65ad949ec2d4e0e88d18255a7d3d96df.ppt
- Количество слайдов: 20
 When to Repair Functional MR The Interventional Cardiologist’s Perspective Ted Feldman, M. D. , FSCAI FACC FESC Evanston Hospital CRT Cardiovacular Research Technologies Washington, D. C. February 23 – 26, 2013
When to Repair Functional MR The Interventional Cardiologist’s Perspective Ted Feldman, M. D. , FSCAI FACC FESC Evanston Hospital CRT Cardiovacular Research Technologies Washington, D. C. February 23 – 26, 2013
 Ted E. Feldman, MD Consulting: Abbott Laboratories Boston Scientific Corporation Edwards Lifesciences, LLC
Ted E. Feldman, MD Consulting: Abbott Laboratories Boston Scientific Corporation Edwards Lifesciences, LLC
 INDICATIONS FOR MITRAL VALVE OPERATION
INDICATIONS FOR MITRAL VALVE OPERATION
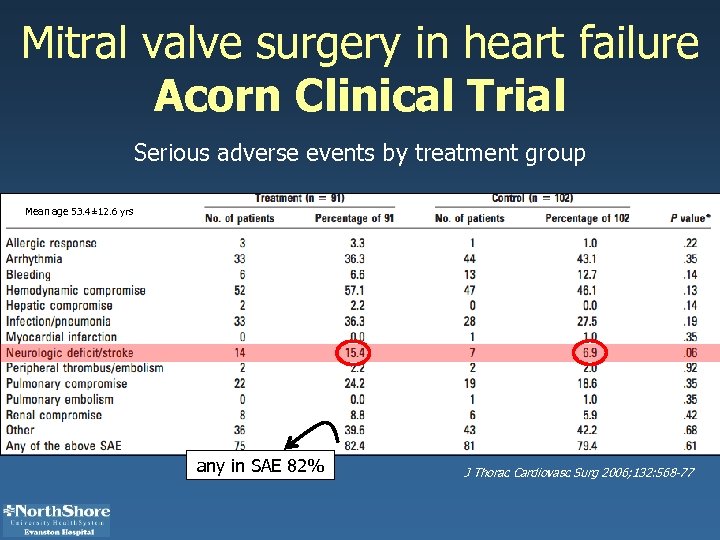 Mitral valve surgery in heart failure Acorn Clinical Trial Serious adverse events by treatment group Mean age 53. 4± 12. 6 yrs any in SAE 82% J Thorac Cardiovasc Surg 2006; 132: 568 -77
Mitral valve surgery in heart failure Acorn Clinical Trial Serious adverse events by treatment group Mean age 53. 4± 12. 6 yrs any in SAE 82% J Thorac Cardiovasc Surg 2006; 132: 568 -77
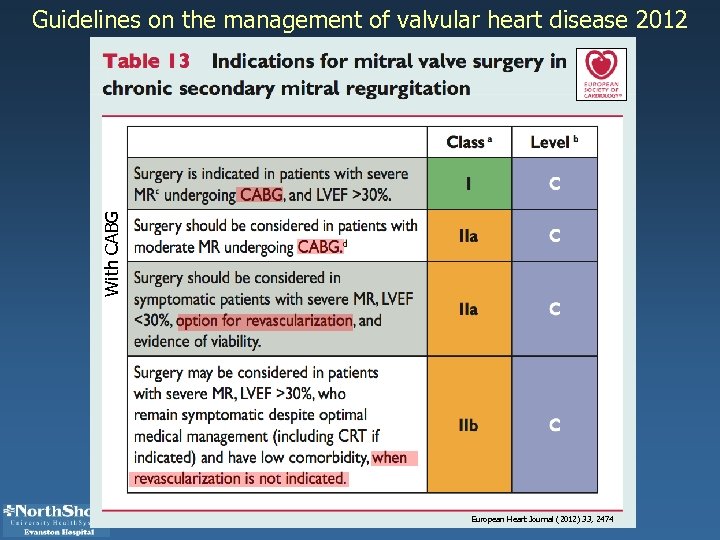 With CABG Guidelines on the management of valvular heart disease 2012 European Heart Journal (2012) 33, 2474
With CABG Guidelines on the management of valvular heart disease 2012 European Heart Journal (2012) 33, 2474
 Patient Selection for MR Therapies § Trial inclusion and exclusion criteria determine patient population § Resultant patient profile variable
