0052eaa45466a844a954ff7df028ac94.ppt
- Количество слайдов: 20

Where do Japan & USA fit into Global Trials Efforts? Keith Dawkins MD FRCP FACC FSCAI Chief Medical Officer Senior Vice President Boston Scientific Corporation

Conflict of Interest Boston Scientific Corporation Employee Stockholder

Why Undertake a Global Device Study? Assess device outcomes in different geographies, populations, ethnicities Accelerate diverse regulatory pathways Early granting of country-specific reimbursement Marketing opportunities etc
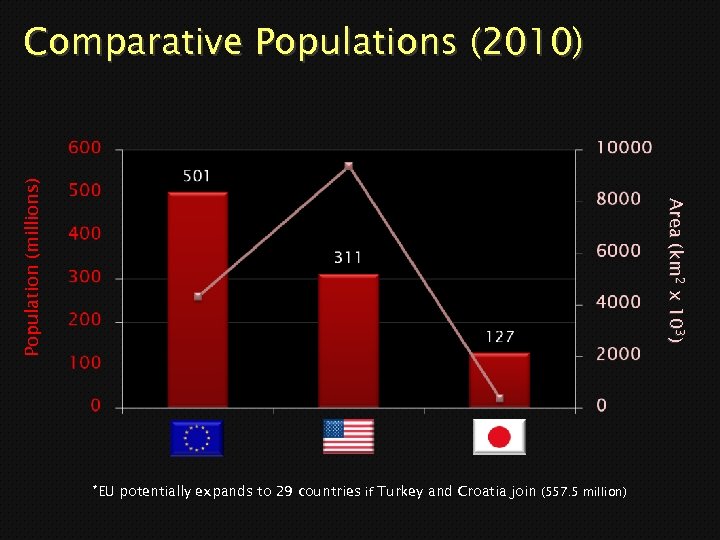
Area (km 2 x 103) Area (km x 10 ) Population (millions) Comparative Populations (2010) *EU potentially expands to 29 countries if Turkey and Croatia join (557. 5 million)
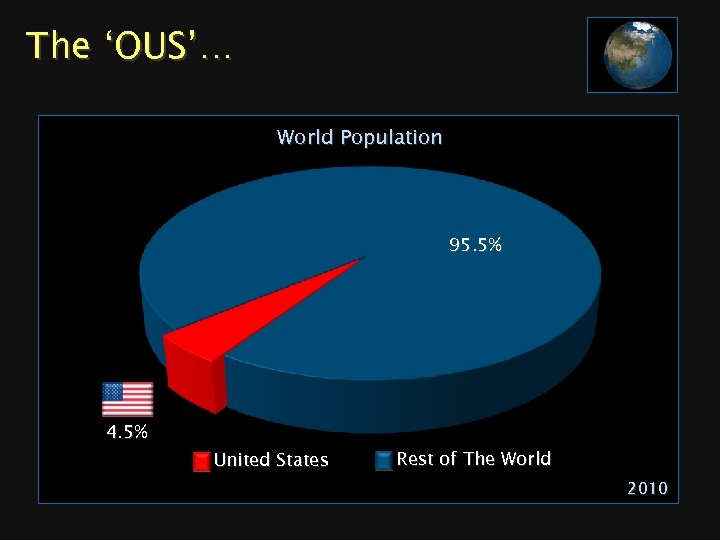
The ‘OUS’… World Population 95. 5% 4. 5% United States Rest of The World 2010
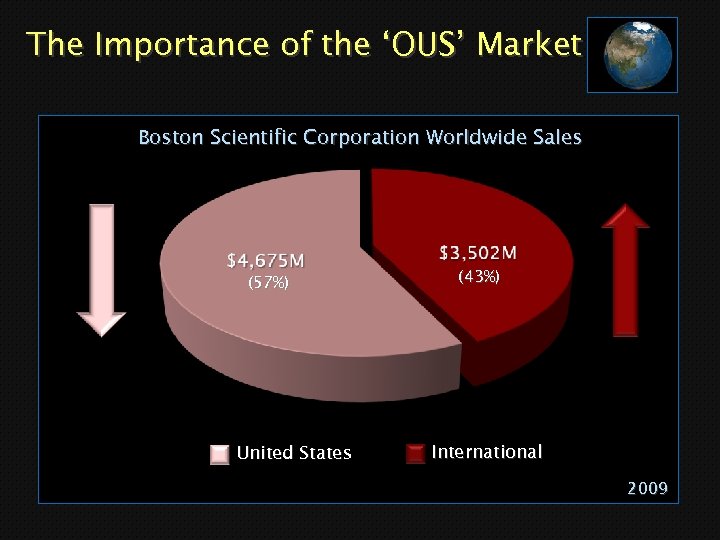
The Importance of the ‘OUS’ Market Boston Scientific Corporation Worldwide Sales (57%) United States (43%) International 2009

