d69a3dcd7198757bbe4a932f1fc5b57f.ppt
- Количество слайдов: 37
 WHEN AND WHY TO CLOSE LAA ? Matteo Montorfano San Raffaele Hospital Milan, Italy
WHEN AND WHY TO CLOSE LAA ? Matteo Montorfano San Raffaele Hospital Milan, Italy
 Magnitude of the Problem: Stroke 1. The World Health Organization estimates that in 2001 there were over 20. 5 million strokes worldwide, 5. 5 million of these were fatal. 1 2. Europe averages approximately 650, 000 deaths due to stroke each year. 2 3. Stroke is the 3 rd leading cause of death behind diseases of the heart and cancer and the 1 st cause of serious long-term disability. 3 4. Stroke social cost accounts approximately for the 3% of total health care expenditures. 4 1. World Health Report 2002 2. International Cardiovascular Disease Statistics 3. 2003 Heart and Stroke Statistical Update, American Heart Association 4. Evers SM, et al. International comparison of stroke cost studies. Stroke. 2004.
Magnitude of the Problem: Stroke 1. The World Health Organization estimates that in 2001 there were over 20. 5 million strokes worldwide, 5. 5 million of these were fatal. 1 2. Europe averages approximately 650, 000 deaths due to stroke each year. 2 3. Stroke is the 3 rd leading cause of death behind diseases of the heart and cancer and the 1 st cause of serious long-term disability. 3 4. Stroke social cost accounts approximately for the 3% of total health care expenditures. 4 1. World Health Report 2002 2. International Cardiovascular Disease Statistics 3. 2003 Heart and Stroke Statistical Update, American Heart Association 4. Evers SM, et al. International comparison of stroke cost studies. Stroke. 2004.
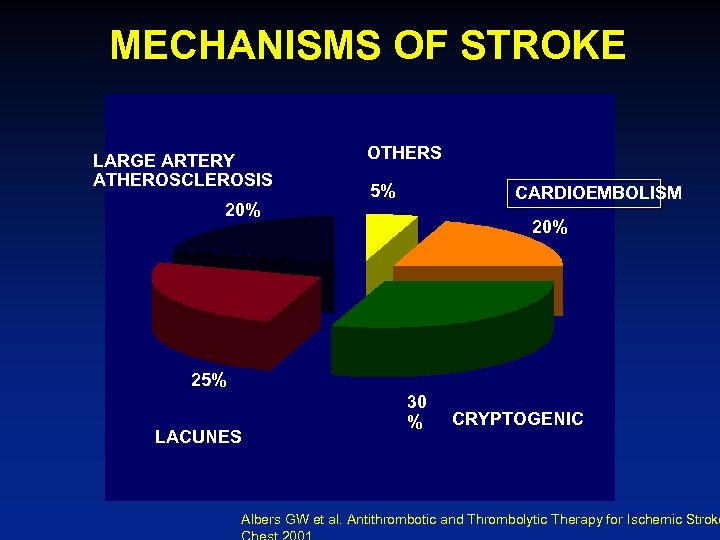 MECHANISMS OF STROKE LARGE ARTERY ATHEROSCLEROSIS 20% OTHERS 5% CARDIOEMBOLISM 20% 25% LACUNES 30 % CRYPTOGENIC Albers GW et al. Antithrombotic and Thrombolytic Therapy for Ischemic Stroke
MECHANISMS OF STROKE LARGE ARTERY ATHEROSCLEROSIS 20% OTHERS 5% CARDIOEMBOLISM 20% 25% LACUNES 30 % CRYPTOGENIC Albers GW et al. Antithrombotic and Thrombolytic Therapy for Ischemic Stroke
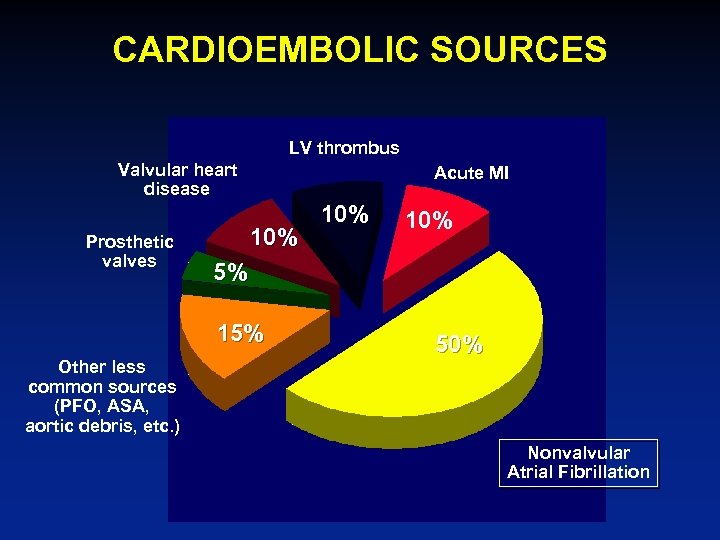 CARDIOEMBOLIC SOURCES LV thrombus Valvular heart disease Prosthetic valves Acute MI 10% 5% 15% Other less common sources (PFO, ASA, aortic debris, etc. ) 10% 50% Nonvalvular Atrial Fibrillation