Patient Selection for MR Therapies § Trial inclusion and exclusion criteria determine patient population § Resultant patient profile variable
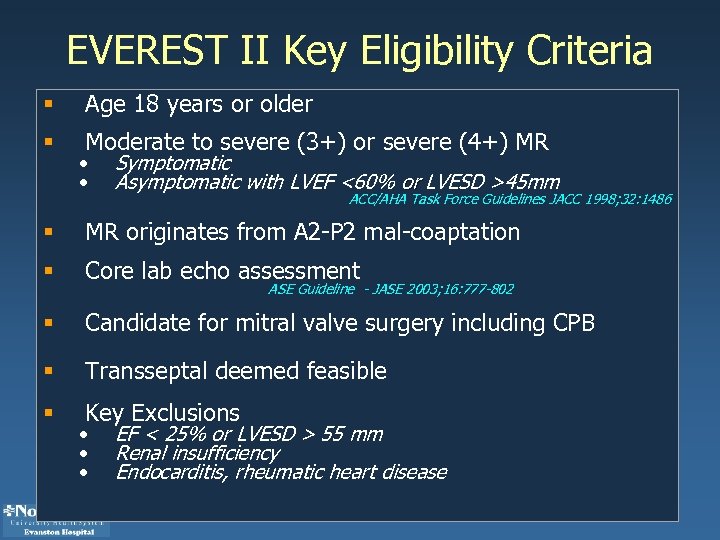 EVEREST II Key Eligibility Criteria § Age 18 years or older § Moderate to severe (3+) or severe (4+) MR • • Symptomatic Asymptomatic with LVEF <60% or LVESD >45 mm ACC/AHA Task Force Guidelines JACC 1998; 32: 1486 § MR originates from A 2 -P 2 mal-coaptation § Core lab echo assessment § Candidate for mitral valve surgery including CPB § Transseptal deemed feasible § Key Exclusions ASE Guideline - JASE 2003; 16: 777 -802 • • • EF < 25% or LVESD > 55 mm Renal insufficiency Endocarditis, rheumatic heart disease
EVEREST II Key Eligibility Criteria § Age 18 years or older § Moderate to severe (3+) or severe (4+) MR • • Symptomatic Asymptomatic with LVEF <60% or LVESD >45 mm ACC/AHA Task Force Guidelines JACC 1998; 32: 1486 § MR originates from A 2 -P 2 mal-coaptation § Core lab echo assessment § Candidate for mitral valve surgery including CPB § Transseptal deemed feasible § Key Exclusions ASE Guideline - JASE 2003; 16: 777 -802 • • • EF < 25% or LVESD > 55 mm Renal insufficiency Endocarditis, rheumatic heart disease
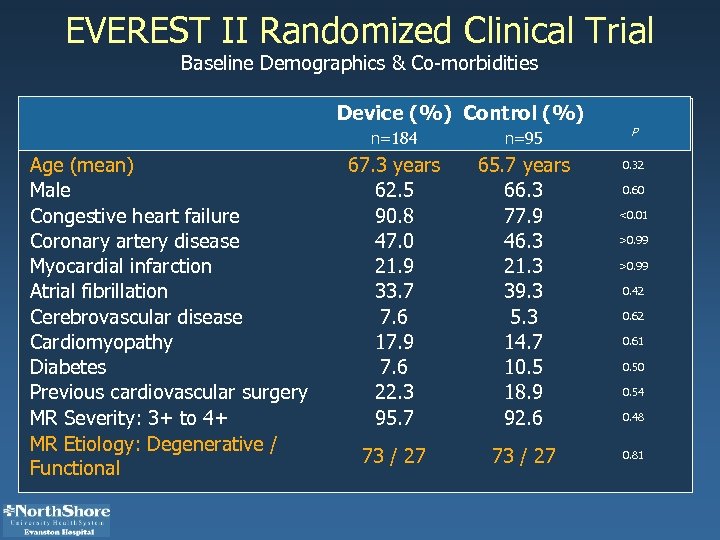 EVEREST II Randomized Clinical Trial Baseline Demographics & Co-morbidities Device (%) Control (%) n=184 Age (mean) Male Congestive heart failure Coronary artery disease Myocardial infarction Atrial fibrillation Cerebrovascular disease Cardiomyopathy Diabetes Previous cardiovascular surgery MR Severity: 3+ to 4+ MR Etiology: Degenerative / Functional n=95 P 67. 