Whereas the term covalent implies sharing of electrons

37754-9-bonding-packing2.ppt
- Количество слайдов: 30
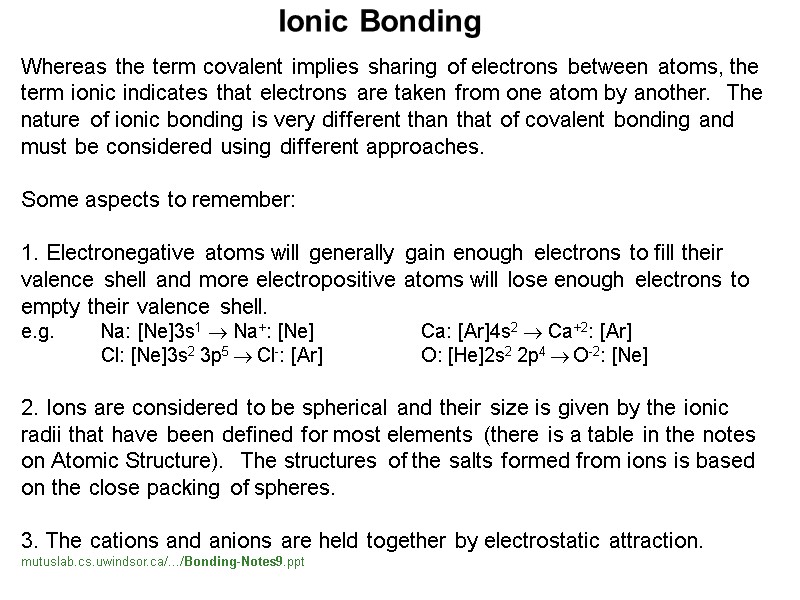
 Whereas the term covalent implies sharing of electrons between atoms, the term ionic indicates that electrons are taken from one atom by another. The nature of ionic bonding is very different than that of covalent bonding and must be considered using different approaches. Some aspects to remember: 1. Electronegative atoms will generally gain enough electrons to fill their valence shell and more electropositive atoms will lose enough electrons to empty their valence shell. e.g. Na: [Ne]3s1 Na+: [Ne] Ca: [Ar]4s2 Ca+2: [Ar] Cl: [Ne]3s2 3p5 Cl-: [Ar] O: [He]2s2 2p4 O-2: [Ne] 2. Ions are considered to be spherical and their size is given by the ionic radii that have been defined for most elements (there is a table in the notes on Atomic Structure). The structures of the salts formed from ions is based on the close packing of spheres. 3. The cations and anions are held together by electrostatic attraction. mutuslab.cs.uwindsor.ca/.../Bonding-Notes9.ppt Ionic Bonding
Whereas the term covalent implies sharing of electrons between atoms, the term ionic indicates that electrons are taken from one atom by another. The nature of ionic bonding is very different than that of covalent bonding and must be considered using different approaches. Some aspects to remember: 1. Electronegative atoms will generally gain enough electrons to fill their valence shell and more electropositive atoms will lose enough electrons to empty their valence shell. e.g. Na: [Ne]3s1 Na+: [Ne] Ca: [Ar]4s2 Ca+2: [Ar] Cl: [Ne]3s2 3p5 Cl-: [Ar] O: [He]2s2 2p4 O-2: [Ne] 2. Ions are considered to be spherical and their size is given by the ionic radii that have been defined for most elements (there is a table in the notes on Atomic Structure). The structures of the salts formed from ions is based on the close packing of spheres. 3. The cations and anions are held together by electrostatic attraction. mutuslab.cs.uwindsor.ca/.../Bonding-Notes9.ppt Ionic Bonding
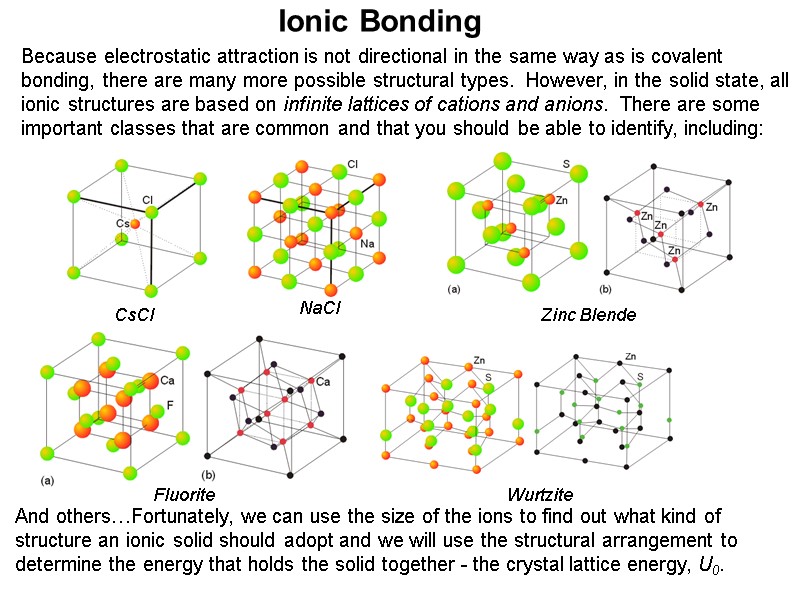
 Ionic Bonding Because electrostatic attraction is not directional in the same way as is covalent bonding, there are many more possible structural types. However, in the solid state, all ionic structures are based on infinite lattices of cations and anions. There are some important classes that are common and that you should be able to identify, including: And others…Fortunately, we can use the size of the ions to find out what kind of structure an ionic solid should adopt and we will use the structural arrangement to determine the energy that holds the solid together - the crystal lattice energy, U0. CsCl NaCl Zinc Blende Fluorite Wurtzite
Ionic Bonding Because electrostatic attraction is not directional in the same way as is covalent bonding, there are many more possible structural types. However, in the solid state, all ionic structures are based on infinite lattices of cations and anions. There are some important classes that are common and that you should be able to identify, including: And others…Fortunately, we can use the size of the ions to find out what kind of structure an ionic solid should adopt and we will use the structural arrangement to determine the energy that holds the solid together - the crystal lattice energy, U0. CsCl NaCl Zinc Blende Fluorite Wurtzite
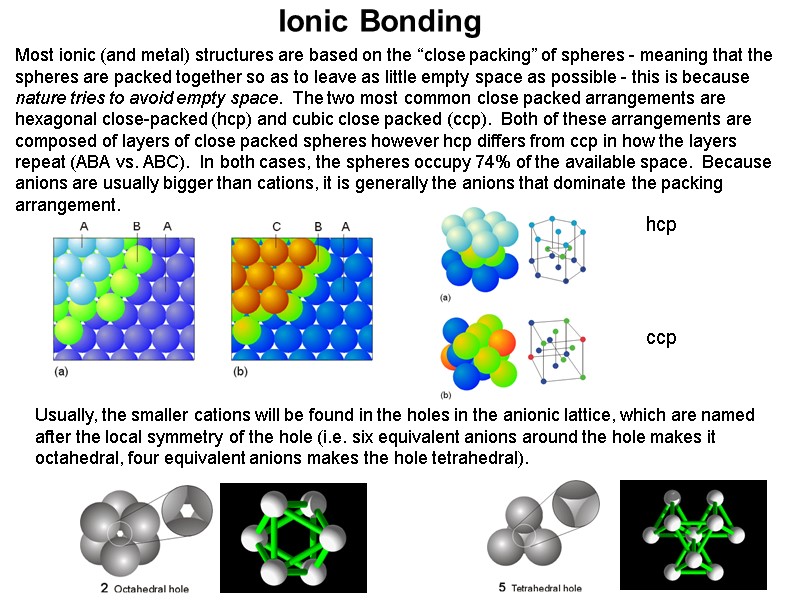
 Ionic Bonding Usually, the smaller cations will be found in the holes in the anionic lattice, which are named after the local symmetry of the hole (i.e. six equivalent anions around the hole makes it octahedral, four equivalent anions makes the hole tetrahedral). hcp ccp Most ionic (and metal) structures are based on the “close packing” of spheres - meaning that the spheres are packed together so as to leave as little empty space as possible - this is because nature tries to avoid empty space. The two most common close packed arrangements are hexagonal close-packed (hcp) and cubic close packed (ccp). Both of these arrangements are composed of layers of close packed spheres however hcp differs from ccp in how the layers repeat (ABA vs. ABC). In both cases, the spheres occupy 74% of the available space. Because anions are usually bigger than cations, it is generally the anions that dominate the packing arrangement.
