2e03a44cf55991ae8f784cd81aea61f0.ppt
- Количество слайдов: 27

WHO Guidelines on Tissue Infectivity Distribution in Transmissible Spongiform Encephalopathies World Health Organization 2006 Proceedings of a Consultation Convened by WHO Geneva 14 -16 September 2005 Summary David M. Asher, MD <david. asher@fda. hhs. gov> Laboratory of Bacterial, Parasitic & Unconventional Agents Division of Emerging & Transfusion-Transmitted Diseases Office of Blood Research & Review Center for Biologics Evaluation & Research US Food & Drug Administration
WHO Guidelines on Tissue Infectivity Distribution in Transmissible Spongiform Encephalopathies http: //www. who. int/bloodproducts/TSEREPORT-Lo. Res. pdf World Health Organization 2006 Proceedings of WHO Consultation Geneva 14 -16 September 2005 Quality and Safety of Plasma Derivatives and Related Substances Department of Medicines Policy and Standards Health Technology and Pharmaceuticals Cluster World Health Organization ------------------------- WHO Secretariat A Padilla (project leader) S Groth L Rago D Wood FX Meslin N Dhingra

Proceedings of a Consultation Convened by WHO Geneva 14 -16 September 2005 Goals of Consultation l Revise WHO 2003 Consultation advice on TSE safety of medicinal products, especially biological products - Vaccines, etc. , prepared with ruminant-derived materials - Products derived from human blood (components, plasma derivatives) - Other human cells and tissues and products derived from them l l Review scientific information on TSEs relevant to safety of medicinal products Compare TSE risk assessments for various countries Provide general advice to “national regulatory authorities with limited resources” about TSE risks associated with medical products and suggest approaches to assess and, if indicated, attempt to reduce risk Special goal: Summarize and evaluate reliability of available information about distribution of infectivity and disease-related prion protein in tissues and body fluids of TSEs of humans and ruminants
WHO TSE Consultation 14 -16 September 2005: Report Issued 2006 Executive summary Recent scientific developments l l Epidemiology, clinical features, Dx criteria of CJD BSE and scrapie Risk of transmission of CJD/v. CJD by human blood, blood products - Recommendations l Tissue infectivity Reducing risk to humans from biological and pharmaceutical products made with ruminant-derived materials - v v v Risk of transmitting v. CJD by blood, blood products Risk assessment Risk reduction: product retrieval/market withdrawal, donor deferral, plasma products, &c. Reducing risk to humans from biological and pharmaceutical products made with human-derived materials - Risk from HCT/Ps - l l Tissue source Tissue removal/processing Production: vaccines, rec. DNA (from banked cells), other Conclusions Annexes 1. 2. TSE infectivity (selected): humans, BSE cattle, scrapie sheep/goats Summary of presentations

26 Countries with BSE in Native Cattle [yr first reported & approx. total cases reported to OIE thro’ 07 Sept 2006] l UK 1986 (>184, 431) [1202 ’ 01; 1144 l Ireland 1989 (1579) Switzerland 1990 (461) France 1991 (971) Portugal 1994 (990) Belgium 1997 (131) Netherlands 1997 (80) Luxembourg 1997 (1) Liechtenstein 1998 (3) Denmark 2000 (14) Germany 2000 (389) Spain 2000 (654) Italy 2000 (132) l l l ’ 02; 611 ’ 03; 343 ’ 04; 225 ’ 05; >61 ’ 06] l l l l Greece 2001 (1) Czech Repub 2001 (24) Slovakia 2001 (23) Japan 2001 (28) Slovenia 2001 (6) Finland 2001 (1) Austria 2001 (5) Poland 2002 (48) Israel 2002 (1) Canada 2003 (1 UK+8) USA 2004 (1 Canada+2) Croatia 2006 (1) Sweden 2006 (1)

Total Reported UK Exports of MBM 1986 – 1995 (unconfirmed review by UK authorities of export records) Legend: (in tonnes) No data 20 - < 100 0 -<5 100 - < 1. 000 5 - < 10 1. 000 - < 10. 000 10 - < 20 > 10. 000