3 years 62. 5 90. 8 47. 0 21. 9 33. 7 7. 6 17. 9 7. 6 22. 3 95. 7 65. 7 years 66. 3 77. 9 46. 3 21. 3 39. 3 5. 3 14. 7 10. 5 18. 9 92. 6 0. 32 73 / 27 0. 60 <0. 01 >0. 99 0. 42 0. 61 0. 50 0. 54 0. 48 0. 81
EVEREST II Randomized Clinical Trial Baseline Demographics & Co-morbidities Device (%) Control (%) n=184 Age (mean) Male Congestive heart failure Coronary artery disease Myocardial infarction Atrial fibrillation Cerebrovascular disease Cardiomyopathy Diabetes Previous cardiovascular surgery MR Severity: 3+ to 4+ MR Etiology: Degenerative / Functional n=95 P 67. 3 years 62. 5 90. 8 47. 0 21. 9 33. 7 7. 6 17. 9 7. 6 22. 3 95. 7 65. 7 years 66. 3 77. 9 46. 3 21. 3 39. 3 5. 3 14. 7 10. 5 18. 9 92. 6 0. 32 73 / 27 0. 60 <0. 01 >0. 99 0. 42 0. 61 0. 50 0. 54 0. 48 0. 81
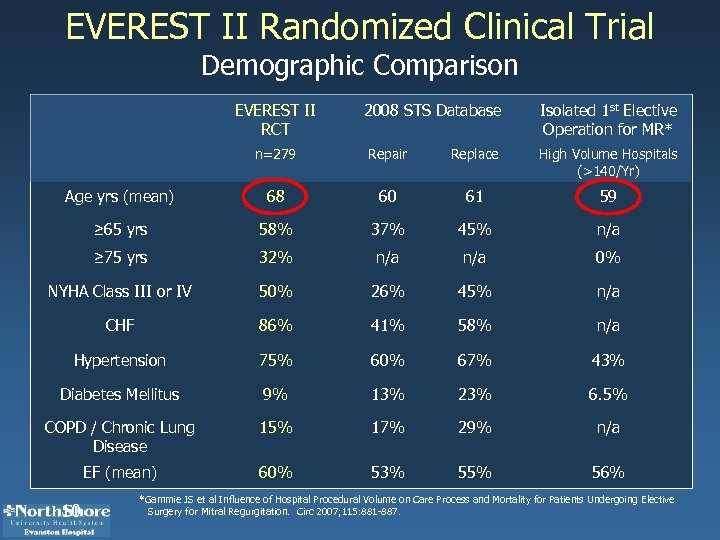 EVEREST II Randomized Clinical Trial Demographic Comparison EVEREST II RCT 2008 STS Database Isolated 1 st Elective Operation for MR* n=279 Repair Replace High Volume Hospitals (>140/Yr) Age yrs (mean) 68 60 61 59 ≥ 65 yrs 58% 37% 45% n/a ≥ 75 yrs 32% n/a 0% NYHA Class III or IV 50% 26% 45% n/a CHF 86% 41% 58% n/a Hypertension 75% 60% 67% 43% Diabetes Mellitus 9% 13% 23% 6. 5% COPD / Chronic Lung Disease 15% 17% 29% n/a EF (mean) 60% 53% 55% 56% 10 *Gammie JS et al Influence of Hospital Procedural Volume on Care Process and Mortality for Patients Undergoing Elective Surgery for Mitral Regurgitation. Circ 2007; 115: 881 -887.