CARDIOEMBOLIC SOURCES LV thrombus Valvular heart disease Prosthetic valves Acute MI 10% 5% 15% Other less common sources (PFO, ASA, aortic debris, etc. ) 10% 50% Nonvalvular Atrial Fibrillation
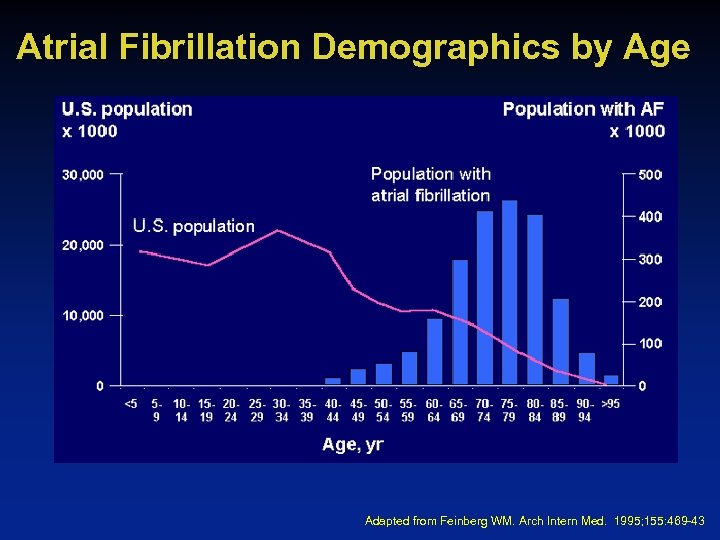 Atrial Fibrillation Demographics by Age Adapted from Feinberg WM. Arch Intern Med. 1995; 155: 469 -43
Atrial Fibrillation Demographics by Age Adapted from Feinberg WM. Arch Intern Med. 1995; 155: 469 -43
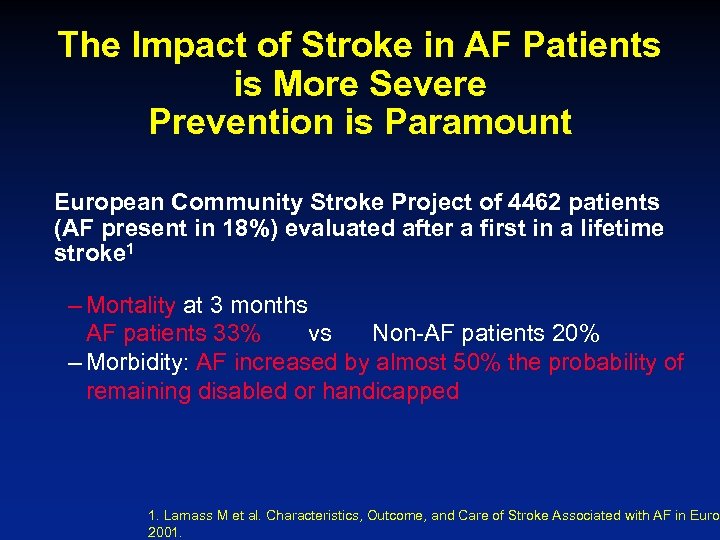 The Impact of Stroke in AF Patients is More Severe Prevention is Paramount European Community Stroke Project of 4462 patients (AF present in 18%) evaluated after a first in a lifetime stroke 1 – Mortality at 3 months AF patients 33% vs Non-AF patients 20% – Morbidity: AF increased by almost 50% the probability of remaining disabled or handicapped 1. Lamass M et al. Characteristics, Outcome, and Care of Stroke Associated with AF in Europ 2001.
The Impact of Stroke in AF Patients is More Severe Prevention is Paramount European Community Stroke Project of 4462 patients (AF present in 18%) evaluated after a first in a lifetime stroke 1 – Mortality at 3 months AF patients 33% vs Non-AF patients 20% – Morbidity: AF increased by almost 50% the probability of remaining disabled or handicapped 1. Lamass M et al. Characteristics, Outcome, and Care of Stroke Associated with AF in Europ 2001.
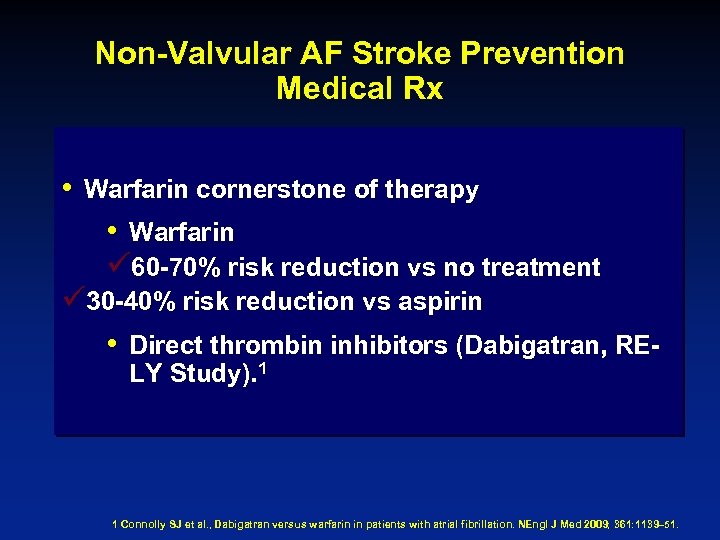 Non-Valvular AF Stroke Prevention Medical Rx • Warfarin cornerstone of therapy • Warfarin ü 60 -70% risk reduction vs no treatment ü 30 -40% risk reduction vs aspirin • Direct thrombin inhibitors (Dabigatran, RELY Study). 1 1 Connolly SJ et al. , Dabigatran versus warfarin in patients with atrial fibrillation. NEngl J Med 2009; 361: 1139– 51.
Non-Valvular AF Stroke Prevention Medical Rx • Warfarin cornerstone of therapy • Warfarin ü 60 -70% risk reduction vs no treatment ü 30 -40% risk reduction vs aspirin • Direct thrombin inhibitors (Dabigatran, RELY Study). 1 1 Connolly SJ et al. , Dabigatran versus warfarin in patients with atrial fibrillation. NEngl J Med 2009; 361: 1139– 51.
