3f976e67ea78a8d6b8d97d76df508fc0.ppt
- Количество слайдов: 24
 WHO Prequalification – Medicines Finished Pharmaceutical Products Hua YIN yinh@who. int
WHO Prequalification – Medicines Finished Pharmaceutical Products Hua YIN yinh@who. int
 Outline • Who can participate? • What requirements and standards do your products have to meet to become prequalified? ü API options ü BE biowaivers • How to submit an application ? • Contact with WHO PQP 2 Shanghai CPHi , June 2014
Outline • Who can participate? • What requirements and standards do your products have to meet to become prequalified? ü API options ü BE biowaivers • How to submit an application ? • Contact with WHO PQP 2 Shanghai CPHi , June 2014
 Who can participate? • Therapeutic areas invited are: – HIV/AIDS – Malaria – Tuberculosis – Reproductive Health (RH) – Influenza – Acute diarrhoea in children (zinc) – Neglected Tropical Diseases (NTDs) • EOIs containing list of invited products published on PQP web site Shanghai CPHi , June 2014 3
Who can participate? • Therapeutic areas invited are: – HIV/AIDS – Malaria – Tuberculosis – Reproductive Health (RH) – Influenza – Acute diarrhoea in children (zinc) – Neglected Tropical Diseases (NTDs) • EOIs containing list of invited products published on PQP web site Shanghai CPHi , June 2014 3
 Who can participate? • Only the products listed in the current EOIs are invited • The strength, dosage forms should be the same as indicated in EOI • EOIs are updated as per treatment requirement—PQ website • Ask if you are not sure, prequalassessment@who. int Shanghai CPHi , June 2014 4
Who can participate? • Only the products listed in the current EOIs are invited • The strength, dosage forms should be the same as indicated in EOI • EOIs are updated as per treatment requirement—PQ website • Ask if you are not sure, prequalassessment@who. int Shanghai CPHi , June 2014 4
 What requirements and standards do your products have to meet? Shanghai CPHi , June 2014 5
What requirements and standards do your products have to meet? Shanghai CPHi , June 2014 5
 Prequalification of FPPs • Assessment of Quality , Safety and Efficacy (Quality and BE) • Inspection of manufacturing sites, FPP, API and CROs • Monitoring of the products after prequalification (variations, requalification, inspections, random QC sampling, investigation of complaints) Shanghai CPHi , June 2014 6
Prequalification of FPPs • Assessment of Quality , Safety and Efficacy (Quality and BE) • Inspection of manufacturing sites, FPP, API and CROs • Monitoring of the products after prequalification (variations, requalification, inspections, random QC sampling, investigation of complaints) Shanghai CPHi , June 2014 6
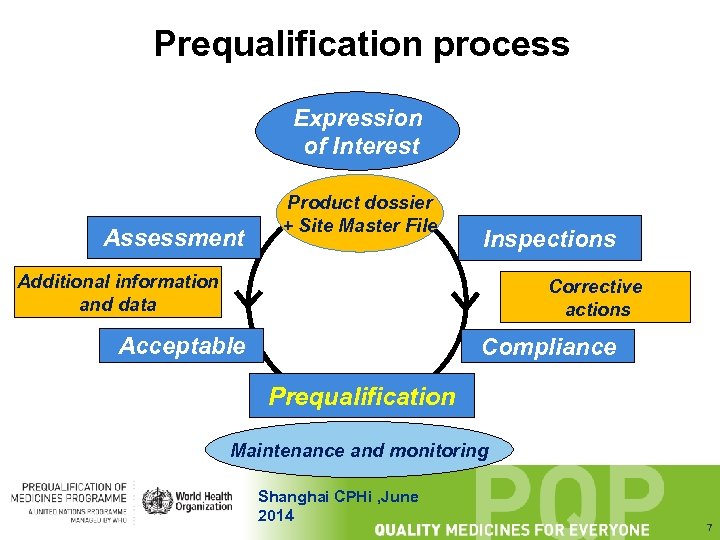 Prequalification process Expression of Interest Assessment Product dossier + Site Master File Inspections Additional information and data Corrective actions Acceptable Compliance Prequalification Maintenance and monitoring Shanghai CPHi , June 2014 7
Prequalification process Expression of Interest Assessment Product dossier + Site Master File Inspections Additional information and data Corrective actions Acceptable Compliance Prequalification Maintenance and monitoring Shanghai CPHi , June 2014 7