EVEREST II Randomized Clinical Trial Demographic Comparison EVEREST II RCT 2008 STS Database Isolated 1 st Elective Operation for MR* n=279 Repair Replace High Volume Hospitals (>140/Yr) Age yrs (mean) 68 60 61 59 ≥ 65 yrs 58% 37% 45% n/a ≥ 75 yrs 32% n/a 0% NYHA Class III or IV 50% 26% 45% n/a CHF 86% 41% 58% n/a Hypertension 75% 60% 67% 43% Diabetes Mellitus 9% 13% 23% 6. 5% COPD / Chronic Lung Disease 15% 17% 29% n/a EF (mean) 60% 53% 55% 56% 10 *Gammie JS et al Influence of Hospital Procedural Volume on Care Process and Mortality for Patients Undergoing Elective Surgery for Mitral Regurgitation. Circ 2007; 115: 881 -887.
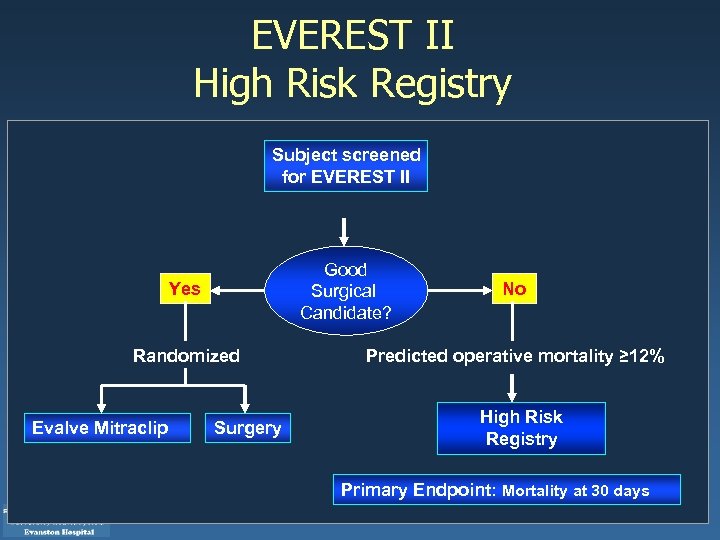 EVEREST II High Risk Registry Subject screened for EVEREST II Good Surgical Candidate? Yes Randomized Evalve Mitraclip Surgery No Predicted operative mortality ≥ 12% High Risk Registry Primary Endpoint: Mortality at 30 days
EVEREST II High Risk Registry Subject screened for EVEREST II Good Surgical Candidate? Yes Randomized Evalve Mitraclip Surgery No Predicted operative mortality ≥ 12% High Risk Registry Primary Endpoint: Mortality at 30 days
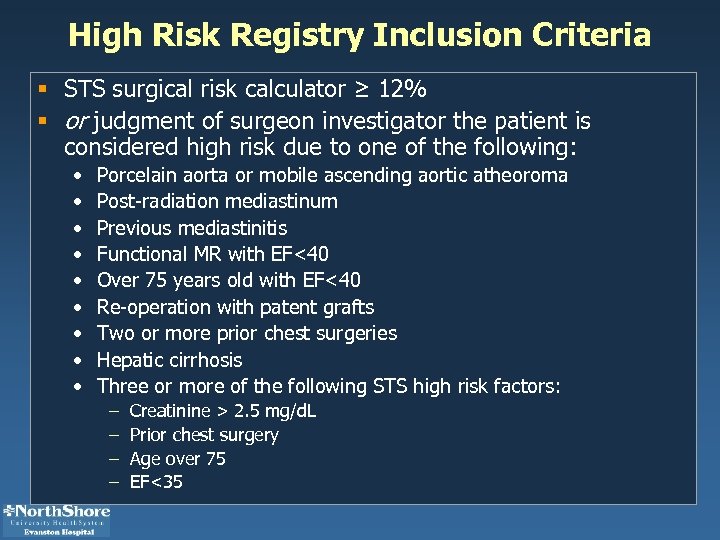 High Risk Registry Inclusion Criteria § STS surgical risk calculator ≥ 12% § or judgment of surgeon investigator the patient is considered high risk due to one of the following: • • • Porcelain aorta or mobile ascending aortic atheoroma Post-radiation mediastinum Previous mediastinitis Functional MR with EF<40 Over 75 years old with EF<40 Re-operation with patent grafts Two or more prior chest surgeries Hepatic cirrhosis Three or more of the following STS high risk factors: – – Creatinine > 2. 5 mg/d. L Prior chest surgery Age over 75 EF<35
High Risk Registry Inclusion Criteria § STS surgical risk calculator ≥ 12% § or judgment of surgeon investigator the patient is considered high risk due to one of the following: • • • Porcelain aorta or mobile ascending aortic atheoroma Post-radiation mediastinum Previous mediastinitis Functional MR with EF<40 Over 75 years old with EF<40 Re-operation with patent grafts Two or more prior chest surgeries Hepatic cirrhosis Three or more of the following STS high risk factors: – – Creatinine > 2. 5 mg/d. L Prior chest surgery Age over 75 EF<35