Ionic Bonding Usually, the smaller cations will be found in the holes in the anionic lattice, which are named after the local symmetry of the hole (i.e. six equivalent anions around the hole makes it octahedral, four equivalent anions makes the hole tetrahedral). hcp ccp Most ionic (and metal) structures are based on the “close packing” of spheres - meaning that the spheres are packed together so as to leave as little empty space as possible - this is because nature tries to avoid empty space. The two most common close packed arrangements are hexagonal close-packed (hcp) and cubic close packed (ccp). Both of these arrangements are composed of layers of close packed spheres however hcp differs from ccp in how the layers repeat (ABA vs. ABC). In both cases, the spheres occupy 74% of the available space. Because anions are usually bigger than cations, it is generally the anions that dominate the packing arrangement.
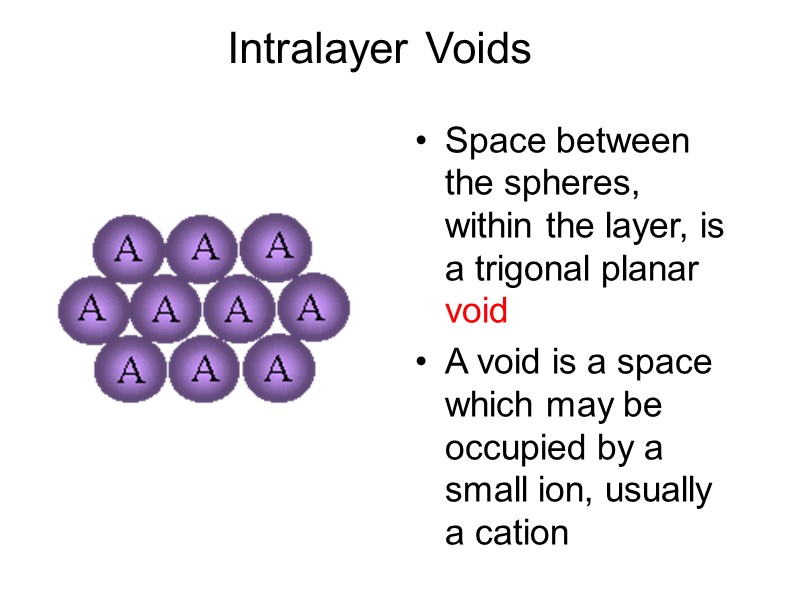
 Intralayer Voids Space between the spheres, within the layer, is a trigonal planar void A void is a space which may be occupied by a small ion, usually a cation
Intralayer Voids Space between the spheres, within the layer, is a trigonal planar void A void is a space which may be occupied by a small ion, usually a cation
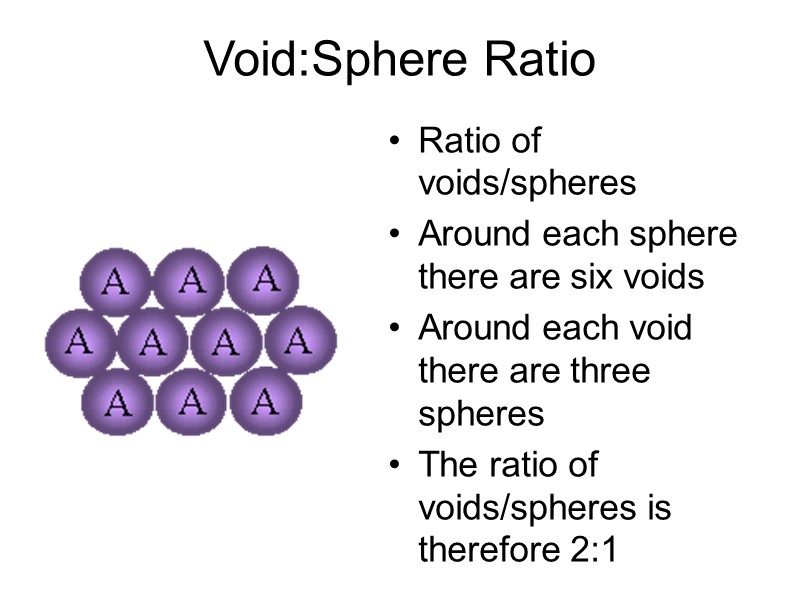
 Void:Sphere Ratio Ratio of voids/spheres Around each sphere there are six voids Around each void there are three spheres The ratio of voids/spheres is therefore 2:1
Void:Sphere Ratio Ratio of voids/spheres Around each sphere there are six voids Around each void there are three spheres The ratio of voids/spheres is therefore 2:1
 Interlayer Voids Both types of packing have empty places (voids) between layers There are two types of voids Each has a different CN
Interlayer Voids Both types of packing have empty places (voids) between layers There are two types of voids Each has a different CN
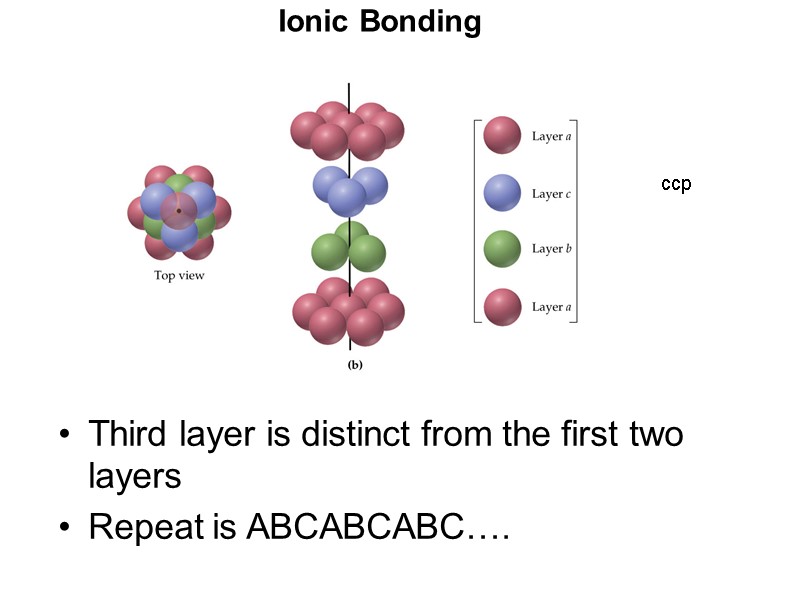
 Ionic Bonding ccp Third layer is distinct from the first two layers Repeat is ABCABCABC….
Ionic Bonding ccp Third layer is distinct from the first two layers Repeat is ABCABCABC….
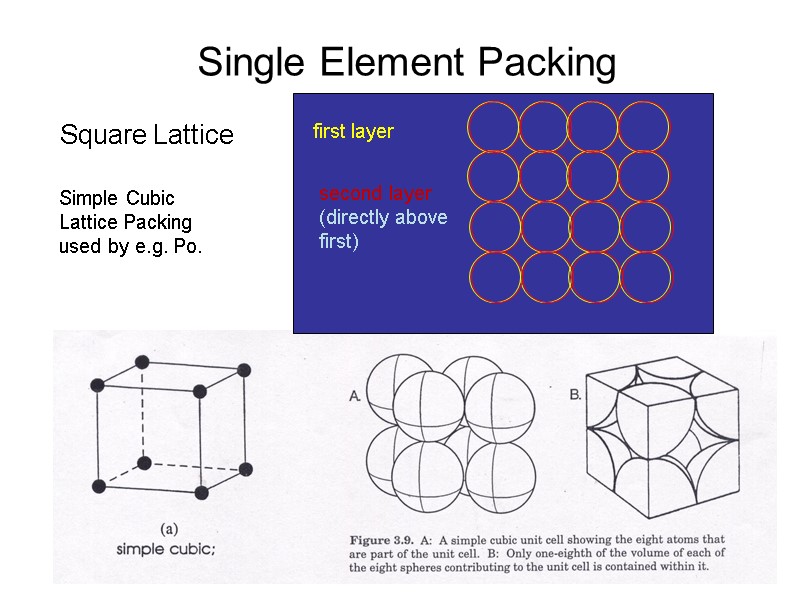
 Single Element Packing Square Lattice Simple Cubic Lattice Packing used by e.g. Po.