 Quality Guidance • WHO PQP guidelines are applied: – Guideline on submission of documentation for a multisource (generic) finished pharmaceutical product for the WHO Prequalification of Medicines Programme: Quality part – Specific guidance, e. g: Guidance for zinc products; magnesium sulfate injection; guidance on BE studies for RH medicines • When WHO guidelines silence: ICH guidance • If needed, other agencies' requirements , such as EMA, USFDA. Shanghai CPHi , June 2014 8
Quality Guidance • WHO PQP guidelines are applied: – Guideline on submission of documentation for a multisource (generic) finished pharmaceutical product for the WHO Prequalification of Medicines Programme: Quality part – Specific guidance, e. g: Guidance for zinc products; magnesium sulfate injection; guidance on BE studies for RH medicines • When WHO guidelines silence: ICH guidance • If needed, other agencies' requirements , such as EMA, USFDA. Shanghai CPHi , June 2014 8
 BE Guidance ¨ WHO – Technical Report Series No. 937, May 2006 Annex 7: Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability Annex 8: Proposal to waive in vivo bioequivalence requirements for WHO Model List of Essential Medicines immediate release, solid oral dosage forms Shanghai CPHi , June 2014 9
BE Guidance ¨ WHO – Technical Report Series No. 937, May 2006 Annex 7: Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability Annex 8: Proposal to waive in vivo bioequivalence requirements for WHO Model List of Essential Medicines immediate release, solid oral dosage forms Shanghai CPHi , June 2014 9
 Inspections (GMP) • Inspection of FPP and API manufacturing sites, and CROs • Inspections conducted by an SRA are taken into account when planning inspections • The need for inspections of API sites and CROs are decided on a case by case risk basis. • WHO GMP, GCP and GLP http: //www. who. int/prequal/ Shanghai CPHi , June 2014 10
Inspections (GMP) • Inspection of FPP and API manufacturing sites, and CROs • Inspections conducted by an SRA are taken into account when planning inspections • The need for inspections of API sites and CROs are decided on a case by case risk basis. • WHO GMP, GCP and GLP http: //www. who. int/prequal/ Shanghai CPHi , June 2014 10
 http: //www. who. int/prequal/ Shanghai CPHi , June 2014 11
http: //www. who. int/prequal/ Shanghai CPHi , June 2014 11
 Documents to be submitted • Covering letter • Product dossier (Quality and Bioequivalent), in CTD format • A product sample • A site master file, for each manufacturing site • In English Shanghai CPHi , June 2014 12
Documents to be submitted • Covering letter • Product dossier (Quality and Bioequivalent), in CTD format • A product sample • A site master file, for each manufacturing site • In English Shanghai CPHi , June 2014 12
 Options for submitting API information Option 1: Confirmation of API Prequalification document (CPQ); Option 2: Certificate of suitability of the European Pharmacopoeia (CEP); C o Option 3: Active pharmaceutical ingredient master file p (APIMF) procedure; e n Option 4: Full API details in the product dossier. ha ge n tr Shanghai CPHi , June 13 ai 2014 13
Options for submitting API information Option 1: Confirmation of API Prequalification document (CPQ); Option 2: Certificate of suitability of the European Pharmacopoeia (CEP); C o Option 3: Active pharmaceutical ingredient master file p (APIMF) procedure; e n Option 4: Full API details in the product dossier. ha ge n tr Shanghai CPHi , June 13 ai 2014 13