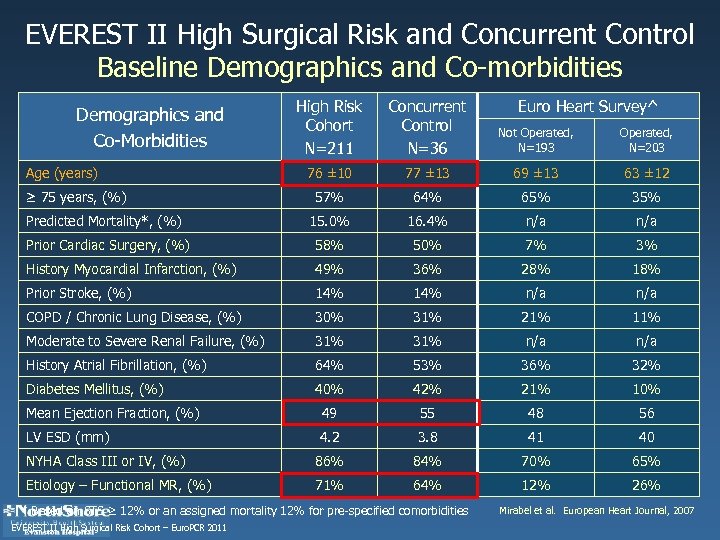 EVEREST II High Surgical Risk and Concurrent Control Baseline Demographics and Co-morbidities High Risk Cohort N=211 Concurrent Control N=36 Not Operated, N=193 Operated, N=203 76 ± 10 77 ± 13 69 ± 13 63 ± 12 57% 64% 65% 35% Predicted Mortality*, (%) 15. 0% 16. 4% n/a Prior Cardiac Surgery, (%) 58% 50% 7% 3% History Myocardial Infarction, (%) 49% 36% 28% 18% Prior Stroke, (%) 14% n/a COPD / Chronic Lung Disease, (%) 30% 31% 21% 11% Moderate to Severe Renal Failure, (%) 31% n/a History Atrial Fibrillation, (%) 64% 53% 36% 32% Diabetes Mellitus, (%) 40% 42% 21% 10% Mean Ejection Fraction, (%) 49 55 48 56 LV ESD (mm) 4. 2 3. 8 41 40 NYHA Class III or IV, (%) 86% 84% 70% 65% Etiology – Functional MR, (%) 71% 64% 12% 26% Demographics and Co-Morbidities Age (years) ≥ 75 years, (%) * Based on STS ≥ 12% or an assigned mortality 12% for pre-specified comorbidities EVEREST II High Surgical Risk Cohort – Euro. PCR 2011 Euro Heart Survey^ Mirabel et al. European Heart Journal, 2007
EVEREST II High Surgical Risk and Concurrent Control Baseline Demographics and Co-morbidities High Risk Cohort N=211 Concurrent Control N=36 Not Operated, N=193 Operated, N=203 76 ± 10 77 ± 13 69 ± 13 63 ± 12 57% 64% 65% 35% Predicted Mortality*, (%) 15. 0% 16. 4% n/a Prior Cardiac Surgery, (%) 58% 50% 7% 3% History Myocardial Infarction, (%) 49% 36% 28% 18% Prior Stroke, (%) 14% n/a COPD / Chronic Lung Disease, (%) 30% 31% 21% 11% Moderate to Severe Renal Failure, (%) 31% n/a History Atrial Fibrillation, (%) 64% 53% 36% 32% Diabetes Mellitus, (%) 40% 42% 21% 10% Mean Ejection Fraction, (%) 49 55 48 56 LV ESD (mm) 4. 2 3. 8 41 40 NYHA Class III or IV, (%) 86% 84% 70% 65% Etiology – Functional MR, (%) 71% 64% 12% 26% Demographics and Co-Morbidities Age (years) ≥ 75 years, (%) * Based on STS ≥ 12% or an assigned mortality 12% for pre-specified comorbidities EVEREST II High Surgical Risk Cohort – Euro. PCR 2011 Euro Heart Survey^ Mirabel et al. European Heart Journal, 2007