Single Element Packing Square Lattice Simple Cubic Lattice Packing used by e.g. Po.
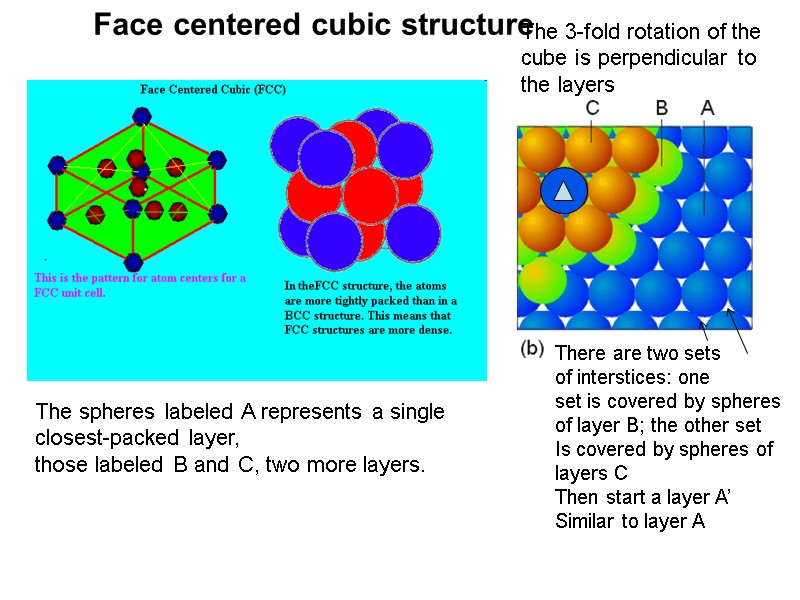
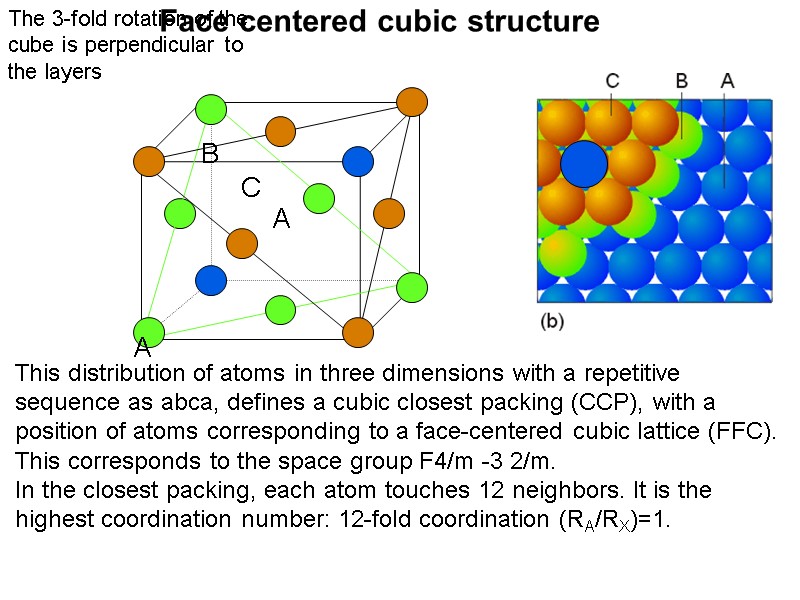
 Face centered cubic structure The spheres labeled A represents a single closest-packed layer, those labeled B and C, two more layers. There are two sets of interstices: one set is covered by spheres of layer B; the other set Is covered by spheres of layers C Then start a layer A’ Similar to layer A The 3-fold rotation of the cube is perpendicular to the layers
Face centered cubic structure The spheres labeled A represents a single closest-packed layer, those labeled B and C, two more layers. There are two sets of interstices: one set is covered by spheres of layer B; the other set Is covered by spheres of layers C Then start a layer A’ Similar to layer A The 3-fold rotation of the cube is perpendicular to the layers
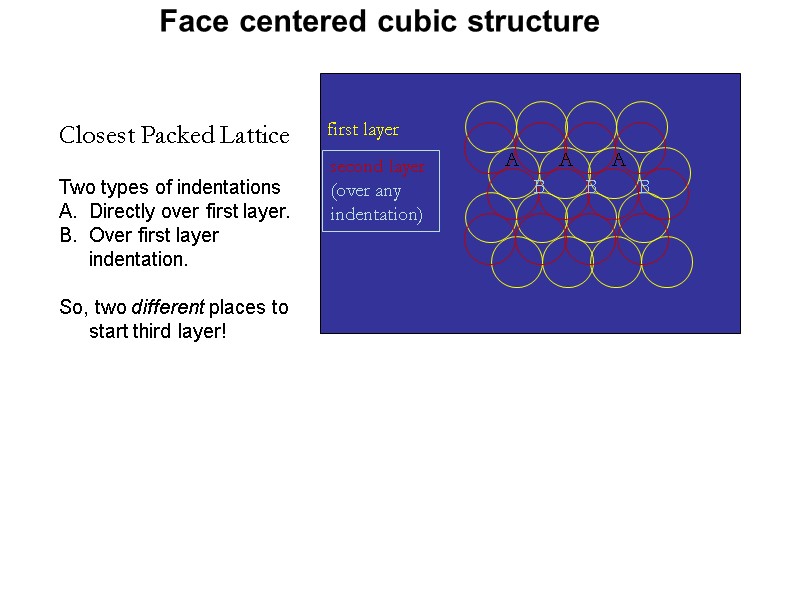
 Closest Packed Lattice first layer second layer (over any indentation) Two types of indentations Directly over first layer. Over first layer indentation. So, two different places to start third layer! Face centered cubic structure
Closest Packed Lattice first layer second layer (over any indentation) Two types of indentations Directly over first layer. Over first layer indentation. So, two different places to start third layer! Face centered cubic structure
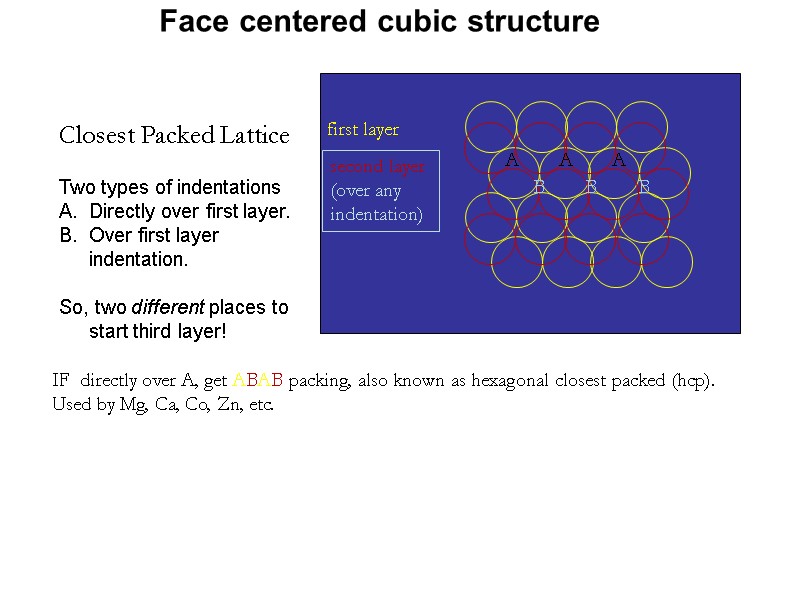
 Closest Packed Lattice first layer second layer (over any indentation) Two types of indentations Directly over first layer. Over first layer indentation. So, two different places to start third layer! IF directly over A, get ABAB packing, also known as hexagonal closest packed (hcp). Used by Mg, Ca, Co, Zn, etc. Face centered cubic structure
Closest Packed Lattice first layer second layer (over any indentation) Two types of indentations Directly over first layer. Over first layer indentation. So, two different places to start third layer! IF directly over A, get ABAB packing, also known as hexagonal closest packed (hcp). Used by Mg, Ca, Co, Zn, etc. Face centered cubic structure
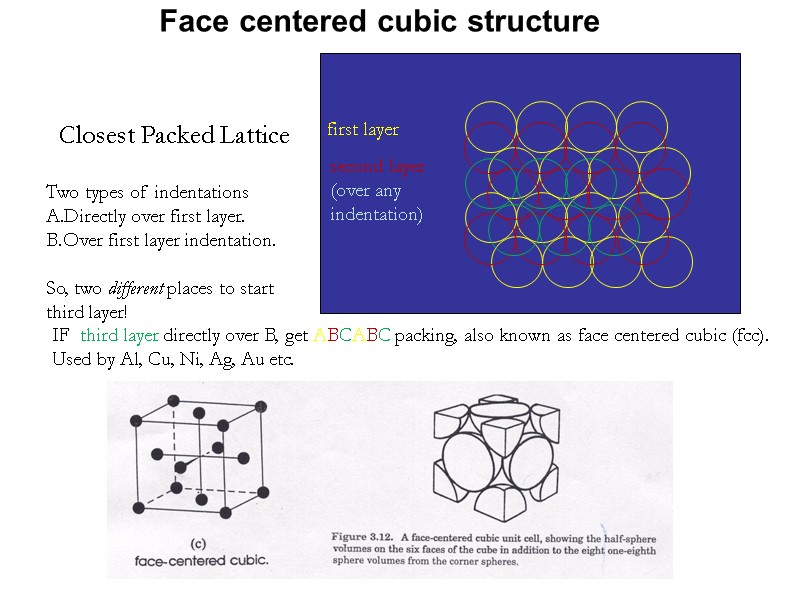
 Closest Packed Lattice first layer second layer (over any indentation) Two types of indentations Directly over first layer. Over first layer indentation. So, two different places to start third layer! IF third layer directly over B, get ABCABC packing, also known as face centered cubic (fcc). Used by Al, Cu, Ni, Ag, Au etc. Face centered cubic structure
