quality improvement collaboratives: systematic review. Schouten et al, BMJ, 2010 Online First • The evidence for collaborative interventions for quality improvement is positive but limited and unpredictable. • Cost effectiveness studies are required
Evidence for the impact of quality improvement collaboratives: systematic review. Schouten et al, BMJ, 2010 Online First • The evidence for collaborative interventions for quality improvement is positive but limited and unpredictable. • Cost effectiveness studies are required
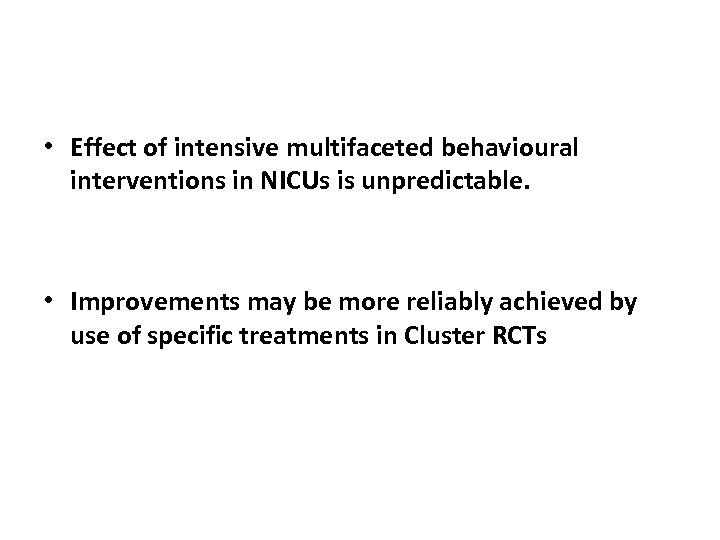 • Effect of intensive multifaceted behavioural interventions in NICUs is unpredictable. • Improvements may be more reliably achieved by use of specific treatments in Cluster RCTs
• Effect of intensive multifaceted behavioural interventions in NICUs is unpredictable. • Improvements may be more reliably achieved by use of specific treatments in Cluster RCTs
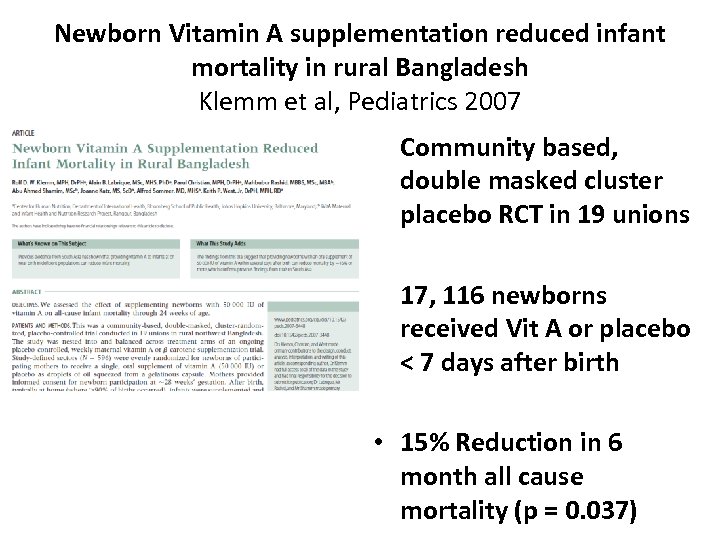 Newborn Vitamin A supplementation reduced infant mortality in rural Bangladesh Klemm et al, Pediatrics 2007 • Community based, double masked cluster placebo RCT in 19 unions • 17, 116 newborns received Vit A or placebo < 7 days after birth • 15% Reduction in 6 month all cause mortality (p = 0. 037)
Newborn Vitamin A supplementation reduced infant mortality in rural Bangladesh Klemm et al, Pediatrics 2007 • Community based, double masked cluster placebo RCT in 19 unions • 17, 116 newborns received Vit A or placebo < 7 days after birth • 15% Reduction in 6 month all cause mortality (p = 0. 037)
 Human cost of delay in adopting effective therapy
Human cost of delay in adopting effective therapy
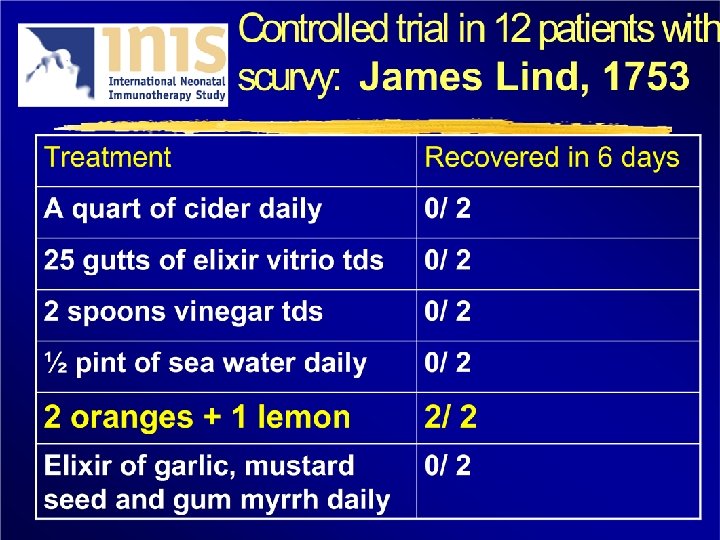
 How long did it take before the Navy Sick and Hurt Board ordered all seamen to be given lemon or lime juice to prevent scurvy?