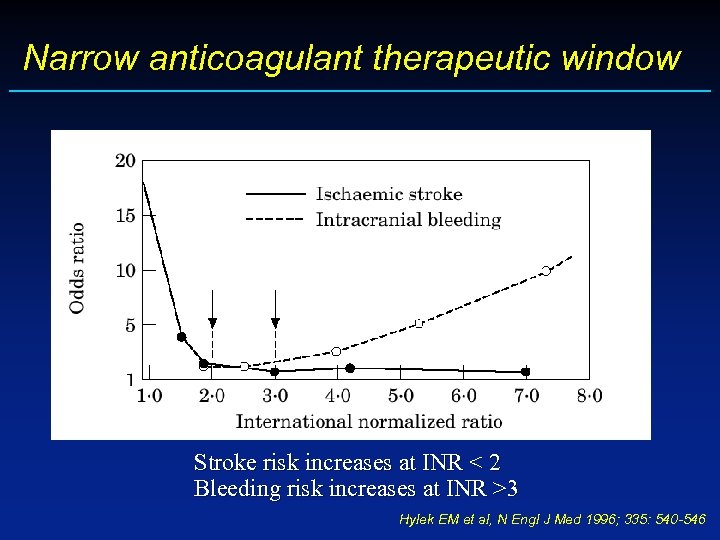 Narrow anticoagulant therapeutic window Stroke risk increases at INR < 2 Bleeding risk increases at INR >3 Hylek EM et al, N Engl J Med 1996; 335: 540 -546
Narrow anticoagulant therapeutic window Stroke risk increases at INR < 2 Bleeding risk increases at INR >3 Hylek EM et al, N Engl J Med 1996; 335: 540 -546
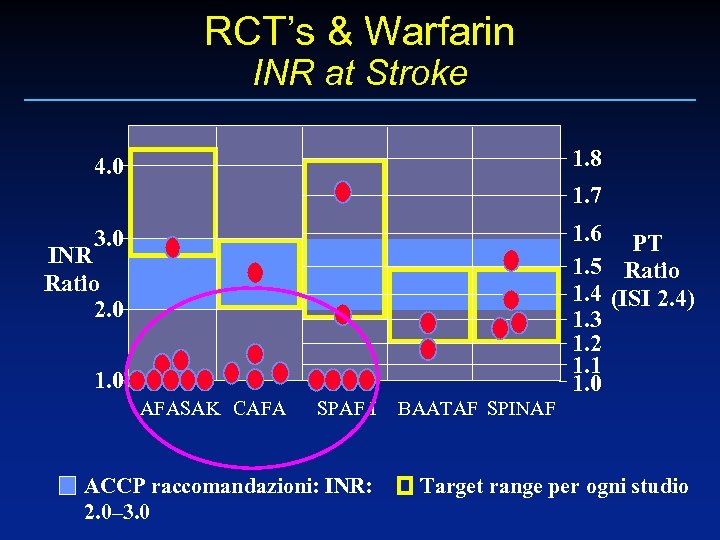 RCT’s & Warfarin INR at Stroke 1. 8 4. 0 1. 7 1. 6 PT 1. 5 Ratio 1. 4 (ISI 2. 4) 1. 3 1. 2 1. 1 1. 0 3. 0 INR Ratio 2. 0 1. 0 AFASAK CAFA SPAF I ACCP raccomandazioni: INR: 2. 0– 3. 0 BAATAF SPINAF Target range per ogni studio
RCT’s & Warfarin INR at Stroke 1. 8 4. 0 1. 7 1. 6 PT 1. 5 Ratio 1. 4 (ISI 2. 4) 1. 3 1. 2 1. 1 1. 0 3. 0 INR Ratio 2. 0 1. 0 AFASAK CAFA SPAF I ACCP raccomandazioni: INR: 2. 0– 3. 0 BAATAF SPINAF Target range per ogni studio
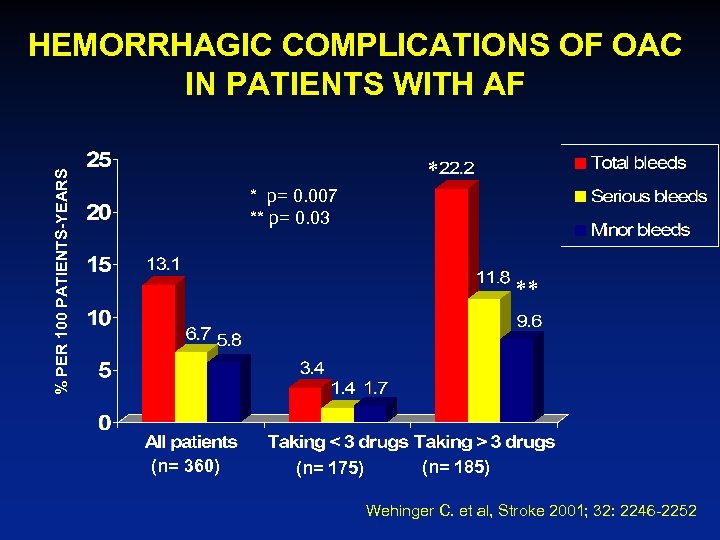 HEMORRHAGIC COMPLICATIONS OF OAC IN PATIENTS WITH AF % PER 100 PATIENTS-YEARS * * p= 0. 007 ** p= 0. 03 ** (n= 360) (n= 175) (n= 185) Wehinger C. et al, Stroke 2001; 32: 2246 -2252
HEMORRHAGIC COMPLICATIONS OF OAC IN PATIENTS WITH AF % PER 100 PATIENTS-YEARS * * p= 0. 007 ** p= 0. 03 ** (n= 360) (n= 175) (n= 185) Wehinger C. et al, Stroke 2001; 32: 2246 -2252
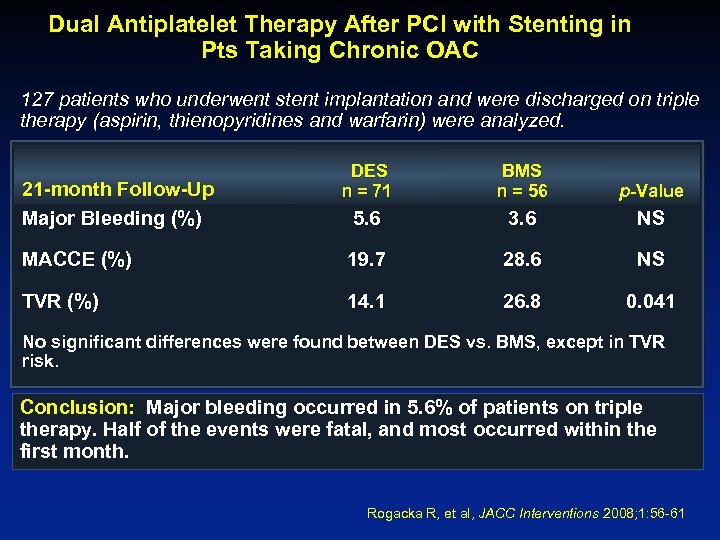 Dual Antiplatelet Therapy After PCI with Stenting in Pts Taking Chronic OAC 127 patients who underwent stent implantation and were discharged on triple therapy (aspirin, thienopyridines and warfarin) were analyzed. DES n = 71 BMS n = 56 p-Value 5. 6 3. 6 NS MACCE (%) 19. 7 28. 6 NS TVR (%) 14. 1 26. 8 0. 041 21 -month Follow-Up Major Bleeding (%) No significant differences were found between DES vs. BMS, except in TVR risk. Conclusion: Major bleeding occurred in 5. 6% of patients on triple therapy. Half of the events were fatal, and most occurred within the first month. Rogacka R, et al, JACC Interventions 2008; 1: 56 -61
Dual Antiplatelet Therapy After PCI with Stenting in Pts Taking Chronic OAC 127 patients who underwent stent implantation and were discharged on triple therapy (aspirin, thienopyridines and warfarin) were analyzed. DES n = 71 BMS n = 56 p-Value 5. 6 3. 6 NS MACCE (%) 19. 7 28. 6 NS TVR (%) 14. 1 26. 8 0. 041 21 -month Follow-Up Major Bleeding (%) No significant differences were found between DES vs. BMS, except in TVR risk. Conclusion: Major bleeding occurred in 5. 6% of patients on triple therapy. Half of the events were fatal, and most occurred within the first month. Rogacka R, et al, JACC Interventions 2008; 1: 56 -61