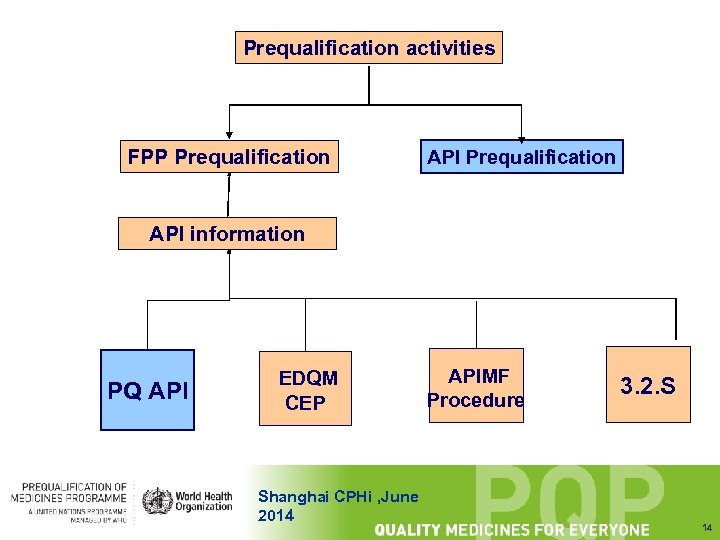 Prequalification activities FPP Prequalification API information PQ API EDQM CEP Shanghai CPHi , June 2014 APIMF Procedure 3. 2. S 14
Prequalification activities FPP Prequalification API information PQ API EDQM CEP Shanghai CPHi , June 2014 APIMF Procedure 3. 2. S 14
 Options for submitting API information • Technical requirements for all options are same, the various processes ensure that ultimately all sections of the module 3. 2. S are assessed. • Documentation to be submitted in an FPP dossier are different for the four API options: C CPQ < CEP < APIMF < Full information o p e • The four options for submitting API-related information also affects the manner in which API changes are n handled after prequalification of the FPP—refer to PQP ha Variation guidance ge n tr Shanghai CPHi , June 15 ai 2014 15
Options for submitting API information • Technical requirements for all options are same, the various processes ensure that ultimately all sections of the module 3. 2. S are assessed. • Documentation to be submitted in an FPP dossier are different for the four API options: C CPQ < CEP < APIMF < Full information o p e • The four options for submitting API-related information also affects the manner in which API changes are n handled after prequalification of the FPP—refer to PQP ha Variation guidance ge n tr Shanghai CPHi , June 15 ai 2014 15
 BE may not necessary • Aqueous solutions – – – Intravenous solutions Intramuscular, subcutaneous solutions Oral solutions Otic or ophthalmic solutions Solutions for nasal administration • Powders for reconstitution as solution Shanghai CPHi , June 2014 16
BE may not necessary • Aqueous solutions – – – Intravenous solutions Intramuscular, subcutaneous solutions Oral solutions Otic or ophthalmic solutions Solutions for nasal administration • Powders for reconstitution as solution Shanghai CPHi , June 2014 16
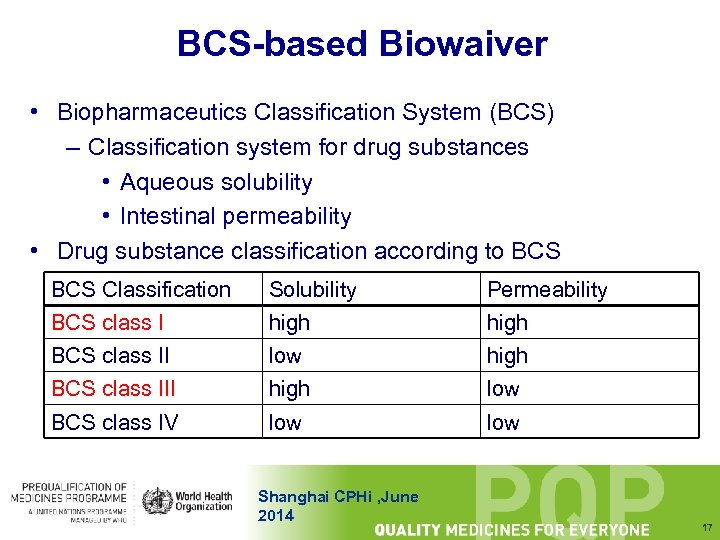 BCS-based Biowaiver • Biopharmaceutics Classification System (BCS) – Classification system for drug substances • Aqueous solubility • Intestinal permeability • Drug substance classification according to BCS Classification BCS class I Solubility high Permeability high BCS class II low high BCS class III high low BCS class IV low Shanghai CPHi , June 2014 17
BCS-based Biowaiver • Biopharmaceutics Classification System (BCS) – Classification system for drug substances • Aqueous solubility • Intestinal permeability • Drug substance classification according to BCS Classification BCS class I Solubility high Permeability high BCS class II low high BCS class III high low BCS class IV low Shanghai CPHi , June 2014 17
 BCS-based Biowaiver • Requirements for BCS-based Biowaiver – General Notes on BCS-based Biowaiver Applications – Biowaiver Application Form: Biopharmaceutics Classification System (BCS) – BCS-based biowaiver applications for RH products • http: //apps. who. int/prequal/info_applicants/info_for_applic ants_BE_implementation. htm Shanghai CPHi , June 2014 18
BCS-based Biowaiver • Requirements for BCS-based Biowaiver – General Notes on BCS-based Biowaiver Applications – Biowaiver Application Form: Biopharmaceutics Classification System (BCS) – BCS-based biowaiver applications for RH products • http: //apps. who. int/prequal/info_applicants/info_for_applic ants_BE_implementation. htm Shanghai CPHi , June 2014 18