How long did it take before the Navy Sick and Hurt Board ordered all seamen to be given lemon or lime juice to prevent scurvy?

 42 years
42 years
 The human costs of failing to cumulate evidence in systematic reviews “Advice on some life saving therapies has been delayed for more than a decade, while other treatments have been recommended long after controlled research has shown them to be harmful. ” Antman et al. JAMA, 1992
The human costs of failing to cumulate evidence in systematic reviews “Advice on some life saving therapies has been delayed for more than a decade, while other treatments have been recommended long after controlled research has shown them to be harmful. ” Antman et al. JAMA, 1992
 Systematic reviews are needed to identify useful treatments efficiently Would any of you have agreed to participate in a placebo controlled trial of prophylactic antibiotics for colorectal surgery after 1975? Chalmers I, 2007
Systematic reviews are needed to identify useful treatments efficiently Would any of you have agreed to participate in a placebo controlled trial of prophylactic antibiotics for colorectal surgery after 1975? Chalmers I, 2007
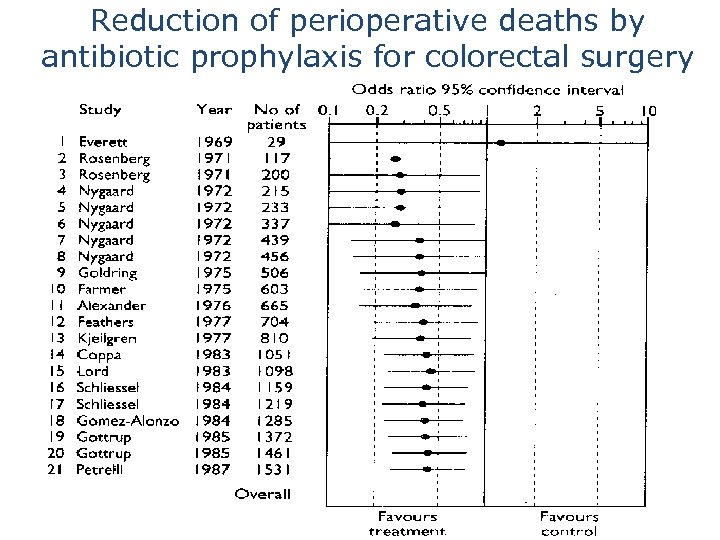 Reduction of perioperative deaths by antibiotic prophylaxis for colorectal surgery
Reduction of perioperative deaths by antibiotic prophylaxis for colorectal surgery

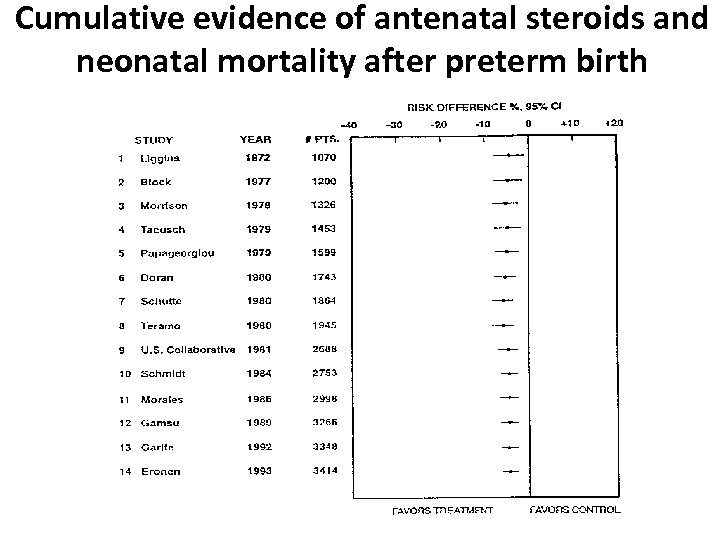 Cumulative evidence of antenatal steroids and neonatal mortality after preterm birth
Cumulative evidence of antenatal steroids and neonatal mortality after preterm birth
 Antenatal steroids in threatened preterm birth • By 1980, 8 RCTs had been done in 1, 945 patients • Cumulative meta analysis showed a 31% reduction in the relative risk of mortality
Antenatal steroids in threatened preterm birth • By 1980, 8 RCTs had been done in 1, 945 patients • Cumulative meta analysis showed a 31% reduction in the relative risk of mortality
 Antenatal steroids in threatened preterm birth • In the next 21 years, 13 more RCTs were reported in another 2, 324 patients (p<0. 00001) • Half of those patients – over 1, 150 received no antenatal steroids.