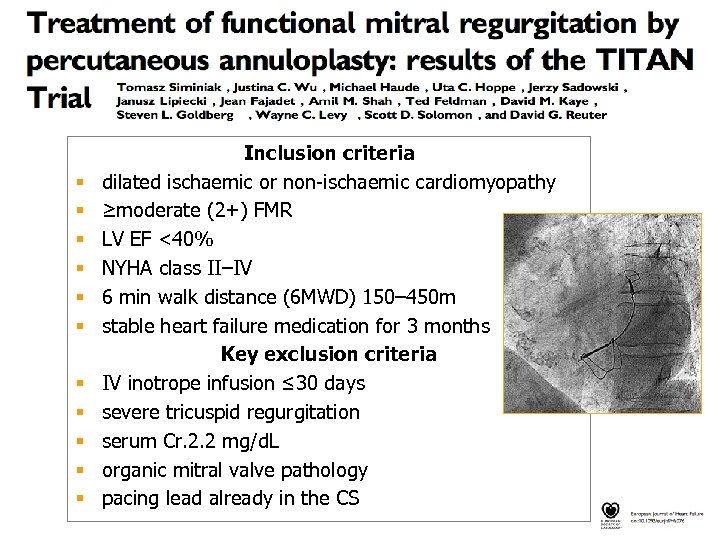 § § § Inclusion criteria dilated ischaemic or non-ischaemic cardiomyopathy ≥moderate (2+) FMR LV EF <40% NYHA class II–IV 6 min walk distance (6 MWD) 150– 450 m stable heart failure medication for 3 months Key exclusion criteria IV inotrope infusion ≤ 30 days severe tricuspid regurgitation serum Cr. 2. 2 mg/d. L organic mitral valve pathology pacing lead already in the CS
§ § § Inclusion criteria dilated ischaemic or non-ischaemic cardiomyopathy ≥moderate (2+) FMR LV EF <40% NYHA class II–IV 6 min walk distance (6 MWD) 150– 450 m stable heart failure medication for 3 months Key exclusion criteria IV inotrope infusion ≤ 30 days severe tricuspid regurgitation serum Cr. 2. 2 mg/d. L organic mitral valve pathology pacing lead already in the CS

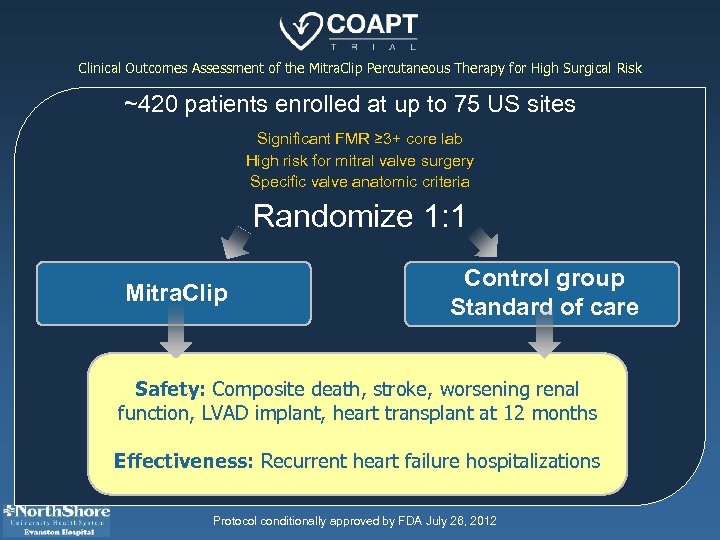 Clinical Outcomes Assessment of the Mitra. Clip Percutaneous Therapy for High Surgical Risk ~420 patients enrolled at up to 75 US sites Significant FMR ≥ 3+ core lab High risk for mitral valve surgery Specific valve anatomic criteria Randomize 1: 1 Mitra. Clip Control group Standard of care Safety: Composite death, stroke, worsening renal function, LVAD implant, heart transplant at 12 months Effectiveness: Recurrent heart failure hospitalizations Protocol conditionally approved by FDA July 26, 2012
Clinical Outcomes Assessment of the Mitra. Clip Percutaneous Therapy for High Surgical Risk ~420 patients enrolled at up to 75 US sites Significant FMR ≥ 3+ core lab High risk for mitral valve surgery Specific valve anatomic criteria Randomize 1: 1 Mitra. Clip Control group Standard of care Safety: Composite death, stroke, worsening renal function, LVAD implant, heart transplant at 12 months Effectiveness: Recurrent heart failure hospitalizations Protocol conditionally approved by FDA July 26, 2012