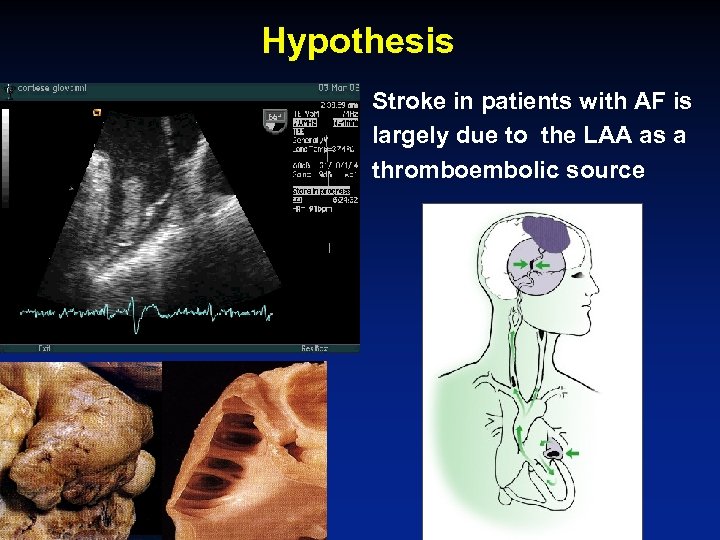 Hypothesis • Stroke in patients with AF is largely due to the LAA as a thromboembolic source
Hypothesis • Stroke in patients with AF is largely due to the LAA as a thromboembolic source
 LAA SURGICAL OBLITERATION During surgery for mitral stenosis “amputation of the left atrial appendage is recommended to reduce the likelihood of postoperative thromboembolic events” ACC/AHA 2006 Guidelines for valvular heart disease Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study “An incomplete LAA ligation during surgery of mitral valve replacement considered together with the absence of LAA ligation, increased risk of embolism at follow-up (up to 11. 9 x)” Garcia-Fernandez MA et al, J Am Coll Cardiol 2003; 42: 1253 -8
LAA SURGICAL OBLITERATION During surgery for mitral stenosis “amputation of the left atrial appendage is recommended to reduce the likelihood of postoperative thromboembolic events” ACC/AHA 2006 Guidelines for valvular heart disease Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study “An incomplete LAA ligation during surgery of mitral valve replacement considered together with the absence of LAA ligation, increased risk of embolism at follow-up (up to 11. 9 x)” Garcia-Fernandez MA et al, J Am Coll Cardiol 2003; 42: 1253 -8
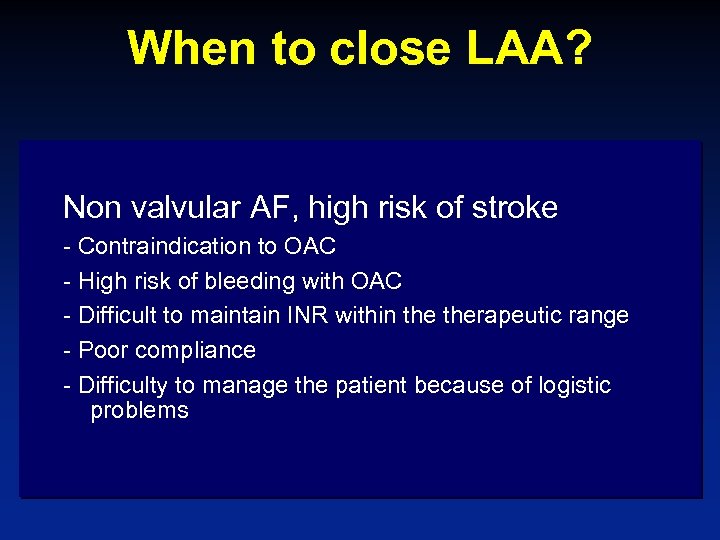 When to close LAA? Non valvular AF, high risk of stroke - Contraindication to OAC - High risk of bleeding with OAC - Difficult to maintain INR within therapeutic range - Poor compliance - Difficulty to manage the patient because of logistic problems
When to close LAA? Non valvular AF, high risk of stroke - Contraindication to OAC - High risk of bleeding with OAC - Difficult to maintain INR within therapeutic range - Poor compliance - Difficulty to manage the patient because of logistic problems
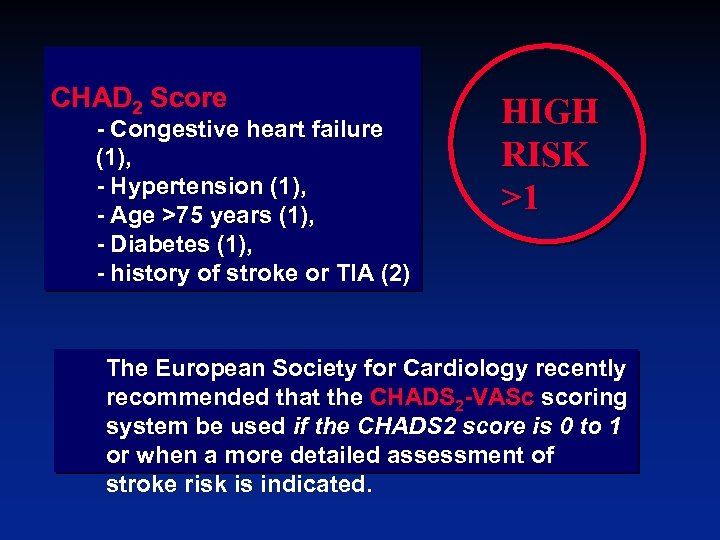 CHAD 2 Score - Congestive heart failure (1), - Hypertension (1), - Age >75 years (1), - Diabetes (1), - history of stroke or TIA (2) HIGH RISK >1 The European Society for Cardiology recently recommended that the CHADS 2 -VASc scoring system be used if the CHADS 2 score is 0 to 1 or when a more detailed assessment of stroke risk is indicated.