The Global Picture Variable requirements for clinical studies Variable end points and follow-up times US EU Japan Variable application of guidance by Notified Bodies Both Pre- and Post-Market requirements increasing Adding time, money and need for resources Need for further alignment and standardization

Global Healthcare Scrutiny…
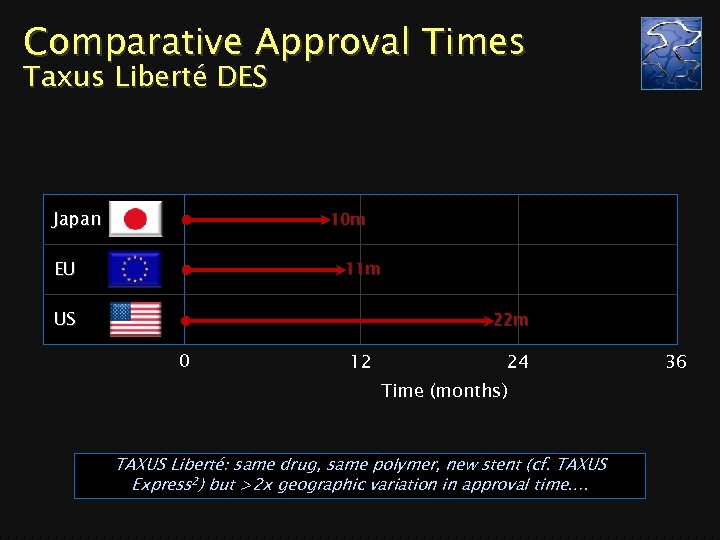
Comparative Approval Times Taxus Liberté DES Japan 10 m EU 11 m US 22 m 0 12 24 Time (months) TAXUS Liberté: same drug, same polymer, new stent (cf. TAXUS Express 2) but >2 x geographic variation in approval time…. 36
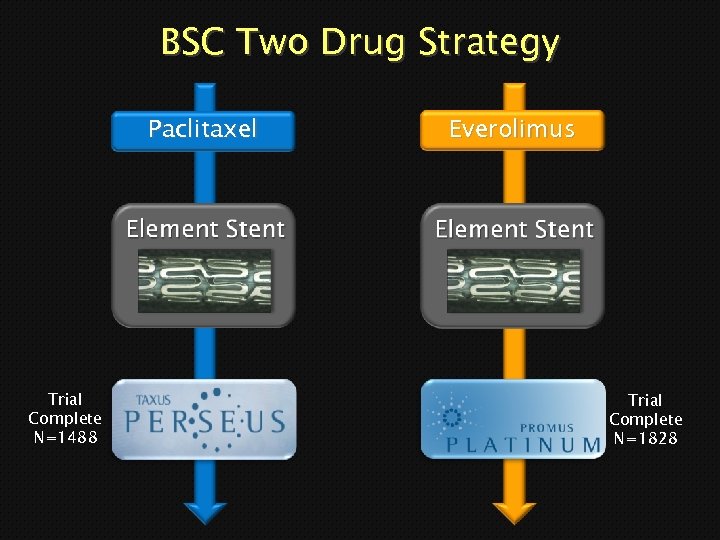
BSC Two Drug Strategy Paclitaxel Trial Complete N=1488 Everolimus Trial Complete N=1828
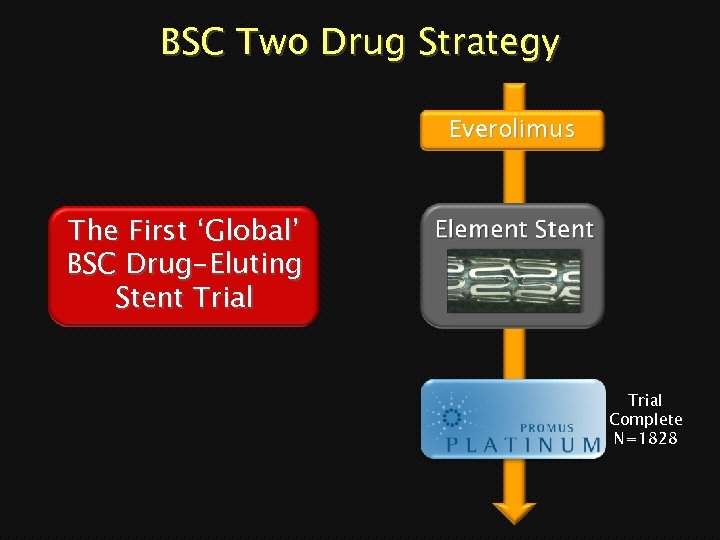
BSC Two Drug Strategy Everolimus The First ‘Global’ BSC Drug-Eluting Stent Trial Complete N=1828