Closest Packed Lattice first layer second layer (over any indentation) Two types of indentations Directly over first layer. Over first layer indentation. So, two different places to start third layer! IF third layer directly over B, get ABCABC packing, also known as face centered cubic (fcc). Used by Al, Cu, Ni, Ag, Au etc. Face centered cubic structure
 The 3-fold rotation of the cube is perpendicular to the layers A A C B This distribution of atoms in three dimensions with a repetitive sequence as abca, defines a cubic closest packing (CCP), with a position of atoms corresponding to a face-centered cubic lattice (FFC). This corresponds to the space group F4/m -3 2/m. In the closest packing, each atom touches 12 neighbors. It is the highest coordination number: 12-fold coordination (RA/RX)=1. Face centered cubic structure
The 3-fold rotation of the cube is perpendicular to the layers A A C B This distribution of atoms in three dimensions with a repetitive sequence as abca, defines a cubic closest packing (CCP), with a position of atoms corresponding to a face-centered cubic lattice (FFC). This corresponds to the space group F4/m -3 2/m. In the closest packing, each atom touches 12 neighbors. It is the highest coordination number: 12-fold coordination (RA/RX)=1. Face centered cubic structure
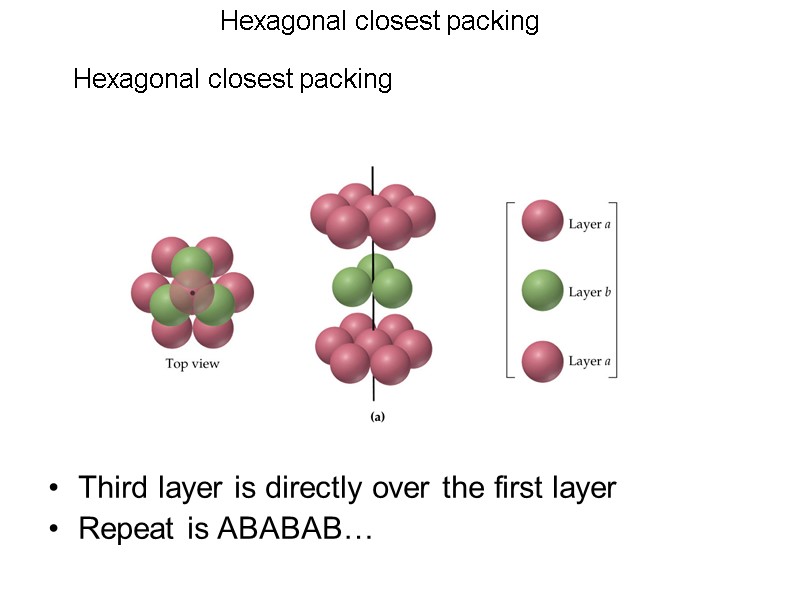
 Hexagonal closest packing Hexagonal closest packing Third layer is directly over the first layer Repeat is ABABAB…
Hexagonal closest packing Hexagonal closest packing Third layer is directly over the first layer Repeat is ABABAB…
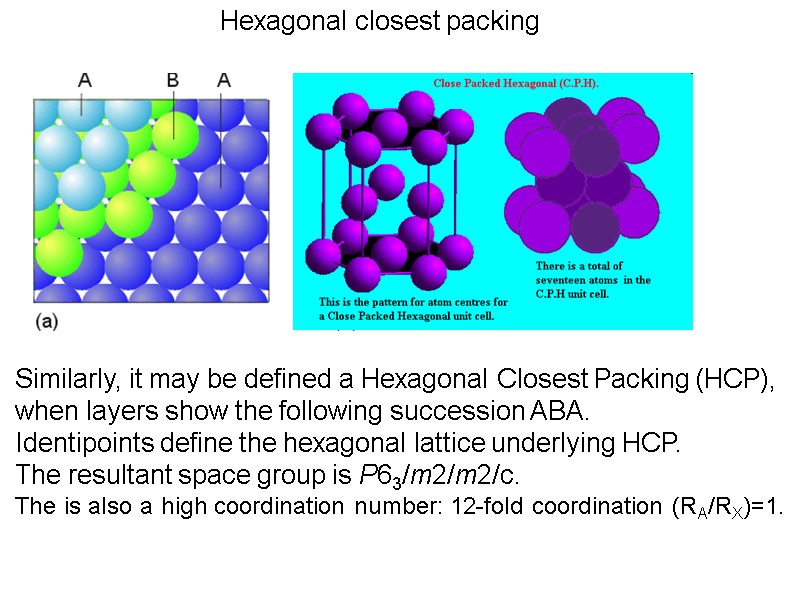
 Hexagonal closest packing Similarly, it may be defined a Hexagonal Closest Packing (HCP), when layers show the following succession ABA. Identipoints define the hexagonal lattice underlying HCP. The resultant space group is P63/m2/m2/c. The is also a high coordination number: 12-fold coordination (RA/RX)=1.
Hexagonal closest packing Similarly, it may be defined a Hexagonal Closest Packing (HCP), when layers show the following succession ABA. Identipoints define the hexagonal lattice underlying HCP. The resultant space group is P63/m2/m2/c. The is also a high coordination number: 12-fold coordination (RA/RX)=1.
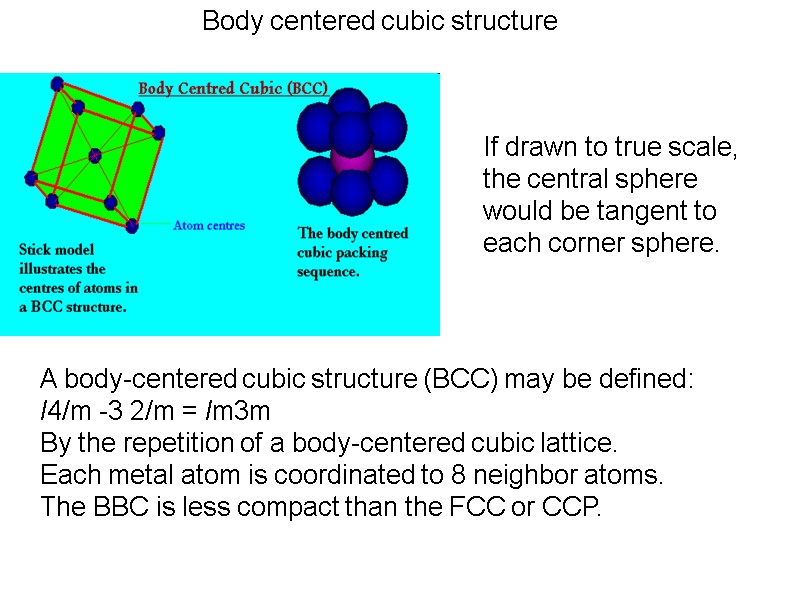
 Body centered cubic structure A body-centered cubic structure (BCC) may be defined: I4/m -3 2/m = Im3m By the repetition of a body-centered cubic lattice. Each metal atom is coordinated to 8 neighbor atoms. The BBC is less compact than the FCC or CCP. If drawn to true scale, the central sphere would be tangent to each corner sphere.