Antenatal steroids in threatened preterm birth • In the next 21 years, 13 more RCTs were reported in another 2, 324 patients (p<0. 00001) • Half of those patients – over 1, 150 received no antenatal steroids.
 Today’s question Would any of you agree for your preterm child to participate in a placebo controlled trial of probiotics after 2009?
Today’s question Would any of you agree for your preterm child to participate in a placebo controlled trial of probiotics after 2009?
 The elephant in the room • Probiotics reduce all cause mortality by more than half (p <0. 00001). • RCTs to compare different strains, and doses, and timing of probiotics are now needed.
The elephant in the room • Probiotics reduce all cause mortality by more than half (p <0. 00001). • RCTs to compare different strains, and doses, and timing of probiotics are now needed.
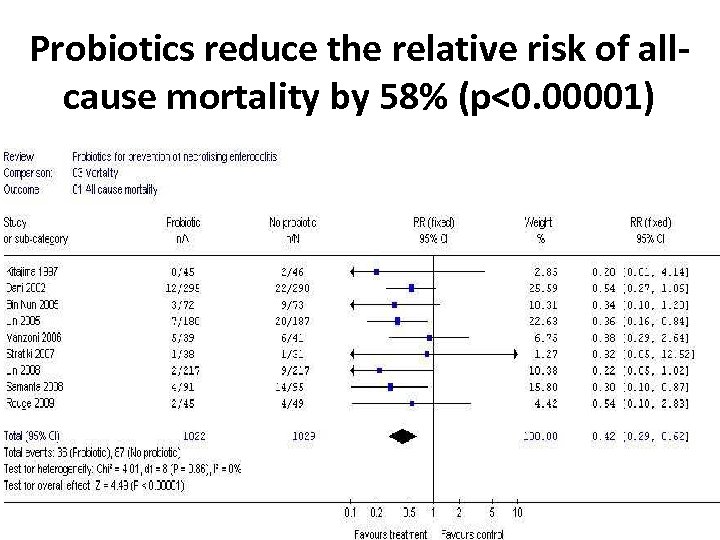 Probiotics reduce the relative risk of allcause mortality by 58% (p<0. 00001)
Probiotics reduce the relative risk of allcause mortality by 58% (p<0. 00001)
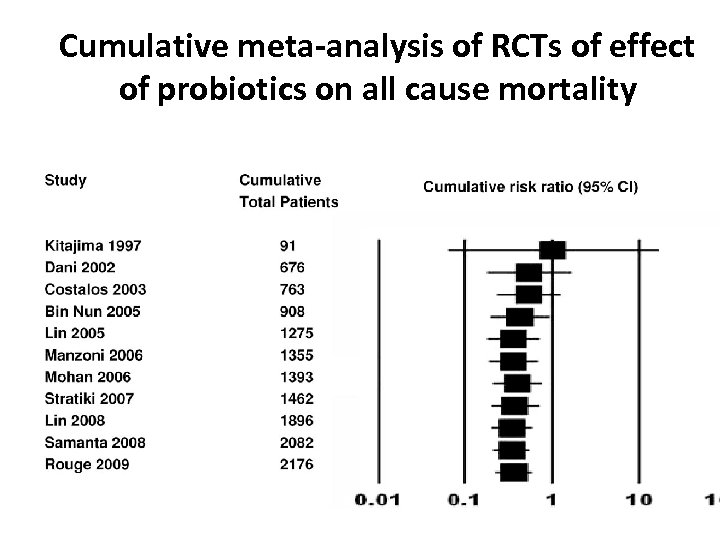 Cumulative meta-analysis of RCTs of effect of probiotics on all cause mortality
Cumulative meta-analysis of RCTs of effect of probiotics on all cause mortality
 Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. • Samanta M, Sarkar M, Ghosh P, Ghosh J, Sinha M, Chatterjee S. J Trop Paediatrics, 2009 Department of Paediatrics, Medical College Kolkata • Our study showed that enteral administration of prophylactic probiotics in neonatal intensive care setup could significantly reduce morbidity due to necrotising enterocolitis in very low birth weight newborn. It also helps in establishing early full enteral feeding and reduces hospital stay.
Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. • Samanta M, Sarkar M, Ghosh P, Ghosh J, Sinha M, Chatterjee S. J Trop Paediatrics, 2009 Department of Paediatrics, Medical College Kolkata • Our study showed that enteral administration of prophylactic probiotics in neonatal intensive care setup could significantly reduce morbidity due to necrotising enterocolitis in very low birth weight newborn. It also helps in establishing early full enteral feeding and reduces hospital stay.