Element Stent Platform Geometry designed for drug delivery Four stent models Consistent surface-to-artery ratios Apex™ balloon Bi-component balloon Multilayer Platinum Chromium Alloy Thin struts Radio-opaque Low recoil High radial strength Cypher 0. 0055” Express 0. 0052” Liberté 0. 0038” Driver 0. 0036” Vision 0. 0032” Element 0. 0032”
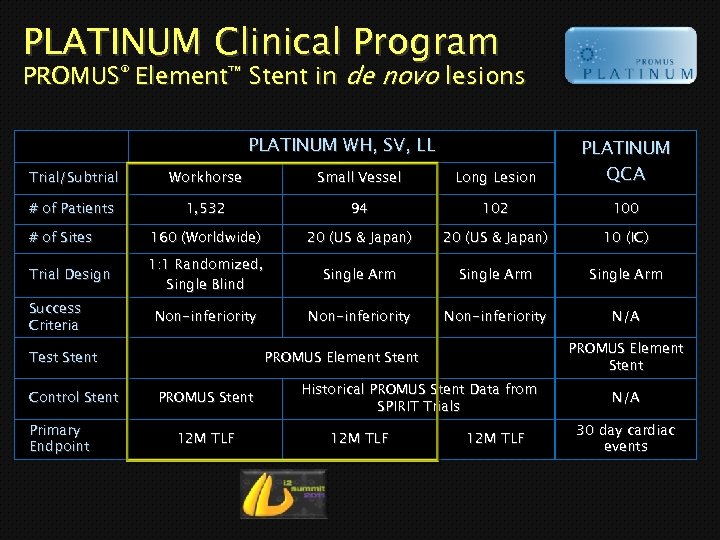
PLATINUM Clinical Program PROMUS® Element™ Stent in de novo lesions PLATINUM WH, SV, LL Trial/Subtrial Workhorse Small Vessel Long Lesion PLATINUM QCA # of Patients 1, 532 94 102 100 # of Sites 160 (Worldwide) 20 (US & Japan) 10 (IC) Trial Design 1: 1 Randomized, Single Blind Single Arm Non-inferiority N/A Success Criteria Test Stent Control Stent Primary Endpoint PROMUS Element Stent PROMUS Stent 12 M TLF Historical PROMUS Stent Data from SPIRIT Trials 12 M TLF N/A 30 day cardiac events
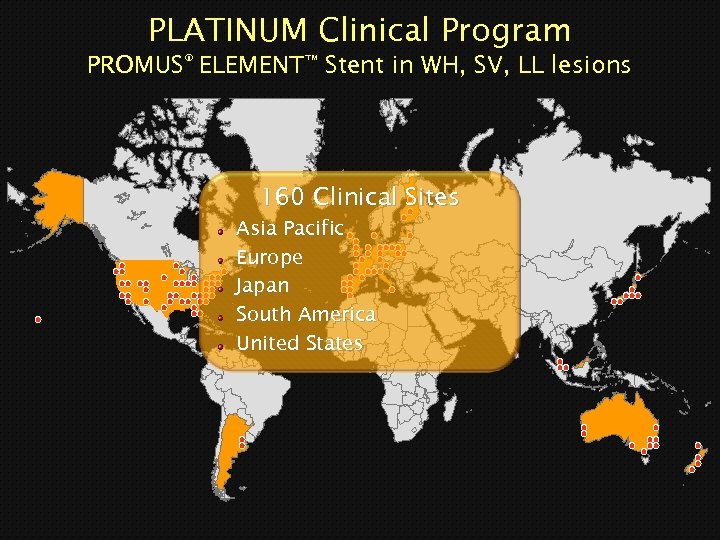
PLATINUM Clinical Program PROMUS® ELEMENT™ Stent in WH, SV, LL lesions 160 Clinical Sites Asia Pacific Europe Japan South America United States
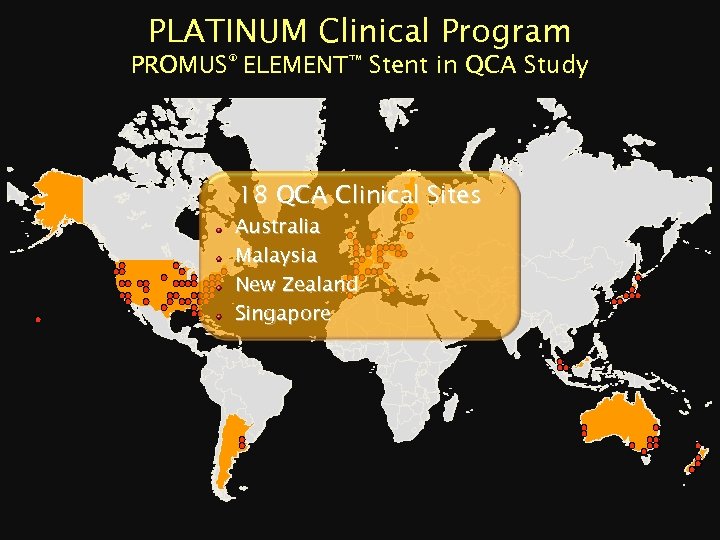