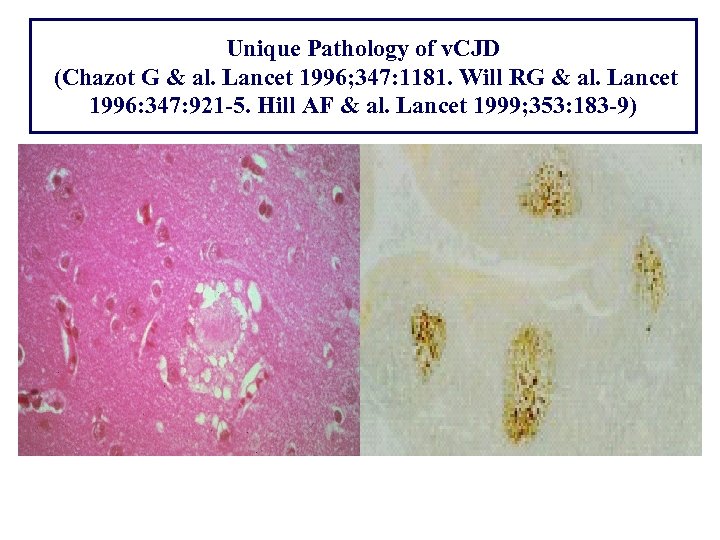
Unique Pathology of v. CJD (Chazot G & al. Lancet 1996; 347: 1181. Will RG & al. Lancet 1996: 347: 921 -5. Hill AF & al. Lancet 1999; 353: 183 -9)
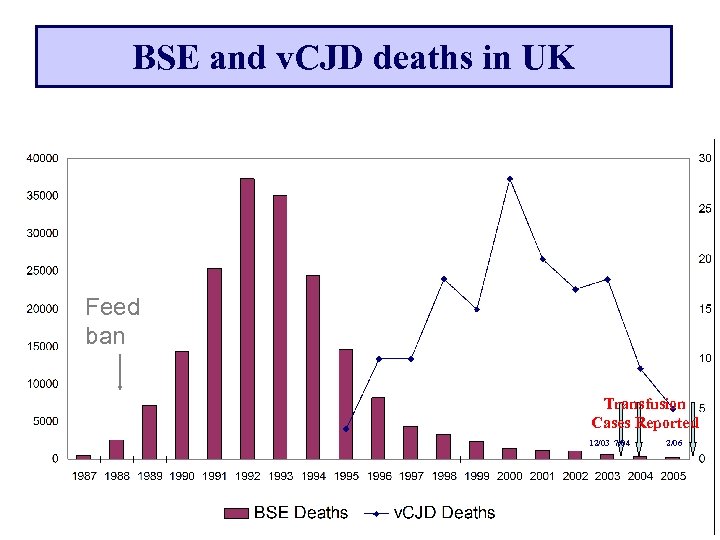
BSE and v. CJD deaths in UK Feed ban Transfusion Cases Reported 12/03 7/04 2/06
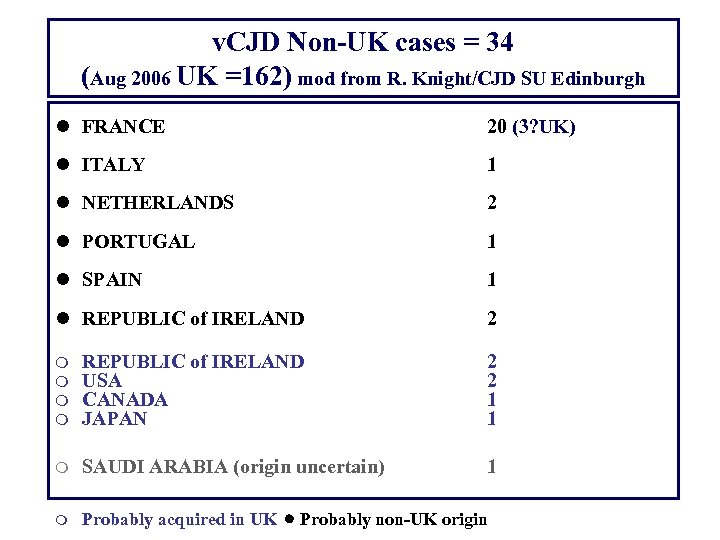
v. CJD Non-UK cases = 34 (Aug 2006 UK =162) mod from R. Knight/CJD SU Edinburgh l FRANCE 20 (3? UK) l ITALY 1 l NETHERLANDS 2 l PORTUGAL 1 l SPAIN 1 l REPUBLIC of IRELAND 2 m m REPUBLIC of IRELAND USA CANADA JAPAN 2 2 1 1 m SAUDI ARABIA (origin uncertain) 1 m Probably acquired in UK Probably non-UK origin