 Key Inclusion Criteria • Functional MR ≥ 3+ – ischemic or non-ischemic cardiomyopathy • Symptomatic – NYHA class II, III or ambulatory IV • STS mortality risk is ≥ 8% or Local Site Heart Team concludes that co-morbidities result in a prohibitive predicted operative risk of stroke or death • ≥ 1 HF hospitalization during prior year – and/or BNP ≥ 400 pg/ml – or n. T-pro. BNP ≥ 1600 pg/ml ≤ 90 days • treated per standards for CAD, LV dysfunction, MR or HF including CRT, revascularization, OMT • primary MR jet originates from malcoaptation of A 2 -P 2 scallops
Key Inclusion Criteria • Functional MR ≥ 3+ – ischemic or non-ischemic cardiomyopathy • Symptomatic – NYHA class II, III or ambulatory IV • STS mortality risk is ≥ 8% or Local Site Heart Team concludes that co-morbidities result in a prohibitive predicted operative risk of stroke or death • ≥ 1 HF hospitalization during prior year – and/or BNP ≥ 400 pg/ml – or n. T-pro. BNP ≥ 1600 pg/ml ≤ 90 days • treated per standards for CAD, LV dysfunction, MR or HF including CRT, revascularization, OMT • primary MR jet originates from malcoaptation of A 2 -P 2 scallops
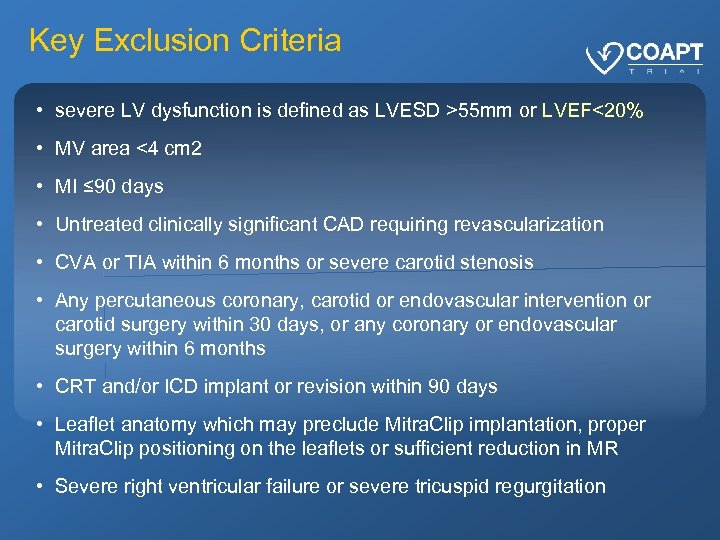 Key Exclusion Criteria • severe LV dysfunction is defined as LVESD >55 mm or LVEF<20% • MV area <4 cm 2 • MI ≤ 90 days • Untreated clinically significant CAD requiring revascularization • CVA or TIA within 6 months or severe carotid stenosis • Any percutaneous coronary, carotid or endovascular intervention or carotid surgery within 30 days, or any coronary or endovascular surgery within 6 months • CRT and/or ICD implant or revision within 90 days • Leaflet anatomy which may preclude Mitra. Clip implantation, proper Mitra. Clip positioning on the leaflets or sufficient reduction in MR • Severe right ventricular failure or severe tricuspid regurgitation