 Pre-review of BE protocol Dr. Matthias Stahl stahlm@who. int or prequalassessment@who. int Shanghai CPHi , June 2014 19
Pre-review of BE protocol Dr. Matthias Stahl stahlm@who. int or prequalassessment@who. int Shanghai CPHi , June 2014 19
 How to Submit an application WHO Geneva: World Health Organization WHO Prequalification Team - Medicines HIS/EMP/RHT Room 613 20 Avenue Appia 1211 Geneva 27 Switzerland UNICEF- Cophenhagen : CONFIDENTIAL Attention: WHO Prequalification Team - Medicines Product Name: UNICEF Supply Division Oceanvej 10 - 12 2150 Nordhavn Copenhagen Denmark Shanghai CPHi , June 2014 20
How to Submit an application WHO Geneva: World Health Organization WHO Prequalification Team - Medicines HIS/EMP/RHT Room 613 20 Avenue Appia 1211 Geneva 27 Switzerland UNICEF- Cophenhagen : CONFIDENTIAL Attention: WHO Prequalification Team - Medicines Product Name: UNICEF Supply Division Oceanvej 10 - 12 2150 Nordhavn Copenhagen Denmark Shanghai CPHi , June 2014 20
 How to Submit an application Step 1: Dossier CD/DVDs (API +FPP +BE) → Geneva After has been accepted for assessment and has been allocated a WHO reference number Step 2: Dossier (paper copies +CD/DVDs )+ samples → Copenhagen Site Master File & Contract Research Organization Master File (CROMF) (hard copies +CD/DVDs) → Geneva 2 1 Shanghai CPHi , June 2014 21
How to Submit an application Step 1: Dossier CD/DVDs (API +FPP +BE) → Geneva After has been accepted for assessment and has been allocated a WHO reference number Step 2: Dossier (paper copies +CD/DVDs )+ samples → Copenhagen Site Master File & Contract Research Organization Master File (CROMF) (hard copies +CD/DVDs) → Geneva 2 1 Shanghai CPHi , June 2014 21
 Contact with PQP • Email to: prequalassessment@who. int prequalinspection@who. int • Technical Meetings with PQP assessors/inspectors – – Pre-submission meeting Any stage following submission Teleconference, videoconference or face to face meeting Meeting request form to be filled (PQ website) Shanghai CPHi , June 2014 22
Contact with PQP • Email to: prequalassessment@who. int prequalinspection@who. int • Technical Meetings with PQP assessors/inspectors – – Pre-submission meeting Any stage following submission Teleconference, videoconference or face to face meeting Meeting request form to be filled (PQ website) Shanghai CPHi , June 2014 22
 Abbreviations • • • 23 PQP: Prequalificaiton of Medicines Programme API: Active Pharmaceutical Ingredient FPP: Finished Pharmaceutical Product APIMF: Active Pharmaceutical Ingredient Master File (DMF) SRA: Stringent Regulatory Authorities CEP: Certificate of Suitability (Co. S) QOS-PD: Quality Overall Summary - Product Dossier QIS: Quality Information Summary FDC: Fixed dose combination CTD: Common technical document BE: Bioequivalent BCS : Biopharmaceutics Classification System
Abbreviations • • • 23 PQP: Prequalificaiton of Medicines Programme API: Active Pharmaceutical Ingredient FPP: Finished Pharmaceutical Product APIMF: Active Pharmaceutical Ingredient Master File (DMF) SRA: Stringent Regulatory Authorities CEP: Certificate of Suitability (Co. S) QOS-PD: Quality Overall Summary - Product Dossier QIS: Quality Information Summary FDC: Fixed dose combination CTD: Common technical document BE: Bioequivalent BCS : Biopharmaceutics Classification System
 Thank you for your attention! Shanghai CPHi , June 2014 24
Thank you for your attention! Shanghai CPHi , June 2014 24