> 360 Iatrogenic Transmissions of CJD/v. CJD by Classes of FDA-regulated Medical Products of Human Origin RBC: the only new class of medical product CJD-implicated past 10 yr Incub’n Average ? 16 mo Incub’n Range 13, 18, 320 mo Product Cornea Cases 3 Dura mater > 168 12 yr 1. 3 -22 yr Pit hormones l Growth l Gonadotropin > 180 4 12 yr 13 yr 5 -39 yr 12 -16 yr 6 (? 7) 19 mo 15 – 28 mo 3 > 6 yr 7. 8 yr, 6. 5 yr, (> 5 yr) Neurosurgical instruments (excluding outlier) (includes electrode) RBC (UK v. CJD)
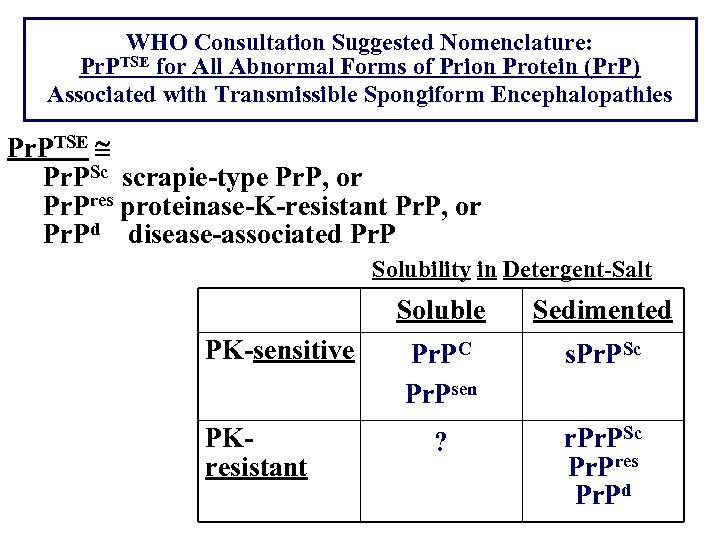
WHO Consultation Suggested Nomenclature: Pr. PTSE for All Abnormal Forms of Prion Protein (Pr. P) Associated with Transmissible Spongiform Encephalopathies Pr. PTSE Pr. PSc scrapie-type Pr. P, or Pr. Pres proteinase-K-resistant Pr. P, or Pr. Pd disease-associated Pr. P Solubility in Detergent-Salt Soluble PK-sensitive PKresistant Sedimented Pr. PC Pr. Psen s. Pr. PSc ? r. PSc Pr. Pres Pr. Pd

Proceedings of a Consultation Convened by WHO Geneva 14 -16 September 2005 Selected Highlights l l … [W]hen feasible, tissues or body fluids of ruminant origin should be avoided in the preparation of biological and pharmaceutical products. When bovine materials must be used, they should be obtained from sources assessed to have negligible risk from the infectious agent of BSE. Most bovine tissues, including bovine muscle, used to manufacture biologicals, if carefully selected by taking into account the geographical distribution of BSE and collected according to guidelines, have little risk of contamination with BSE agent … No country except UK and Ireland has reported more than 1500 BSE cases. Recent findings of disease-associated proteins in muscles of sheep with scrapie (… not known to infect humans) and the recognition of BSE itself in a goat, reinforce the need for manufacturers of biologicals to maintain … precautionary safety measures …