Body centered cubic structure A body-centered cubic structure (BCC) may be defined: I4/m -3 2/m = Im3m By the repetition of a body-centered cubic lattice. Each metal atom is coordinated to 8 neighbor atoms. The BBC is less compact than the FCC or CCP. If drawn to true scale, the central sphere would be tangent to each corner sphere.
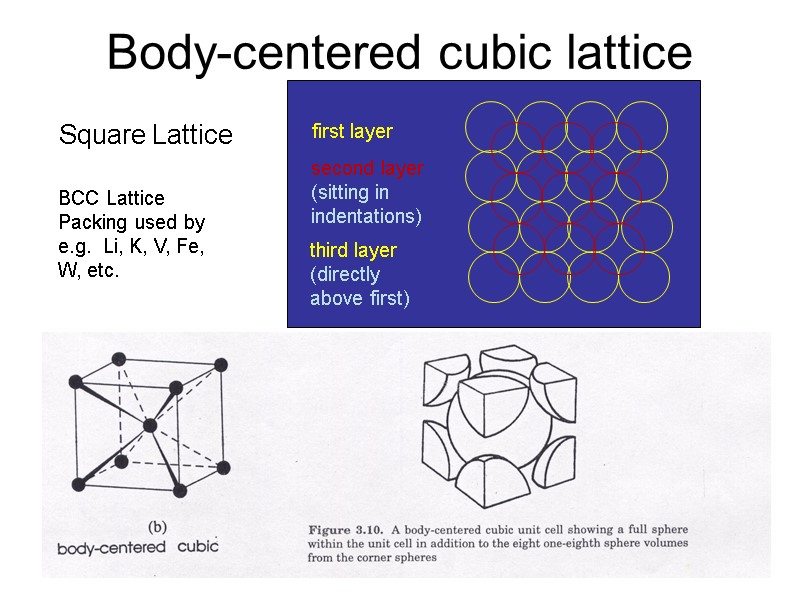
 Body-centered cubic lattice Square Lattice first layer second layer (sitting in indentations) third layer (directly above first) BCC Lattice Packing used by e.g. Li, K, V, Fe, W, etc.
Body-centered cubic lattice Square Lattice first layer second layer (sitting in indentations) third layer (directly above first) BCC Lattice Packing used by e.g. Li, K, V, Fe, W, etc.
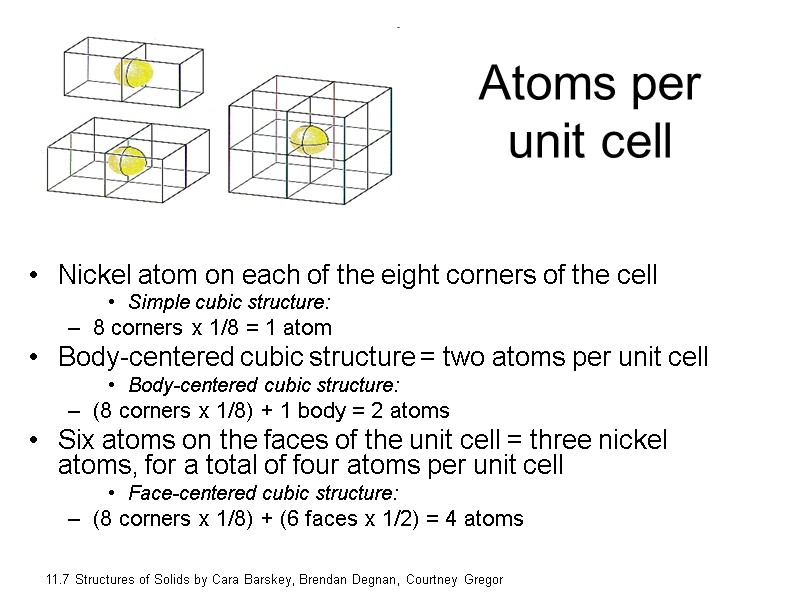
 Atoms per unit cell Nickel atom on each of the eight corners of the cell Simple cubic structure: 8 corners x 1/8 = 1 atom Body-centered cubic structure = two atoms per unit cell Body-centered cubic structure: (8 corners x 1/8) + 1 body = 2 atoms Six atoms on the faces of the unit cell = three nickel atoms, for a total of four atoms per unit cell Face-centered cubic structure: (8 corners x 1/8) + (6 faces x 1/2) = 4 atoms 11.7 Structures of Solids by Cara Barskey, Brendan Degnan, Courtney Gregor
Atoms per unit cell Nickel atom on each of the eight corners of the cell Simple cubic structure: 8 corners x 1/8 = 1 atom Body-centered cubic structure = two atoms per unit cell Body-centered cubic structure: (8 corners x 1/8) + 1 body = 2 atoms Six atoms on the faces of the unit cell = three nickel atoms, for a total of four atoms per unit cell Face-centered cubic structure: (8 corners x 1/8) + (6 faces x 1/2) = 4 atoms 11.7 Structures of Solids by Cara Barskey, Brendan Degnan, Courtney Gregor
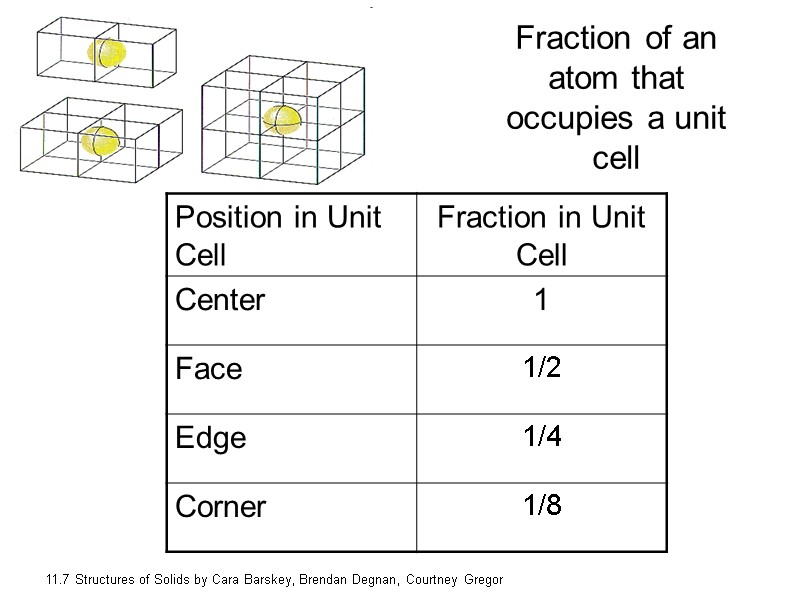
 Fraction of an atom that occupies a unit cell 11.7 Structures of Solids by Cara Barskey, Brendan Degnan, Courtney Gregor
Fraction of an atom that occupies a unit cell 11.7 Structures of Solids by Cara Barskey, Brendan Degnan, Courtney Gregor
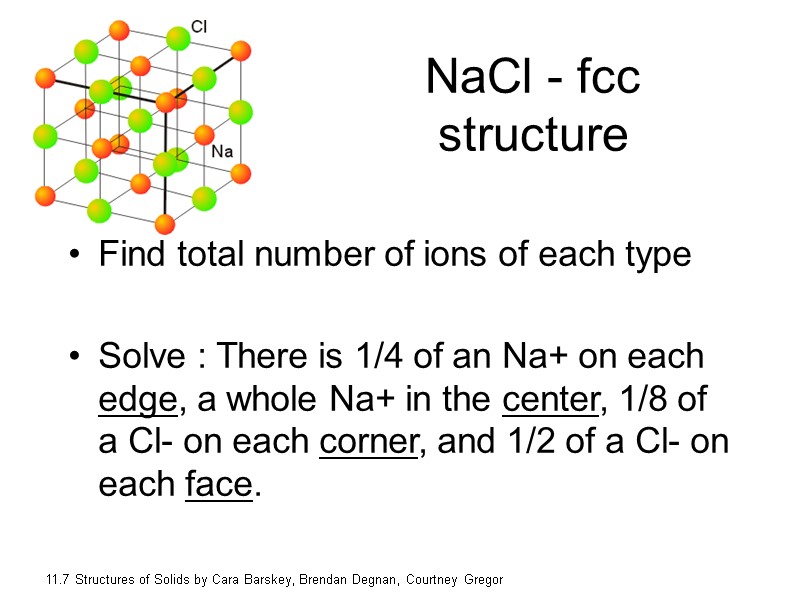
 NaCl - fcc structure Find total number of ions of each type Solve : There is 1/4 of an Na+ on each edge, a whole Na+ in the center, 1/8 of a Cl- on each corner, and 1/2 of a Cl- on each face. 11.7 Structures of Solids by Cara Barskey, Brendan Degnan, Courtney Gregor
NaCl - fcc structure Find total number of ions of each type Solve : There is 1/4 of an Na+ on each edge, a whole Na+ in the center, 1/8 of a Cl- on each corner, and 1/2 of a Cl- on each face. 11.7 Structures of Solids by Cara Barskey, Brendan Degnan, Courtney Gregor
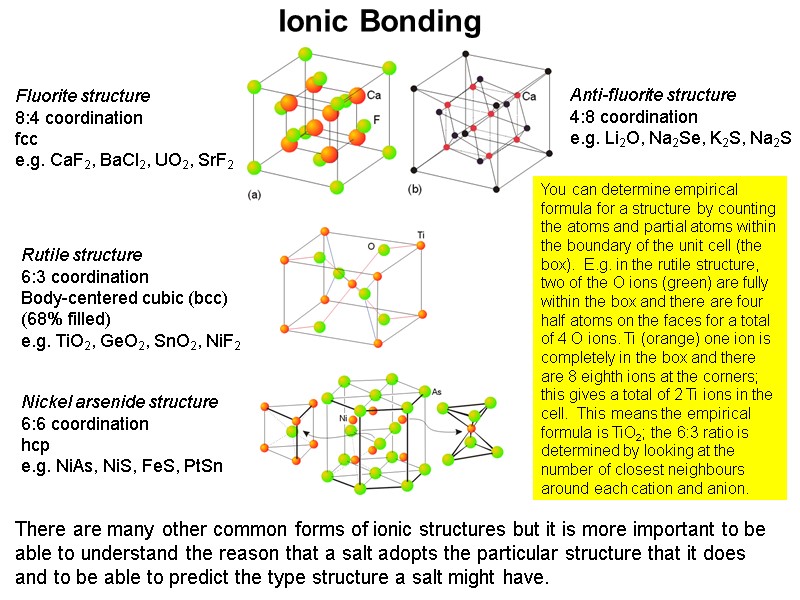