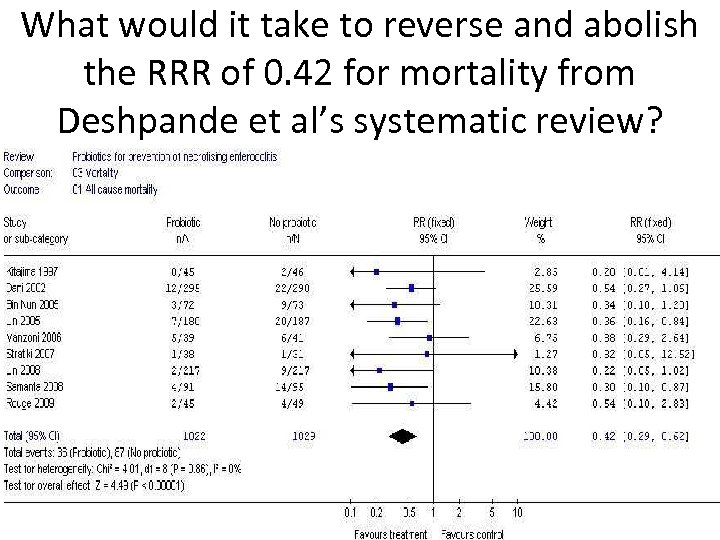 What would it take to reverse and abolish the RRR of 0. 42 for mortality from Deshpande et al’s systematic review?
What would it take to reverse and abolish the RRR of 0. 42 for mortality from Deshpande et al’s systematic review?
 • Broadly speaking, it would take about the same number of patients [i. e. about 2000] and/ or trials showing an opposite effect for the result from the current updated systematic review to disappear. • This would be unprecedented in neonatal/ perinatal medicine.
• Broadly speaking, it would take about the same number of patients [i. e. about 2000] and/ or trials showing an opposite effect for the result from the current updated systematic review to disappear. • This would be unprecedented in neonatal/ perinatal medicine.
 Neonatal sepsis and multiresistance: global priorities
Neonatal sepsis and multiresistance: global priorities
 Background • Each year, over 1. 6 million babies die from preventable sepsis. The vast majority of these deaths are in developing countries. • Over two thirds of these deaths are in preterm or low birth weight infants.
Background • Each year, over 1. 6 million babies die from preventable sepsis. The vast majority of these deaths are in developing countries. • Over two thirds of these deaths are in preterm or low birth weight infants.
 • Effective treatments are likely to have greater effects in infants at higher risk. • So a RCT in VLBW infants is likely to have greater power.
• Effective treatments are likely to have greater effects in infants at higher risk. • So a RCT in VLBW infants is likely to have greater power.
 Multi-resistant bacteria – a global threat to public health • Decades after discovering antibiotics, widespread over-use in NICUs - and in paediatric and adult wards - has selected highly resistant bacteria. • This has created a global threat to public health
Multi-resistant bacteria – a global threat to public health • Decades after discovering antibiotics, widespread over-use in NICUs - and in paediatric and adult wards - has selected highly resistant bacteria. • This has created a global threat to public health
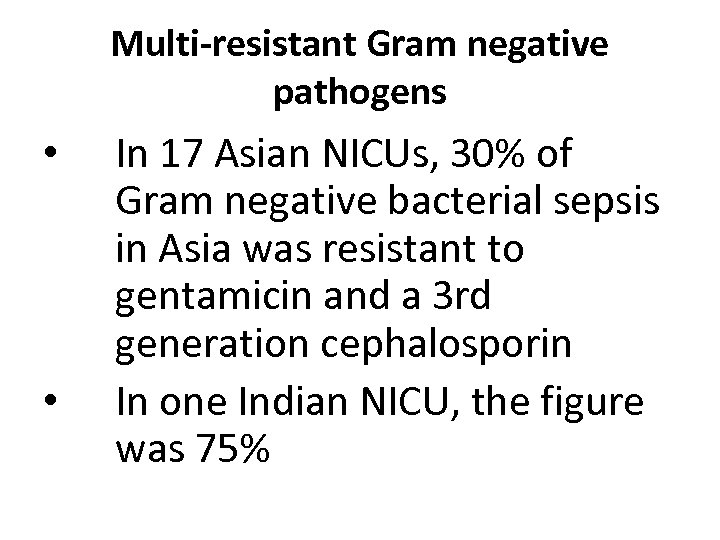 Multi-resistant Gram negative pathogens • • In 17 Asian NICUs, 30% of Gram negative bacterial sepsis in Asia was resistant to gentamicin and a 3 rd generation cephalosporin In one Indian NICU, the figure was 75%
Multi-resistant Gram negative pathogens • • In 17 Asian NICUs, 30% of Gram negative bacterial sepsis in Asia was resistant to gentamicin and a 3 rd generation cephalosporin In one Indian NICU, the figure was 75%

 ‘Infections now occur that are resistant to all current antibacterial options. … there is an urgent, immediate need for new agents with activity against these panresistant organisms. ”
‘Infections now occur that are resistant to all current antibacterial options. … there is an urgent, immediate need for new agents with activity against these panresistant organisms. ”
 Lactoferrin – antimicrobial and antioxidant
Lactoferrin – antimicrobial and antioxidant
 Lactoferrin – antimicrobial and antioxidant • Mammalian milk glycoprotein, also present in plasma, tears, CSF and other secretions – highly conserved component of the innate response to sepsis – antioxidant – immunomodulator
Lactoferrin – antimicrobial and antioxidant • Mammalian milk glycoprotein, also present in plasma, tears, CSF and other secretions – highly conserved component of the innate response to sepsis – antioxidant – immunomodulator