Proceedings of a Consultation Convened by WHO Geneva 14 -16 September 2005 Selected Highlights l l l In naturally affected cattle, BSE infectivity, detected by assay in mice, has been demonstrated only in brain, spinal cord and retina … [and] in a pool of nictitating membranes but not in pools of lymph nodes or spleen. Recently infectivity was detected in some peripheral nerves and a solitary muscle, of a single case of BSE in a German cow … In cattle experimentally exposed by the oral route, BSE infectivity has been detected by mouse assay in the distal ileum through much of the disease course from six months post exposure and in the … CNS … and sensory ganglia of the peripheral nervous system from late in the incubation period. Infectivity has also been found in palatine tonsil, at a single time point … only by assay in cattle and not by the mouse assay. BSE has been experimentally transmitted via the oral route to sheep and goats. [T]here is recent evidence that one goat [and perhaps another] has been naturally infected with BSE.
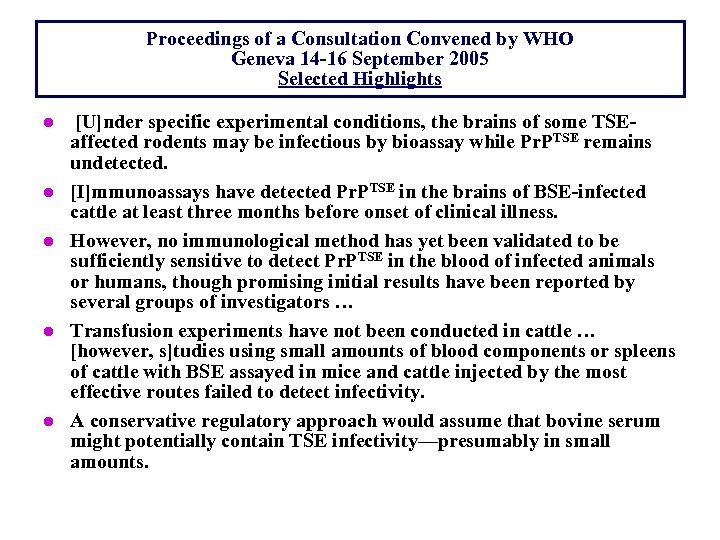
Proceedings of a Consultation Convened by WHO Geneva 14 -16 September 2005 Selected Highlights l l l [U]nder specific experimental conditions, the brains of some TSEaffected rodents may be infectious by bioassay while Pr. PTSE remains undetected. [I]mmunoassays have detected Pr. PTSE in the brains of BSE-infected cattle at least three months before onset of clinical illness. However, no immunological method has yet been validated to be sufficiently sensitive to detect Pr. PTSE in the blood of infected animals or humans, though promising initial results have been reported by several groups of investigators … Transfusion experiments have not been conducted in cattle … [however, s]tudies using small amounts of blood components or spleens of cattle with BSE assayed in mice and cattle injected by the most effective routes failed to detect infectivity. A conservative regulatory approach would assume that bovine serum might potentially contain TSE infectivity—presumably in small amounts.

Proceedings of a Consultation Convened by WHO Geneva 14 -16 September 2005 Selected Highlights l l l Ruminant blood and blood derivatives, such as fetal calf serum in cell cultures media and bovine serum albumin stabilizers … [have] not been identified as a source of infection, and properly collected fetal bovine serum has a negligible risk. However, blood of sheep with experimental BSE or natural scrapie can be infectious and, because scrapie and BSE agents behave similarly in sheep and goats, the blood of small ruminants should either be avoided in preparing biologicals or selected very carefully from sources known to be free of TSEs. There is a continuing need to ensure that all national regulatory authorities with limited resources have ready access to reliable and upto-date information when assessing TSE risks and evaluating [medicinal] product safety.