 Ionic Bonding Fluorite structure 8:4 coordination fcc e.g. CaF2, BaCl2, UO2, SrF2 Anti-fluorite structure 4:8 coordination e.g. Li2O, Na2Se, K2S, Na2S Rutile structure 6:3 coordination Body-centered cubic (bcc) (68% filled) e.g. TiO2, GeO2, SnO2, NiF2 There are many other common forms of ionic structures but it is more important to be able to understand the reason that a salt adopts the particular structure that it does and to be able to predict the type structure a salt might have. Nickel arsenide structure 6:6 coordination hcp e.g. NiAs, NiS, FeS, PtSn You can determine empirical formula for a structure by counting the atoms and partial atoms within the boundary of the unit cell (the box). E.g. in the rutile structure, two of the O ions (green) are fully within the box and there are four half atoms on the faces for a total of 4 O ions. Ti (orange) one ion is completely in the box and there are 8 eighth ions at the corners; this gives a total of 2 Ti ions in the cell. This means the empirical formula is TiO2; the 6:3 ratio is determined by looking at the number of closest neighbours around each cation and anion.
Ionic Bonding Fluorite structure 8:4 coordination fcc e.g. CaF2, BaCl2, UO2, SrF2 Anti-fluorite structure 4:8 coordination e.g. Li2O, Na2Se, K2S, Na2S Rutile structure 6:3 coordination Body-centered cubic (bcc) (68% filled) e.g. TiO2, GeO2, SnO2, NiF2 There are many other common forms of ionic structures but it is more important to be able to understand the reason that a salt adopts the particular structure that it does and to be able to predict the type structure a salt might have. Nickel arsenide structure 6:6 coordination hcp e.g. NiAs, NiS, FeS, PtSn You can determine empirical formula for a structure by counting the atoms and partial atoms within the boundary of the unit cell (the box). E.g. in the rutile structure, two of the O ions (green) are fully within the box and there are four half atoms on the faces for a total of 4 O ions. Ti (orange) one ion is completely in the box and there are 8 eighth ions at the corners; this gives a total of 2 Ti ions in the cell. This means the empirical formula is TiO2; the 6:3 ratio is determined by looking at the number of closest neighbours around each cation and anion.
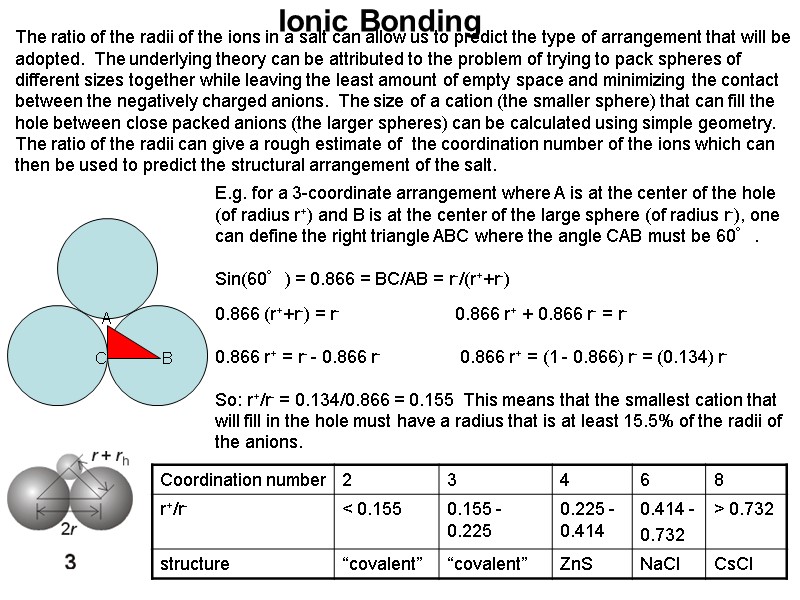
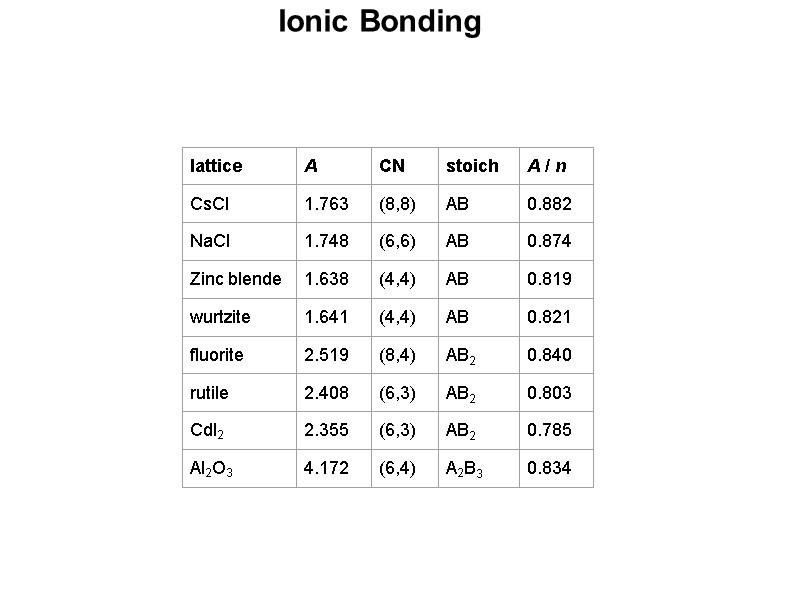
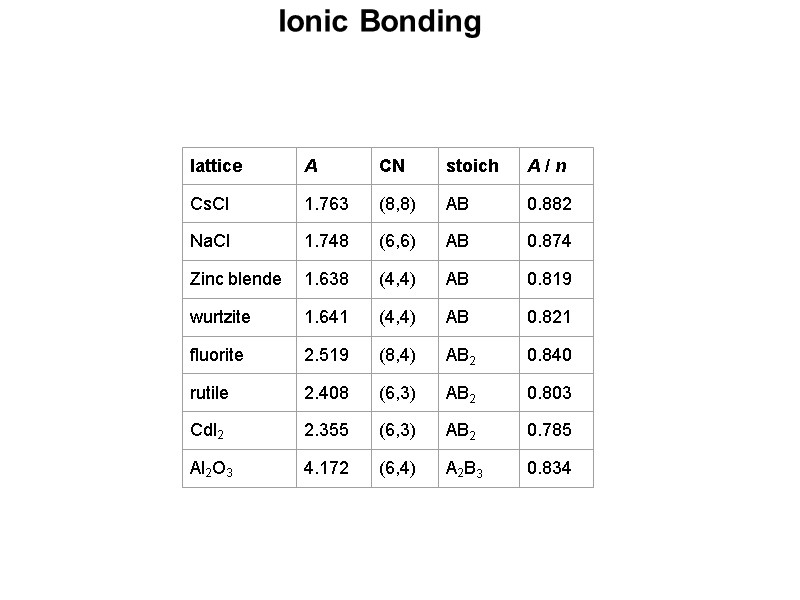
 Ionic Bonding The ratio of the radii of the ions in a salt can allow us to predict the type of arrangement that will be adopted. The underlying theory can be attributed to the problem of trying to pack spheres of different sizes together while leaving the least amount of empty space and minimizing the contact between the negatively charged anions. The size of a cation (the smaller sphere) that can fill the hole between close packed anions (the larger spheres) can be calculated using simple geometry. The ratio of the radii can give a rough estimate of the coordination number of the ions which can then be used to predict the structural arrangement of the salt. E.g. for a 3-coordinate arrangement where A is at the center of the hole (of radius r+) and B is at the center of the large sphere (of radius r-), one can define the right triangle ABC where the angle CAB must be 60°. Sin(60°) = 0.866 = BC/AB = r-/(r++r-) 0.866 (r++r-) = r- 0.866 r+ + 0.866 r- = r- 0.866 r+ = r- - 0.866 r- 0.866 r+ = (1 - 0.866) r- = (0.134) r- So: r+/r- = 0.134/0.866 = 0.155 This means that the smallest cation that will fill in the hole must have a radius that is at least 15.5% of the radii of the anions.