CHAD 2 Score - Congestive heart failure (1), - Hypertension (1), - Age >75 years (1), - Diabetes (1), - history of stroke or TIA (2) HIGH RISK >1 The European Society for Cardiology recently recommended that the CHADS 2 -VASc scoring system be used if the CHADS 2 score is 0 to 1 or when a more detailed assessment of stroke risk is indicated.
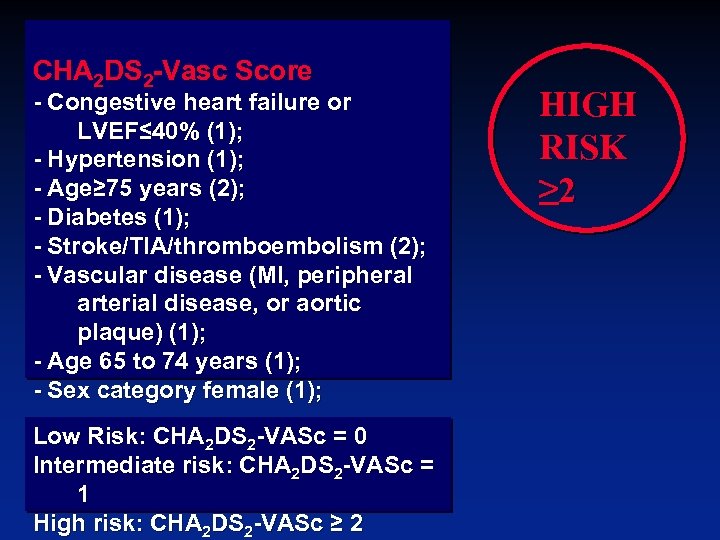 CHA 2 DS 2 -Vasc Score - Congestive heart failure or LVEF≤ 40% (1); - Hypertension (1); - Age≥ 75 years (2); - Diabetes (1); - Stroke/TIA/thromboembolism (2); - Vascular disease (MI, peripheral arterial disease, or aortic plaque) (1); - Age 65 to 74 years (1); - Sex category female (1); Low Risk: CHA 2 DS 2 -VASc = 0 Intermediate risk: CHA 2 DS 2 -VASc = 1 High risk: CHA 2 DS 2 -VASc ≥ 2 HIGH RISK ≥ 2
CHA 2 DS 2 -Vasc Score - Congestive heart failure or LVEF≤ 40% (1); - Hypertension (1); - Age≥ 75 years (2); - Diabetes (1); - Stroke/TIA/thromboembolism (2); - Vascular disease (MI, peripheral arterial disease, or aortic plaque) (1); - Age 65 to 74 years (1); - Sex category female (1); Low Risk: CHA 2 DS 2 -VASc = 0 Intermediate risk: CHA 2 DS 2 -VASc = 1 High risk: CHA 2 DS 2 -VASc ≥ 2 HIGH RISK ≥ 2
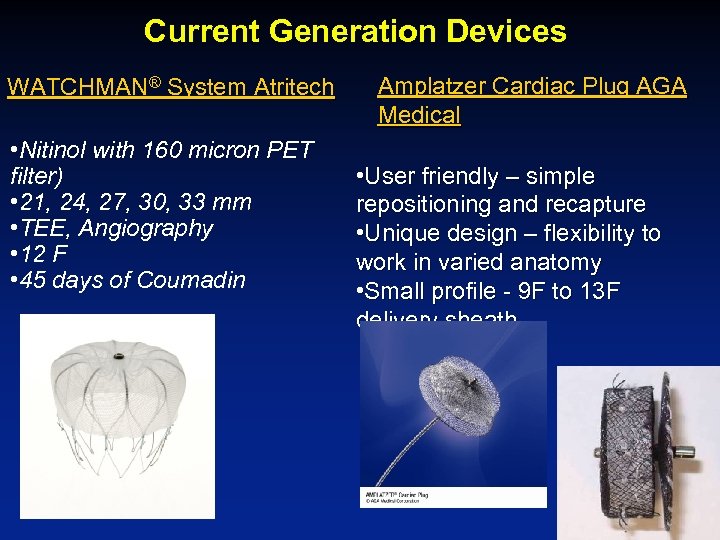
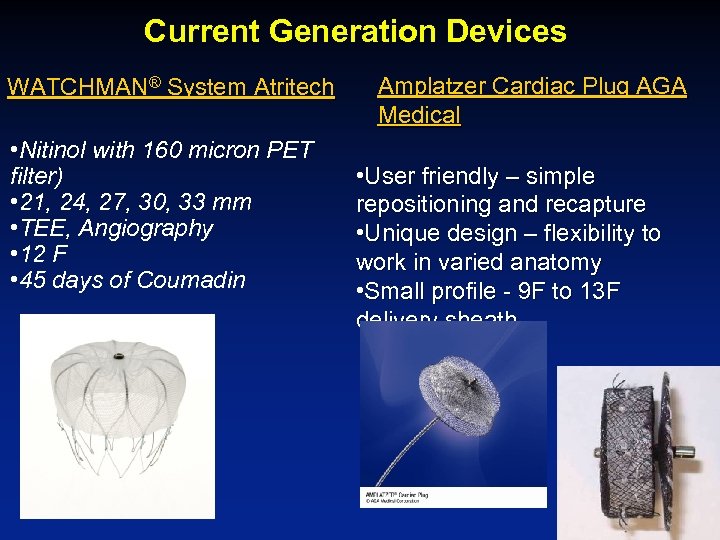 Current Generation Devices WATCHMAN® System Atritech • Nitinol with 160 micron PET filter) • 21, 24, 27, 30, 33 mm • TEE, Angiography • 12 F • 45 days of Coumadin Amplatzer Cardiac Plug AGA Medical • User friendly – simple repositioning and recapture • Unique design – flexibility to work in varied anatomy • Small profile - 9 F to 13 F delivery sheath
Current Generation Devices WATCHMAN® System Atritech • Nitinol with 160 micron PET filter) • 21, 24, 27, 30, 33 mm • TEE, Angiography • 12 F • 45 days of Coumadin Amplatzer Cardiac Plug AGA Medical • User friendly – simple repositioning and recapture • Unique design – flexibility to work in varied anatomy • Small profile - 9 F to 13 F delivery sheath
 PROTECT AF TRIAL • Randomized, controlled, statistically valid study to evaluate the WATCHMAN device compared to warfarin. • In PROTECT AF: • Noninferiority for all strokes – 26% lower in device group • Superiority for hemorrhagic stroke – 91% lower in device group • Noninferiority for mortality rate – 39% lower rate in device group In PROTECT AF, there are early safety adverse events, specifically pericardial effusion; these events have decreased over time Holmes DR, et al. The Lancet 2009; 374: 534 -542.