Proceedings of a Consultation Convened by WHO Geneva 14 -16 September 2005 Results of National Risk Assessments for v. CJD and Blood l UK (M Turner, P Bennett) - Labile components: 1/120, 000 transfusions may be from donors incubating v. CJD, but —if strict indications for transfusion observed—benefits clearly exceed risk - Plasma-derived products: minimal risk - Surgical/dental instruments, HCT/Ps: highly uncertain risk l l France (J-H Trouvin): Independent assessment yielded results very similar to those for UK (for both labile components and plasma-derived products) Australia (A Farrugia): FVIII has highest risk among PDs Canada (S El. Saadany): Due to uncertainties, risk communication is difficult Germany (J Löwer) - Under realistic conditions for Germany, v. CJD should not become an endemic infection maintained only by blood transfusion. - Deferring transfused donors only slightly reduces v. CJD risk in Germany. - German conclusions should not be applied to countries with different BSE/v. CJD risks. l USA (D Scott): Due to uncertainties in assumptions, risk assessments to date allow no confident predictions regarding probability of v. CJD infections and clinical illnesses in individuals exposed to various blood components and plasma derivatives.

WHO TSE Consultation Report: Annex 1 TSE Infectivity and Pr. PTSE Detected in Human and Animal Tissues, Other Materials (WHO tables converted to tile table by OA Maximova, CBER, FDA) l l l IA. High Infectivity - CNS tissues with high titers of infectivity in late stages of all TSEs - Certain tissues anatomically associated with CNS IB. Lower Infectivity - Peripheral tissues with infectivity and/or Pr. PTSE in at least one TSE IC. No Detected Infectivity Table Entries
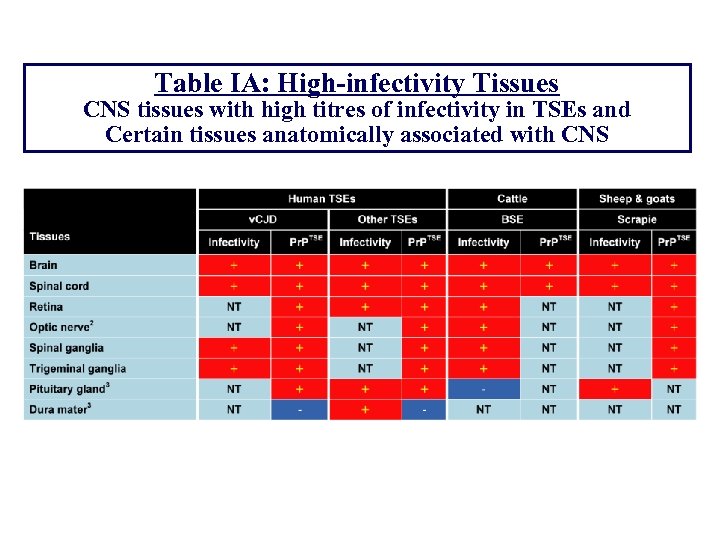
Table IA: High-infectivity Tissues CNS tissues with high titres of infectivity in TSEs and Certain tissues anatomically associated with CNS
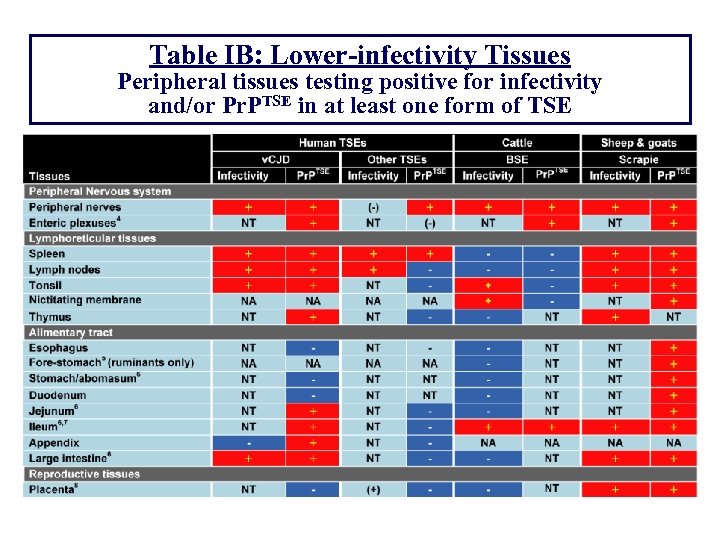
Table IB: Lower-infectivity Tissues Peripheral tissues testing positive for infectivity and/or Pr. PTSE in at least one form of TSE