PLATINUM Clinical Program PROMUS® ELEMENT™ Stent in QCA Study 18 QCA Clinical Sites Australia Malaysia New Zealand Singapore
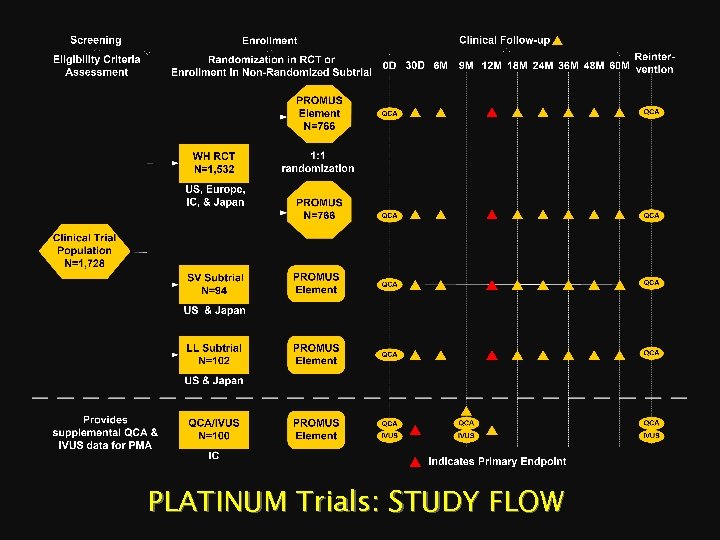
PLATINUM Trials: STUDY FLOW
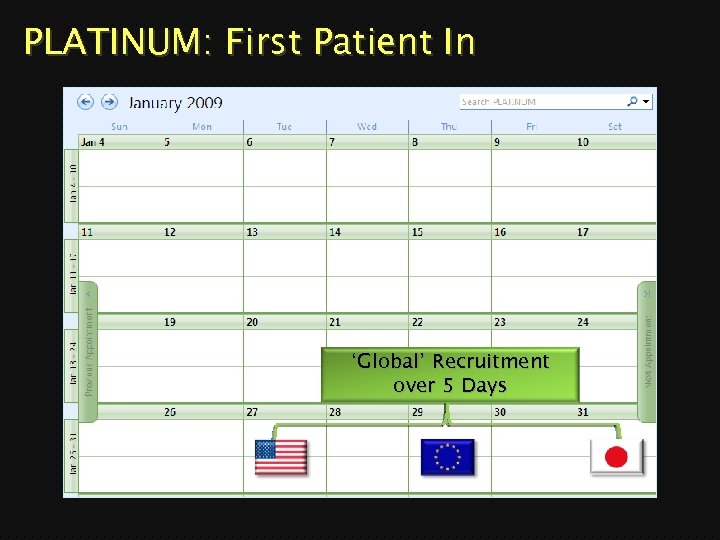
PLATINUM: First Patient In ‘Global’ Recruitment over 5 Days

Difficulties with Running a Global Trial Working across multiple time zones Multiple languages, cultures Investigator training Multiple IRBs Inventory control etc

Why should a Medical Device Manufacturer Market a Product in a Specific Country? High population at risk High procedural volumes Well developed selling organization Acceptable Average Selling Price (ASP) Navigable local regulatory hurdle Intellectual Property (IP) recognition Best business governance/practices

Conclusions Japan and the USA are key markets for the Medical Device Industry The PLATINUM trial confirmed that a ‘Global’ DES trial is feasible and can be executed and completed obtaining robust clinical data BUT Country-specific investment will follow adequate device reimbursement without excessive operational costs We are operating in a very dynamic business environment and strategic priorities will change…
0052eaa45466a844a954ff7df028ac94.ppt