PROTECT AF TRIAL • Randomized, controlled, statistically valid study to evaluate the WATCHMAN device compared to warfarin. • In PROTECT AF: • Noninferiority for all strokes – 26% lower in device group • Superiority for hemorrhagic stroke – 91% lower in device group • Noninferiority for mortality rate – 39% lower rate in device group In PROTECT AF, there are early safety adverse events, specifically pericardial effusion; these events have decreased over time Holmes DR, et al. The Lancet 2009; 374: 534 -542.
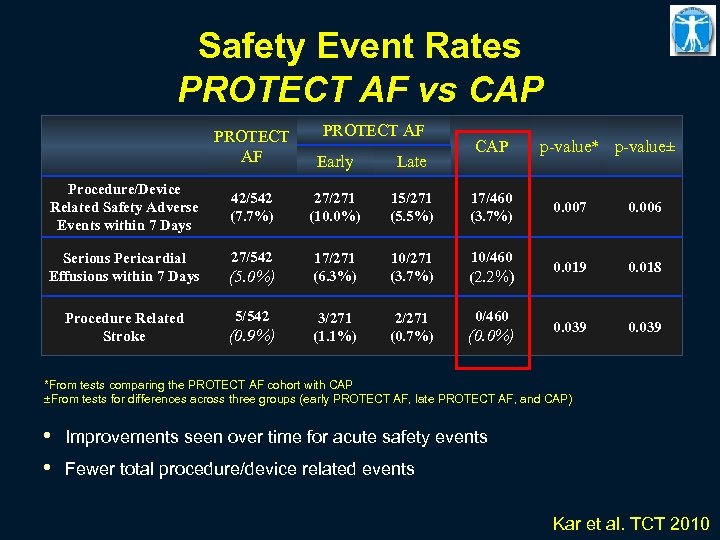 Safety Event Rates PROTECT AF vs CAP PROTECT AF Procedure/Device Related Safety Adverse Events within 7 Days Serious Pericardial Effusions within 7 Days Procedure Related Stroke Early Late 42/542 (7. 7%) 27/271 (10. 0%) 15/271 (5. 5%) 17/460 (3. 7%) 0. 007 0. 006 27/542 17/271 (6. 3%) 10/271 (3. 7%) 10/460 (2. 2%) 0. 019 0. 018 3/271 (1. 1%) 2/271 (0. 7%) 0/460 0. 039 (5. 0%) 5/542 (0. 9%) CAP (0. 0%) p-value* p-value± *From tests comparing the PROTECT AF cohort with CAP ±From tests for differences across three groups (early PROTECT AF, late PROTECT AF, and CAP) • • Improvements seen over time for acute safety events Fewer total procedure/device related events Kar et al. TCT 2010
Safety Event Rates PROTECT AF vs CAP PROTECT AF Procedure/Device Related Safety Adverse Events within 7 Days Serious Pericardial Effusions within 7 Days Procedure Related Stroke Early Late 42/542 (7. 7%) 27/271 (10. 0%) 15/271 (5. 5%) 17/460 (3. 7%) 0. 007 0. 006 27/542 17/271 (6. 3%) 10/271 (3. 7%) 10/460 (2. 2%) 0. 019 0. 018 3/271 (1. 1%) 2/271 (0. 7%) 0/460 0. 039 (5. 0%) 5/542 (0. 9%) CAP (0. 0%) p-value* p-value± *From tests comparing the PROTECT AF cohort with CAP ±From tests for differences across three groups (early PROTECT AF, late PROTECT AF, and CAP) • • Improvements seen over time for acute safety events Fewer total procedure/device related events Kar et al. TCT 2010
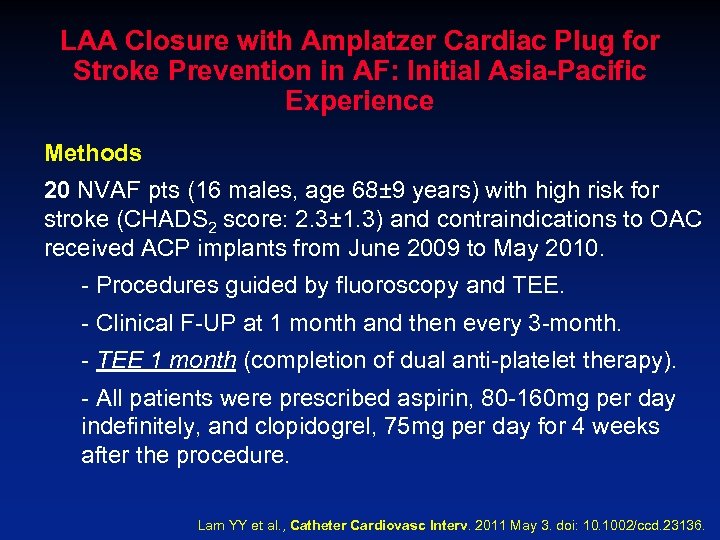 LAA Closure with Amplatzer Cardiac Plug for Stroke Prevention in AF: Initial Asia-Pacific Experience Methods 20 NVAF pts (16 males, age 68± 9 years) with high risk for stroke (CHADS 2 score: 2. 3± 1. 3) and contraindications to OAC received ACP implants from June 2009 to May 2010. - Procedures guided by fluoroscopy and TEE. - Clinical F-UP at 1 month and then every 3 -month. - TEE 1 month (completion of dual anti-platelet therapy). - All patients were prescribed aspirin, 80 -160 mg per day indefinitely, and clopidogrel, 75 mg per day for 4 weeks after the procedure. Lam YY et al. , Catheter Cardiovasc Interv. 2011 May 3. doi: 10. 1002/ccd. 23136.