Table IB: Lower-infectivity Tissues (Cont. ) Peripheral tissues testing positive for infectivity and/or Pr. PTSE in at least one form of TSE
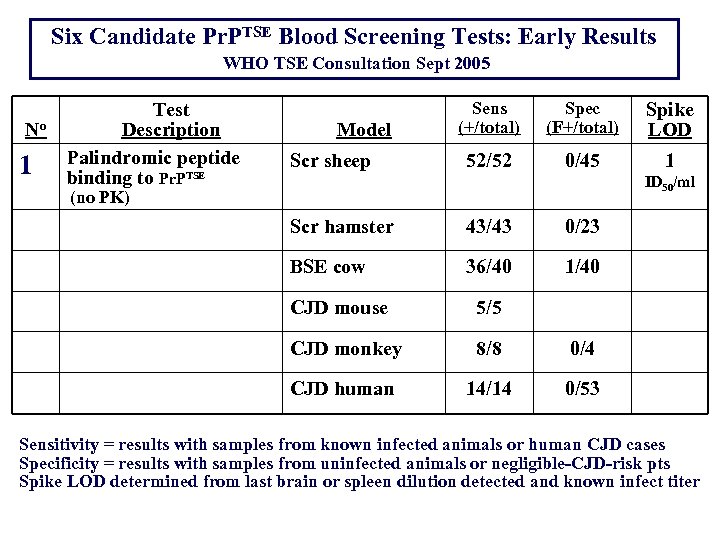
Six Candidate Pr. PTSE Blood Screening Tests: Early Results WHO TSE Consultation Sept 2005 No 1 Test Description Palindromic peptide binding to Pr. PTSE Model Scr sheep Sens (+/total) Spec (F+/total) Spike LOD 52/52 0/45 1 ID 50/ml (no PK) Scr hamster 43/43 0/23 BSE cow 36/40 1/40 CJD mouse 5/5 CJD monkey 8/8 0/4 CJD human 14/14 0/53 Sensitivity = results with samples from known infected animals or human CJD cases Specificity = results with samples from uninfected animals or negligible-CJD-risk pts Spike LOD determined from last brain or spleen dilution detected and known infect titer
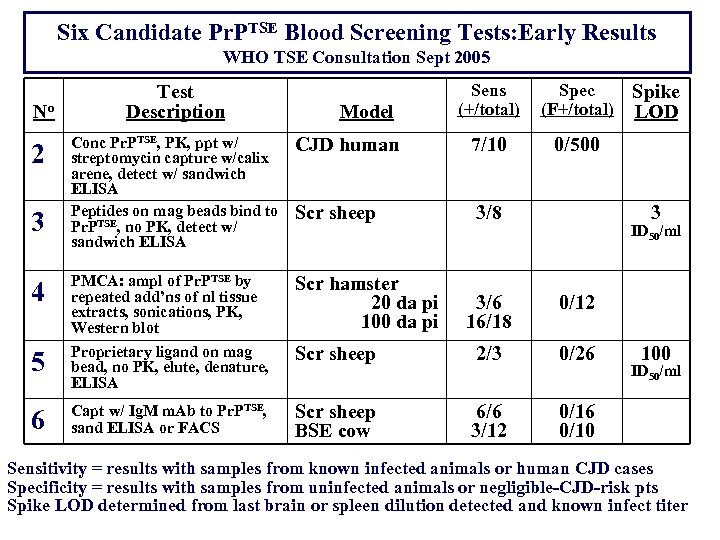
Six Candidate Pr. PTSE Blood Screening Tests: Early Results WHO TSE Consultation Sept 2005 No Test Description Model Sens (+/total) Spec (F+/total) 0/500 Conc Pr. PTSE, PK, ppt w/ streptomycin capture w/calix arene, detect w/ sandwich ELISA Peptides on mag beads bind to Pr. PTSE, no PK, detect w/ sandwich ELISA CJD human 7/10 Scr sheep 3/8 4 PMCA: ampl of Pr. PTSE by repeated add’ns of nl tissue extracts, sonications, PK, Western blot Scr hamster 20 da pi 100 da pi 5 Proprietary ligand on mag bead, no PK, elute, denature, ELISA 6 Capt w/ Ig. M m. Ab to Pr. PTSE, sand ELISA or FACS 2 3 Spike LOD 3 ID 50/ml 3/6 16/18 0/12 Scr sheep 2/3 0/26 Scr sheep BSE cow 6/6 3/12 0/16 0/10 100 ID 50/ml Sensitivity = results with samples from known infected animals or human CJD cases Specificity = results with samples from uninfected animals or negligible-CJD-risk pts Spike LOD determined from last brain or spleen dilution detected and known infect titer