 • Despite 300 million years of interaction, it retains potent antimicrobial activity by sequestering iron and disrupting cellular membrane metabolism of all four major classes of pathogen – bacteria – fungi – viruses (including HIV) – Protozoa (including Plasmodium)
• Despite 300 million years of interaction, it retains potent antimicrobial activity by sequestering iron and disrupting cellular membrane metabolism of all four major classes of pathogen – bacteria – fungi – viruses (including HIV) – Protozoa (including Plasmodium)
 • Lactoferrin is broken down by acid proteolysis in the stomach into lactoferricin, which has enhanced antimicrobial activity. • This may partly explain why antacid therapy with proton pump inhibitors and H 2 antagonists are associated with increased sepsis
• Lactoferrin is broken down by acid proteolysis in the stomach into lactoferricin, which has enhanced antimicrobial activity. • This may partly explain why antacid therapy with proton pump inhibitors and H 2 antagonists are associated with increased sepsis
 Lactoferrin
Lactoferrin
 Anti-oxidant • Lactoferrin behaves like an iron scavenger, snapping up free iron, which may help to inhibit proliferation of pathogenic bacteria free radical disease. • Interestingly, the structural cavities where iron binding takes place on the lactoferrin molecule are much larger than needed, suggesting that it may also have evolved to deal with certain toxins and foreign substances.
Anti-oxidant • Lactoferrin behaves like an iron scavenger, snapping up free iron, which may help to inhibit proliferation of pathogenic bacteria free radical disease. • Interestingly, the structural cavities where iron binding takes place on the lactoferrin molecule are much larger than needed, suggesting that it may also have evolved to deal with certain toxins and foreign substances.
 Anti-oxidant • Free iron, in formula, fortifiers or iron supplements, catalyzes free radical oxidative damage to cells, which may promote diseases linked to free radical damage, such as ROP, BPD, Brain injury.
Anti-oxidant • Free iron, in formula, fortifiers or iron supplements, catalyzes free radical oxidative damage to cells, which may promote diseases linked to free radical damage, such as ROP, BPD, Brain injury.
 Anti-oxidant • Raghuveer et al, Pediatr Res 2002, demonstrated increased free radical activity in human milk and formula after addition of elemental iron. • This free radical activity was suppressed by concurrent administration of Human Lactoferrin. • They postulated that lactoferrin may ameliorate diseases associated with free radical activity, such as ROP, BPD, NEC.
Anti-oxidant • Raghuveer et al, Pediatr Res 2002, demonstrated increased free radical activity in human milk and formula after addition of elemental iron. • This free radical activity was suppressed by concurrent administration of Human Lactoferrin. • They postulated that lactoferrin may ameliorate diseases associated with free radical activity, such as ROP, BPD, NEC.
 Bovine lactoferrin (BLF) • • • 77% homology with human lactoferrin costs <$1 per day, cheaper if made locally powder stable without refrigeration ≤ 2 years given in breast milk or formula by cup and spoon, bottle, or gastric tube.
Bovine lactoferrin (BLF) • • • 77% homology with human lactoferrin costs <$1 per day, cheaper if made locally powder stable without refrigeration ≤ 2 years given in breast milk or formula by cup and spoon, bottle, or gastric tube.