LAA Closure with Amplatzer Cardiac Plug for Stroke Prevention in AF: Initial Asia-Pacific Experience Methods 20 NVAF pts (16 males, age 68± 9 years) with high risk for stroke (CHADS 2 score: 2. 3± 1. 3) and contraindications to OAC received ACP implants from June 2009 to May 2010. - Procedures guided by fluoroscopy and TEE. - Clinical F-UP at 1 month and then every 3 -month. - TEE 1 month (completion of dual anti-platelet therapy). - All patients were prescribed aspirin, 80 -160 mg per day indefinitely, and clopidogrel, 75 mg per day for 4 weeks after the procedure. Lam YY et al. , Catheter Cardiovasc Interv. 2011 May 3. doi: 10. 1002/ccd. 23136.
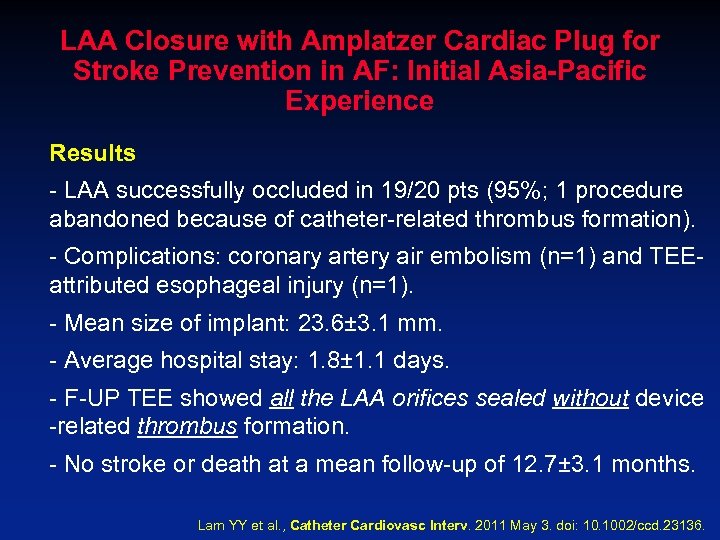 LAA Closure with Amplatzer Cardiac Plug for Stroke Prevention in AF: Initial Asia-Pacific Experience Results - LAA successfully occluded in 19/20 pts (95%; 1 procedure abandoned because of catheter-related thrombus formation). - Complications: coronary artery air embolism (n=1) and TEEattributed esophageal injury (n=1). - Mean size of implant: 23. 6± 3. 1 mm. - Average hospital stay: 1. 8± 1. 1 days. - F-UP TEE showed all the LAA orifices sealed without device -related thrombus formation. - No stroke or death at a mean follow-up of 12. 7± 3. 1 months. Lam YY et al. , Catheter Cardiovasc Interv. 2011 May 3. doi: 10. 1002/ccd. 23136.
LAA Closure with Amplatzer Cardiac Plug for Stroke Prevention in AF: Initial Asia-Pacific Experience Results - LAA successfully occluded in 19/20 pts (95%; 1 procedure abandoned because of catheter-related thrombus formation). - Complications: coronary artery air embolism (n=1) and TEEattributed esophageal injury (n=1). - Mean size of implant: 23. 6± 3. 1 mm. - Average hospital stay: 1. 8± 1. 1 days. - F-UP TEE showed all the LAA orifices sealed without device -related thrombus formation. - No stroke or death at a mean follow-up of 12. 7± 3. 1 months. Lam YY et al. , Catheter Cardiovasc Interv. 2011 May 3. doi: 10. 1002/ccd. 23136.
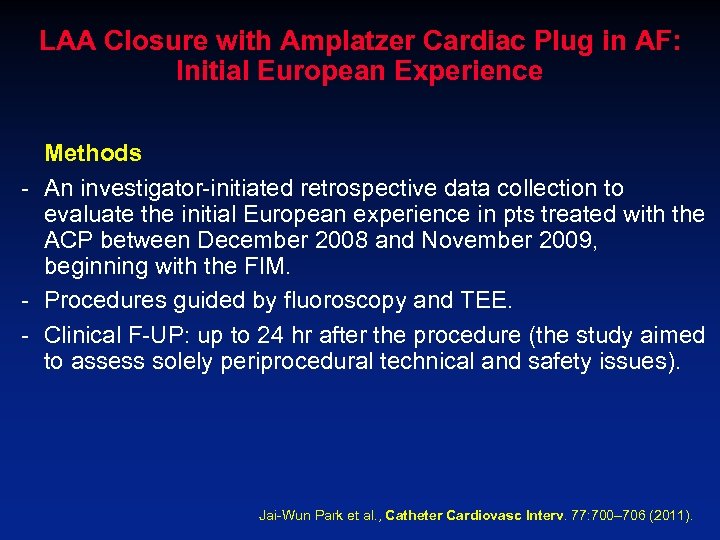 LAA Closure with Amplatzer Cardiac Plug in AF: Initial European Experience Methods - An investigator-initiated retrospective data collection to evaluate the initial European experience in pts treated with the ACP between December 2008 and November 2009, beginning with the FIM. - Procedures guided by fluoroscopy and TEE. - Clinical F-UP: up to 24 hr after the procedure (the study aimed to assess solely periprocedural technical and safety issues). Jai-Wun Park et al. , Catheter Cardiovasc Interv. 77: 700– 706 (2011).
LAA Closure with Amplatzer Cardiac Plug in AF: Initial European Experience Methods - An investigator-initiated retrospective data collection to evaluate the initial European experience in pts treated with the ACP between December 2008 and November 2009, beginning with the FIM. - Procedures guided by fluoroscopy and TEE. - Clinical F-UP: up to 24 hr after the procedure (the study aimed to assess solely periprocedural technical and safety issues). Jai-Wun Park et al. , Catheter Cardiovasc Interv. 77: 700– 706 (2011).