Ionic Bonding The ratio of the radii of the ions in a salt can allow us to predict the type of arrangement that will be adopted. The underlying theory can be attributed to the problem of trying to pack spheres of different sizes together while leaving the least amount of empty space and minimizing the contact between the negatively charged anions. The size of a cation (the smaller sphere) that can fill the hole between close packed anions (the larger spheres) can be calculated using simple geometry. The ratio of the radii can give a rough estimate of the coordination number of the ions which can then be used to predict the structural arrangement of the salt. E.g. for a 3-coordinate arrangement where A is at the center of the hole (of radius r+) and B is at the center of the large sphere (of radius r-), one can define the right triangle ABC where the angle CAB must be 60°. Sin(60°) = 0.866 = BC/AB = r-/(r++r-) 0.866 (r++r-) = r- 0.866 r+ + 0.866 r- = r- 0.866 r+ = r- - 0.866 r- 0.866 r+ = (1 - 0.866) r- = (0.134) r- So: r+/r- = 0.134/0.866 = 0.155 This means that the smallest cation that will fill in the hole must have a radius that is at least 15.5% of the radii of the anions.
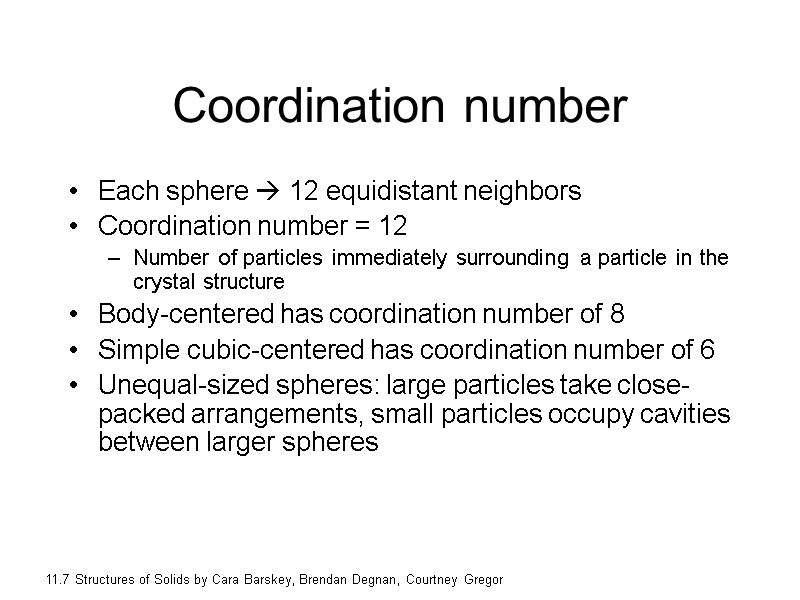
 Coordination number Each sphere 12 equidistant neighbors Coordination number = 12 Number of particles immediately surrounding a particle in the crystal structure Body-centered has coordination number of 8 Simple cubic-centered has coordination number of 6 Unequal-sized spheres: large particles take close-packed arrangements, small particles occupy cavities between larger spheres 11.7 Structures of Solids by Cara Barskey, Brendan Degnan, Courtney Gregor
Coordination number Each sphere 12 equidistant neighbors Coordination number = 12 Number of particles immediately surrounding a particle in the crystal structure Body-centered has coordination number of 8 Simple cubic-centered has coordination number of 6 Unequal-sized spheres: large particles take close-packed arrangements, small particles occupy cavities between larger spheres 11.7 Structures of Solids by Cara Barskey, Brendan Degnan, Courtney Gregor
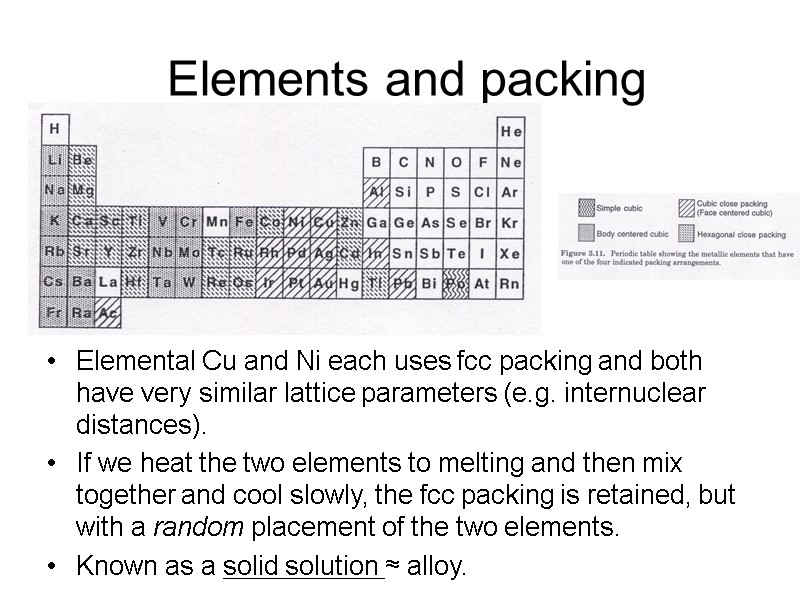
 Elements and packing Elemental Cu and Ni each uses fcc packing and both have very similar lattice parameters (e.g. internuclear distances). If we heat the two elements to melting and then mix together and cool slowly, the fcc packing is retained, but with a random placement of the two elements. Known as a solid solution ≈ alloy.