 Safety and Toxicity The US Federal Drugs Agency has • registered BLF as Generally Regarded as Safe (GRAS) • approved it as a spray on beef carcasses to kill E. coli 0157: H 7, a particularly virulent disease causing bacterium
Safety and Toxicity The US Federal Drugs Agency has • registered BLF as Generally Regarded as Safe (GRAS) • approved it as a spray on beef carcasses to kill E. coli 0157: H 7, a particularly virulent disease causing bacterium
 Previous RCT of BLF in VLBW Infants Manzoni et al, JAMA 2009. • 3 arm RCT in 472 VLBW infants in 11 Italian NICUs, treated daily with – Bovine Lactoferrin 100 mg OR – Bovine lactoferrin 100 mg + probiotic (L. Rhamnosus GG) OR – Placebo (2 mls 5% Glucose)
Previous RCT of BLF in VLBW Infants Manzoni et al, JAMA 2009. • 3 arm RCT in 472 VLBW infants in 11 Italian NICUs, treated daily with – Bovine Lactoferrin 100 mg OR – Bovine lactoferrin 100 mg + probiotic (L. Rhamnosus GG) OR – Placebo (2 mls 5% Glucose)

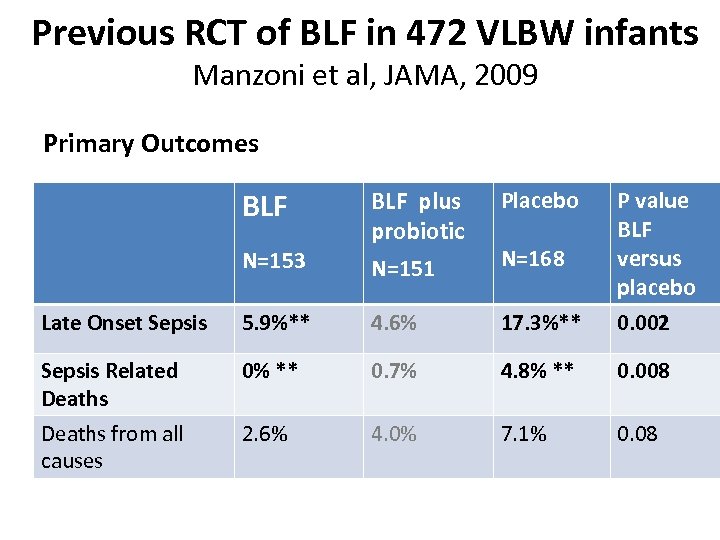 Previous RCT of BLF in 472 VLBW infants Manzoni et al, JAMA, 2009 Primary Outcomes BLF Placebo N=153 BLF plus probiotic N=151 Late Onset Sepsis 5. 9%** 4. 6% 17. 3%** P value BLF versus placebo 0. 002 Sepsis Related Deaths 0% ** 0. 7% 4. 8% ** 0. 008 Deaths from all causes 2. 6% 4. 0% 7. 1% 0. 08 N=168
Previous RCT of BLF in 472 VLBW infants Manzoni et al, JAMA, 2009 Primary Outcomes BLF Placebo N=153 BLF plus probiotic N=151 Late Onset Sepsis 5. 9%** 4. 6% 17. 3%** P value BLF versus placebo 0. 002 Sepsis Related Deaths 0% ** 0. 7% 4. 8% ** 0. 008 Deaths from all causes 2. 6% 4. 0% 7. 1% 0. 08 N=168
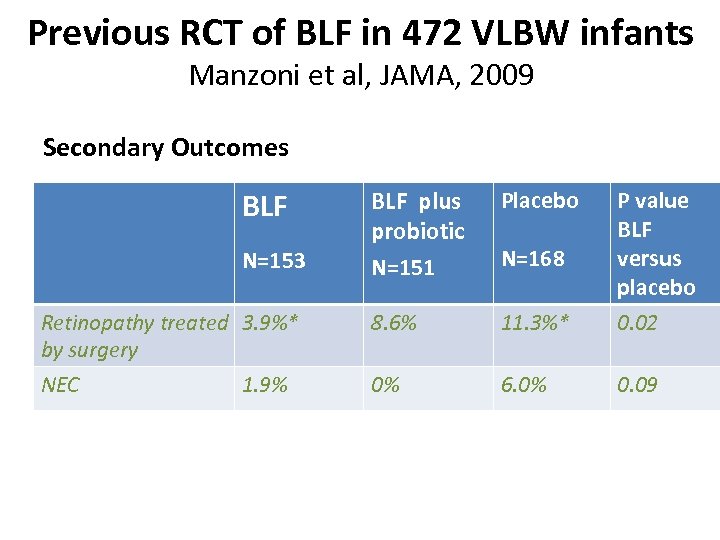 Previous RCT of BLF in 472 VLBW infants Manzoni et al, JAMA, 2009 Secondary Outcomes BLF plus probiotic N=151 Placebo Retinopathy treated 3. 9%* by surgery 8. 6% 11. 3%* P value BLF versus placebo 0. 02 NEC 0% 6. 0% 0. 09 N=153 1. 9% N=168
Previous RCT of BLF in 472 VLBW infants Manzoni et al, JAMA, 2009 Secondary Outcomes BLF plus probiotic N=151 Placebo Retinopathy treated 3. 9%* by surgery 8. 6% 11. 3%* P value BLF versus placebo 0. 02 NEC 0% 6. 0% 0. 09 N=153 1. 9% N=168
 Topics and priorities for possible future collaboration
Topics and priorities for possible future collaboration
 • Delayed cord clamping in preterm infants • Early probiotics in preterm infants within one hour of birth versus 24 hours after initial feeds are tolerated • Pharmacodynamics of BLF in preterm infants Bovine lactoferrin as adjunctive therapy in neonatal sepsis ? ?