Key Exclusion Criteria • severe LV dysfunction is defined as LVESD >55 mm or LVEF<20% • MV area <4 cm 2 • MI ≤ 90 days • Untreated clinically significant CAD requiring revascularization • CVA or TIA within 6 months or severe carotid stenosis • Any percutaneous coronary, carotid or endovascular intervention or carotid surgery within 30 days, or any coronary or endovascular surgery within 6 months • CRT and/or ICD implant or revision within 90 days • Leaflet anatomy which may preclude Mitra. Clip implantation, proper Mitra. Clip positioning on the leaflets or sufficient reduction in MR • Severe right ventricular failure or severe tricuspid regurgitation
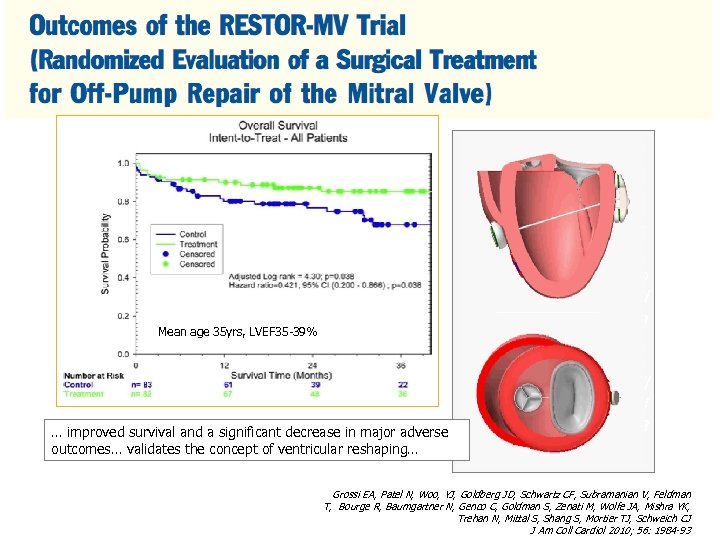 Mean age 35 yrs, LVEF 35 -39% … improved survival and a significant decrease in major adverse outcomes… validates the concept of ventricular reshaping… Grossi EA, Patel N, Woo, YJ, Goldberg JD, Schwartz CF, Subramanian V, Feldman T, Bourge R, Baumgartner N, Genco C, Goldman S, Zenati M, Wolfe JA, Mishra YK, Trehan N, Mittal S, Shang S, Mortier TJ, Schweich CJ J Am Coll Cardiol 2010; 56: 1984 -93 Grossi EA, Patel N, Woo, YJ, Goldberg JD, Schwartz CF, Subramanian V, Feldman T, Bourge R, Baumgartner N, Genco C, Goldman S, Zenati M, Wolfe JA, Mishra YK, Trehan N, Mittal S, Shang S, Mortier TJ, Schweich CJ J Am Coll Cardiol 2010; 56: 1984 -93
Mean age 35 yrs, LVEF 35 -39% … improved survival and a significant decrease in major adverse outcomes… validates the concept of ventricular reshaping… Grossi EA, Patel N, Woo, YJ, Goldberg JD, Schwartz CF, Subramanian V, Feldman T, Bourge R, Baumgartner N, Genco C, Goldman S, Zenati M, Wolfe JA, Mishra YK, Trehan N, Mittal S, Shang S, Mortier TJ, Schweich CJ J Am Coll Cardiol 2010; 56: 1984 -93 Grossi EA, Patel N, Woo, YJ, Goldberg JD, Schwartz CF, Subramanian V, Feldman T, Bourge R, Baumgartner N, Genco C, Goldman S, Zenati M, Wolfe JA, Mishra YK, Trehan N, Mittal S, Shang S, Mortier TJ, Schweich CJ J Am Coll Cardiol 2010; 56: 1984 -93