Elements and packing Elemental Cu and Ni each uses fcc packing and both have very similar lattice parameters (e.g. internuclear distances). If we heat the two elements to melting and then mix together and cool slowly, the fcc packing is retained, but with a random placement of the two elements. Known as a solid solution ≈ alloy.
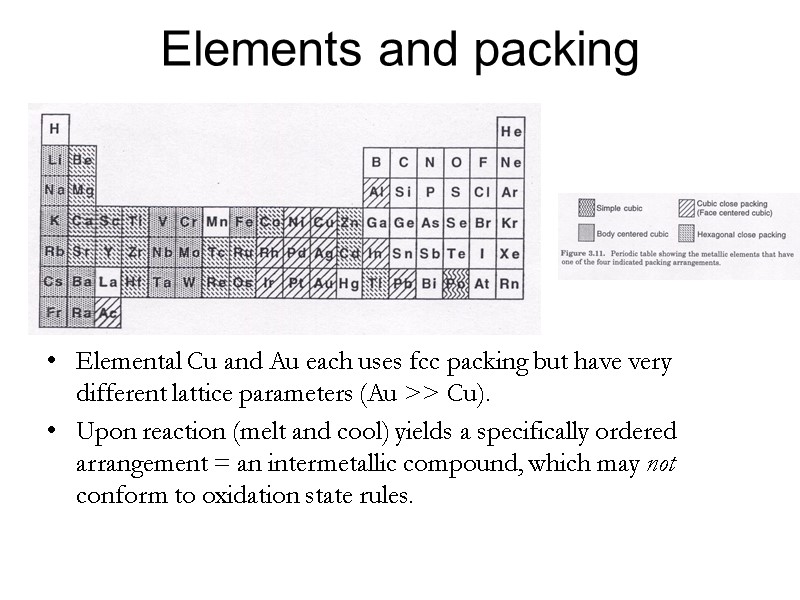
 Elements and packing Elemental Cu and Au each uses fcc packing but have very different lattice parameters (Au >> Cu). Upon reaction (melt and cool) yields a specifically ordered arrangement = an intermetallic compound, which may not conform to oxidation state rules.
Elements and packing Elemental Cu and Au each uses fcc packing but have very different lattice parameters (Au >> Cu). Upon reaction (melt and cool) yields a specifically ordered arrangement = an intermetallic compound, which may not conform to oxidation state rules.
 Ionic Bonding
Ionic Bonding
 Ionic Bonding
Ionic Bonding
 Binary Compounds (MX) Which elements do we need to be concerned about? ignore noble gases; no known extended structures. ignore radioactive elements. this leaves about 80 elements of possible interest. mathematically, this results in ~3,160 possible binary elemental combinations (not taking into account various stoichiometries, AB, AB2, A2.3B3, etcetera). 90% of known binary compounds have simple stoichiometries: MX, MX2, MX3, M3X5, etcetera. For MX there are 20 common structure types (we’ll look at 3). For MX2 there are 26 common structure types (we’ll look at 2). Each of these structural types can be thought of as starting from single element packing lattices.
Binary Compounds (MX) Which elements do we need to be concerned about? ignore noble gases; no known extended structures. ignore radioactive elements. this leaves about 80 elements of possible interest. mathematically, this results in ~3,160 possible binary elemental combinations (not taking into account various stoichiometries, AB, AB2, A2.3B3, etcetera). 90% of known binary compounds have simple stoichiometries: MX, MX2, MX3, M3X5, etcetera. For MX there are 20 common structure types (we’ll look at 3). For MX2 there are 26 common structure types (we’ll look at 2). Each of these structural types can be thought of as starting from single element packing lattices.
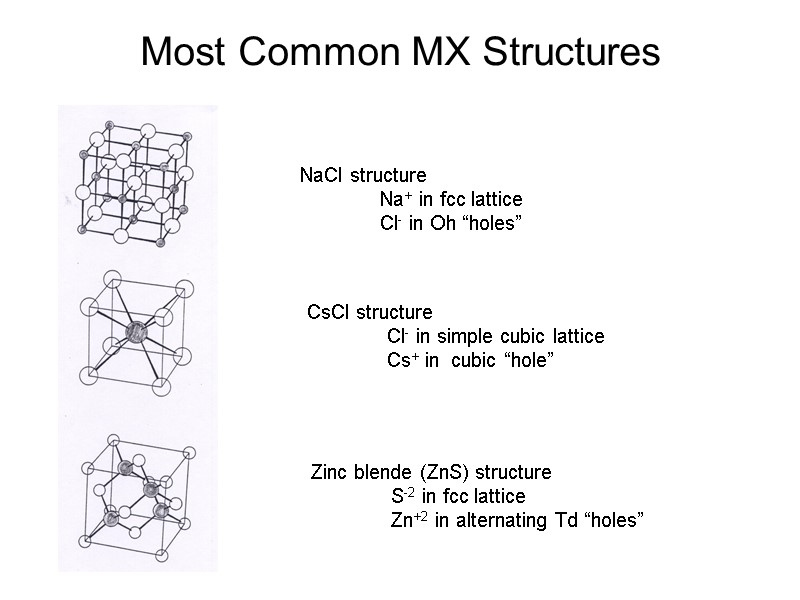
 Most Common MX Structures NaCl structure Na+ in fcc lattice Cl- in Oh “holes” CsCl structure Cl- in simple cubic lattice Cs+ in cubic “hole” Zinc blende (ZnS) structure S-2 in fcc lattice Zn+2 in alternating Td “holes”
Most Common MX Structures NaCl structure Na+ in fcc lattice Cl- in Oh “holes” CsCl structure Cl- in simple cubic lattice Cs+ in cubic “hole” Zinc blende (ZnS) structure S-2 in fcc lattice Zn+2 in alternating Td “holes”
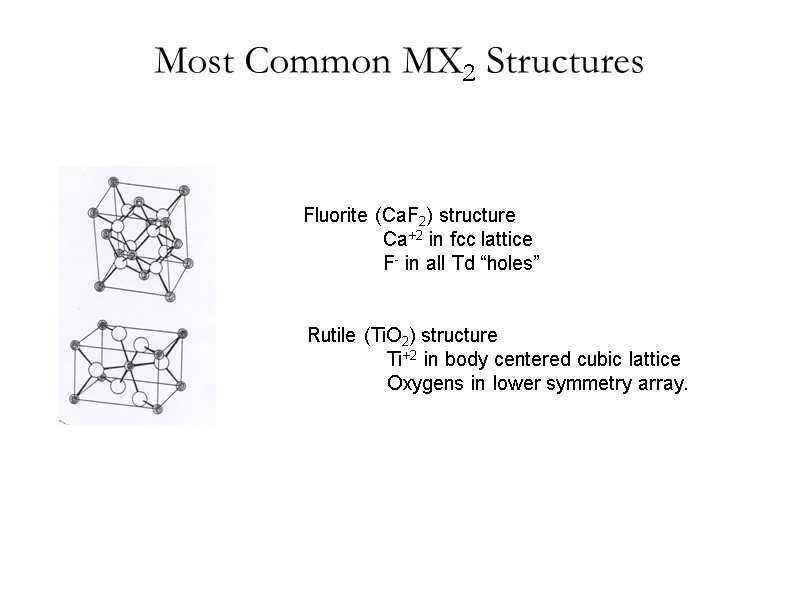
 Most Common MX2 Structures Rutile (TiO2) structure Ti+2 in body centered cubic lattice Oxygens in lower symmetry array. Fluorite (CaF2) structure Ca+2 in fcc lattice F- in all Td “holes”
Most Common MX2 Structures Rutile (TiO2) structure Ti+2 in body centered cubic lattice Oxygens in lower symmetry array. Fluorite (CaF2) structure Ca+2 in fcc lattice F- in all Td “holes”