• Delayed cord clamping in preterm infants • Early probiotics in preterm infants within one hour of birth versus 24 hours after initial feeds are tolerated • Pharmacodynamics of BLF in preterm infants Bovine lactoferrin as adjunctive therapy in neonatal sepsis ? ?
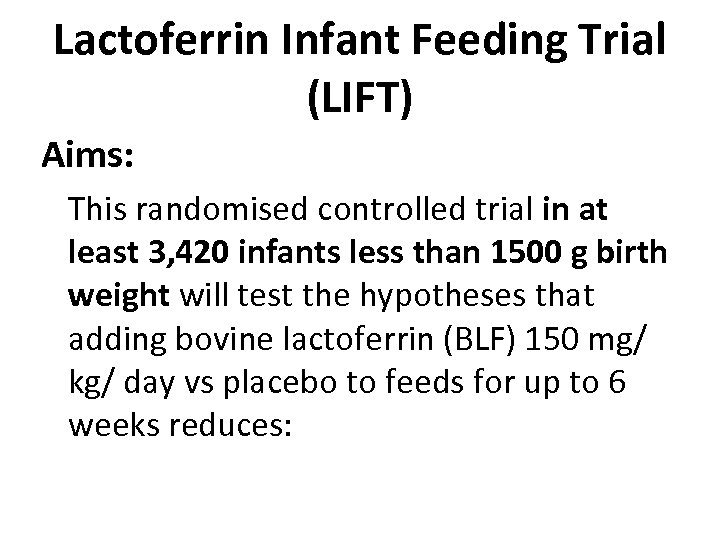 Lactoferrin Infant Feeding Trial (LIFT) Aims: This randomised controlled trial in at least 3, 420 infants less than 1500 g birth weight will test the hypotheses that adding bovine lactoferrin (BLF) 150 mg/ kg/ day vs placebo to feeds for up to 6 weeks reduces:
Lactoferrin Infant Feeding Trial (LIFT) Aims: This randomised controlled trial in at least 3, 420 infants less than 1500 g birth weight will test the hypotheses that adding bovine lactoferrin (BLF) 150 mg/ kg/ day vs placebo to feeds for up to 6 weeks reduces:
 I: Primary composite outcome: (a) death or major morbidity before and up to 40 weeks gestation
I: Primary composite outcome: (a) death or major morbidity before and up to 40 weeks gestation
 II: Secondary outcomes: (a) death before 40 weeks gestation (b) death related to sepsis (c) major morbidity – – – sepsis brain injury chronic lung disease severe retinopathy necrotizing enterocolitis (d) use of an antibiotic more than 7 days after birth
II: Secondary outcomes: (a) death before 40 weeks gestation (b) death related to sepsis (c) major morbidity – – – sepsis brain injury chronic lung disease severe retinopathy necrotizing enterocolitis (d) use of an antibiotic more than 7 days after birth
 Cost Effectiveness Study Assessment of cost per additional life and per additional life free from major morbidity saved, with sensitivity analyses for degree of compliance with recommended policies for infection control and antibiotic restriction (Isaacs et al).
Cost Effectiveness Study Assessment of cost per additional life and per additional life free from major morbidity saved, with sensitivity analyses for degree of compliance with recommended policies for infection control and antibiotic restriction (Isaacs et al).
 Eligibility (a) doctor and parents are substantially uncertain if BLF is indicated or contra indicated and (b) < 1500 g birth weight and (c) <7 days old and (d) parent gives written informed consent. Babies not receiving feeds <7 days old are eligible and will begin study supplements when feeds are tolerated. Babies with severe congenital anomalies are ineligible.
Eligibility (a) doctor and parents are substantially uncertain if BLF is indicated or contra indicated and (b) < 1500 g birth weight and (c) <7 days old and (d) parent gives written informed consent. Babies not receiving feeds <7 days old are eligible and will begin study supplements when feeds are tolerated. Babies with severe congenital anomalies are ineligible.
 Outcomes: • The primary outcome is death or major morbidity. • In NICUs that anticipate >94% follow up, written parental consent will be sought for neuro developmental assessment at 3 years, for which funding will be sought for a separate study. • At least 1, 350 infants are expected to be followed for 3 years in that study.
Outcomes: • The primary outcome is death or major morbidity. • In NICUs that anticipate >94% follow up, written parental consent will be sought for neuro developmental assessment at 3 years, for which funding will be sought for a separate study. • At least 1, 350 infants are expected to be followed for 3 years in that study.