Table IC: Tissues with No Detected Infectivity Tissues examined for infectivity and/or Pr. PTSE with negative results
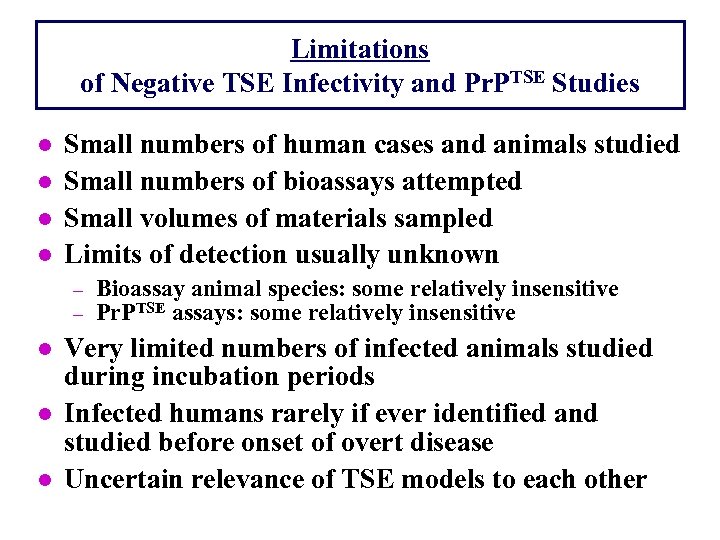
Limitations of Negative TSE Infectivity and Pr. PTSE Studies l l Small numbers of human cases and animals studied Small numbers of bioassays attempted Small volumes of materials sampled Limits of detection usually unknown - l l l Bioassay animal species: some relatively insensitive Pr. PTSE assays: some relatively insensitive Very limited numbers of infected animals studied during incubation periods Infected humans rarely if ever identified and studied before onset of overt disease Uncertain relevance of TSE models to each other
WHO Guidelines on Tissue Infectivity Distribution in TSEs Partial Credits Consultation Chairman WG van Aken, Netherlands Draft Report DM Asher, USA J-P Deslys, France E Griffiths, Canada A Padilla, WHO R Bradley UK M Pocchiari, Italy RG Rohwer, USA J-H Trouvin, France GAH Wells, UK RG Will, UK Annex 1 Working Group P Brown, USA (chairman) R Bradley, GAH Wells, UK (animal TSEs)
2e03a44cf55991ae8f784cd81aea61f0.ppt