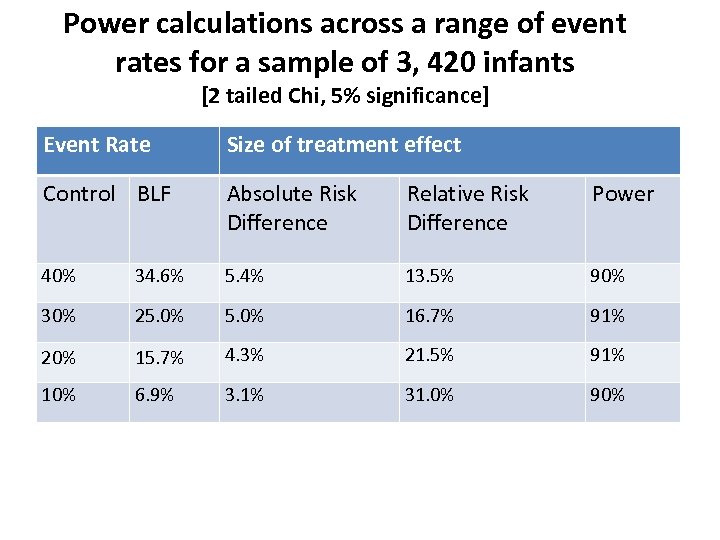 Power calculations across a range of event rates for a sample of 3, 420 infants [2 tailed Chi, 5% significance] Event Rate Size of treatment effect Control BLF Absolute Risk Difference Relative Risk Difference Power 40% 34. 6% 5. 4% 13. 5% 90% 30% 25. 0% 16. 7% 91% 20% 15. 7% 4. 3% 21. 5% 91% 10% 6. 9% 3. 1% 31. 0% 90%
Power calculations across a range of event rates for a sample of 3, 420 infants [2 tailed Chi, 5% significance] Event Rate Size of treatment effect Control BLF Absolute Risk Difference Relative Risk Difference Power 40% 34. 6% 5. 4% 13. 5% 90% 30% 25. 0% 16. 7% 91% 20% 15. 7% 4. 3% 21. 5% 91% 10% 6. 9% 3. 1% 31. 0% 90%
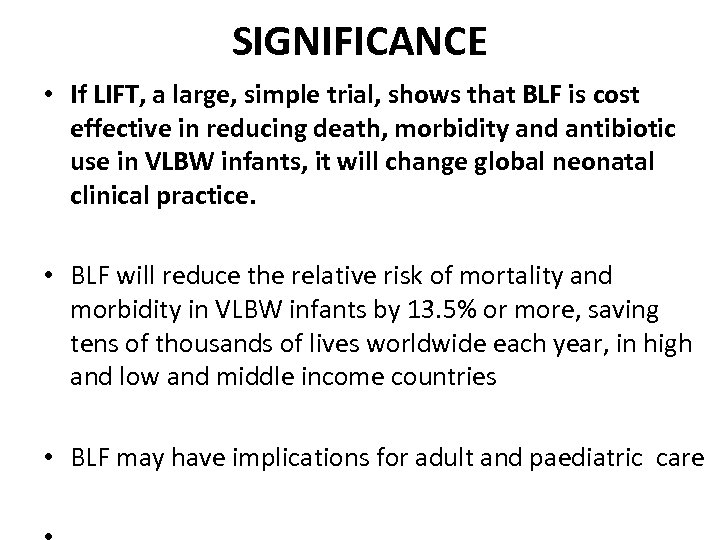 SIGNIFICANCE • If LIFT, a large, simple trial, shows that BLF is cost effective in reducing death, morbidity and antibiotic use in VLBW infants, it will change global neonatal clinical practice. • BLF will reduce the relative risk of mortality and morbidity in VLBW infants by 13. 5% or more, saving tens of thousands of lives worldwide each year, in high and low and middle income countries • BLF may have implications for adult and paediatric care
SIGNIFICANCE • If LIFT, a large, simple trial, shows that BLF is cost effective in reducing death, morbidity and antibiotic use in VLBW infants, it will change global neonatal clinical practice. • BLF will reduce the relative risk of mortality and morbidity in VLBW infants by 13. 5% or more, saving tens of thousands of lives worldwide each year, in high and low and middle income countries • BLF may have implications for adult and paediatric care
 • Large scale international collaboration to win healthy survival for the weakest in society is a mark of true civilisation. • In this, India has a leading role to play.
• Large scale international collaboration to win healthy survival for the weakest in society is a mark of true civilisation. • In this, India has a leading role to play.
 Outline • • Current constraints on achieving large trials Human cost of delay in identifying effective therapy. Rationale for large, simple, low cost trials RCTs and cluster RCTs What is a mega trial and why do we need them? Need for international collaboration Can we create a global collaboration for perinatal mega trials?
Outline • • Current constraints on achieving large trials Human cost of delay in identifying effective therapy. Rationale for large, simple, low cost trials RCTs and cluster RCTs What is a mega trial and why do we need them? Need for international collaboration Can we create a global collaboration for perinatal mega trials?