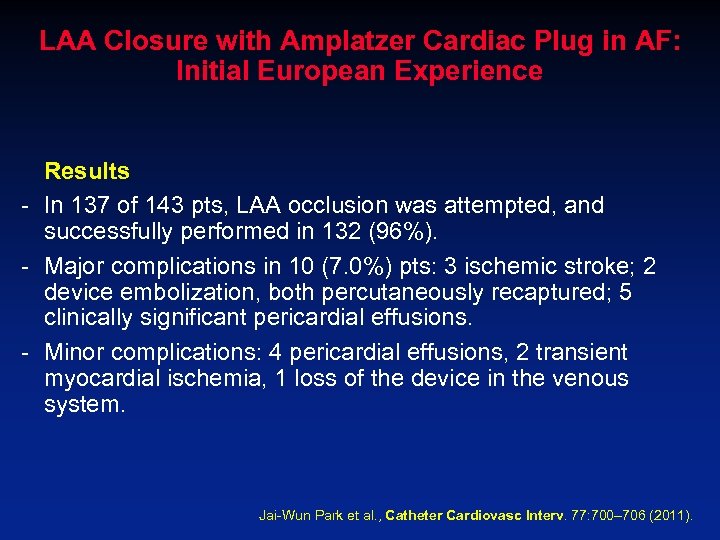 LAA Closure with Amplatzer Cardiac Plug in AF: Initial European Experience Results - In 137 of 143 pts, LAA occlusion was attempted, and successfully performed in 132 (96%). - Major complications in 10 (7. 0%) pts: 3 ischemic stroke; 2 device embolization, both percutaneously recaptured; 5 clinically significant pericardial effusions. - Minor complications: 4 pericardial effusions, 2 transient myocardial ischemia, 1 loss of the device in the venous system. Jai-Wun Park et al. , Catheter Cardiovasc Interv. 77: 700– 706 (2011).
LAA Closure with Amplatzer Cardiac Plug in AF: Initial European Experience Results - In 137 of 143 pts, LAA occlusion was attempted, and successfully performed in 132 (96%). - Major complications in 10 (7. 0%) pts: 3 ischemic stroke; 2 device embolization, both percutaneously recaptured; 5 clinically significant pericardial effusions. - Minor complications: 4 pericardial effusions, 2 transient myocardial ischemia, 1 loss of the device in the venous system. Jai-Wun Park et al. , Catheter Cardiovasc Interv. 77: 700– 706 (2011).
 Current Generation Devices WATCHMAN® System Atritech • Nitinol with 160 micron PET filter) • 21, 24, 27, 30, 33 mm • TEE, Angiography • 12 F • 45 days of Coumadin Amplatzer Cardiac Plug AGA Medical • User friendly – simple repositioning and recapture • Unique design – flexibility to work in varied anatomy • Small profile - 9 F to 13 F delivery sheath
Current Generation Devices WATCHMAN® System Atritech • Nitinol with 160 micron PET filter) • 21, 24, 27, 30, 33 mm • TEE, Angiography • 12 F • 45 days of Coumadin Amplatzer Cardiac Plug AGA Medical • User friendly – simple repositioning and recapture • Unique design – flexibility to work in varied anatomy • Small profile - 9 F to 13 F delivery sheath
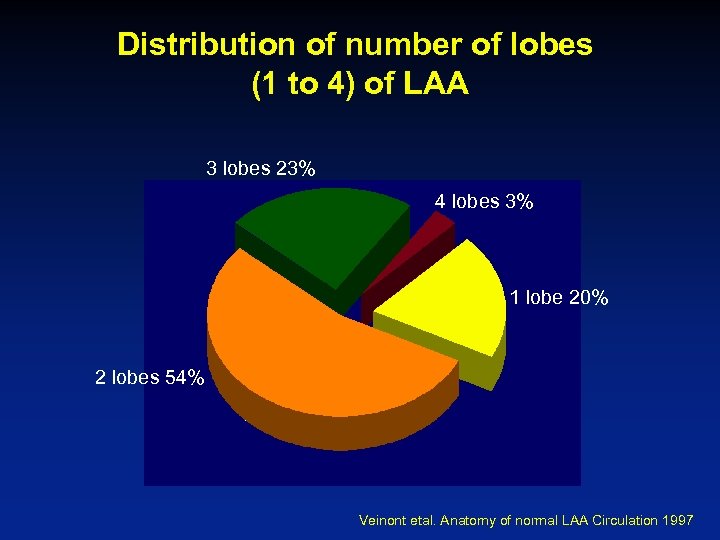 Distribution of number of lobes (1 to 4) of LAA 3 lobes 23% 4 lobes 3% 1 lobe 20% 2 lobes 54% Veinont etal. Anatomy of normal LAA Circulation 1997
Distribution of number of lobes (1 to 4) of LAA 3 lobes 23% 4 lobes 3% 1 lobe 20% 2 lobes 54% Veinont etal. Anatomy of normal LAA Circulation 1997
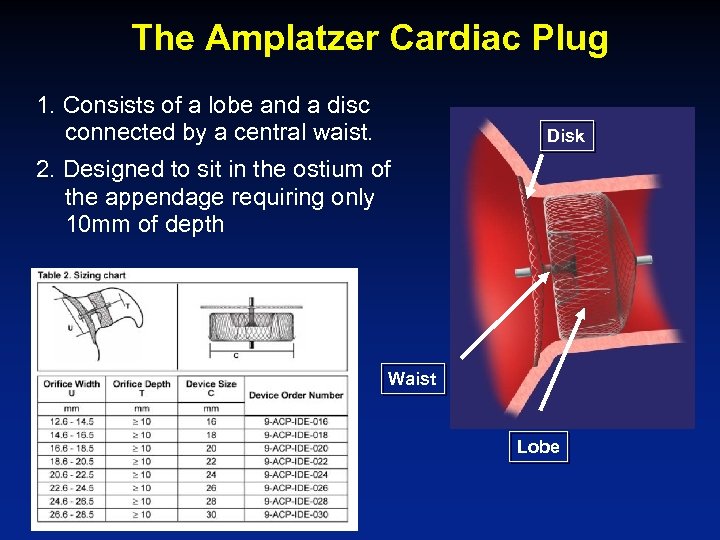 The Amplatzer Cardiac Plug 1. Consists of a lobe and a disc connected by a central waist. Disk 2. Designed to sit in the ostium of the appendage requiring only 10 mm of depth Waist Lobe
The Amplatzer Cardiac Plug 1. Consists of a lobe and a disc connected by a central waist. Disk 2. Designed to sit in the ostium of the appendage requiring only 10 mm of depth Waist Lobe
 Catheter Delivery 9 F, 10 F & 13 Delivery Catheter – 100 cm length – 3 dimensional curve to facilitate access to left atrial appendage. – 0. 035 guide wire compatible dilator • Alignment during device delivery • Where to place transseptal puncture
Catheter Delivery 9 F, 10 F & 13 Delivery Catheter – 100 cm length – 3 dimensional curve to facilitate access to left atrial appendage. – 0. 035 guide wire compatible dilator • Alignment during device delivery • Where to place transseptal puncture
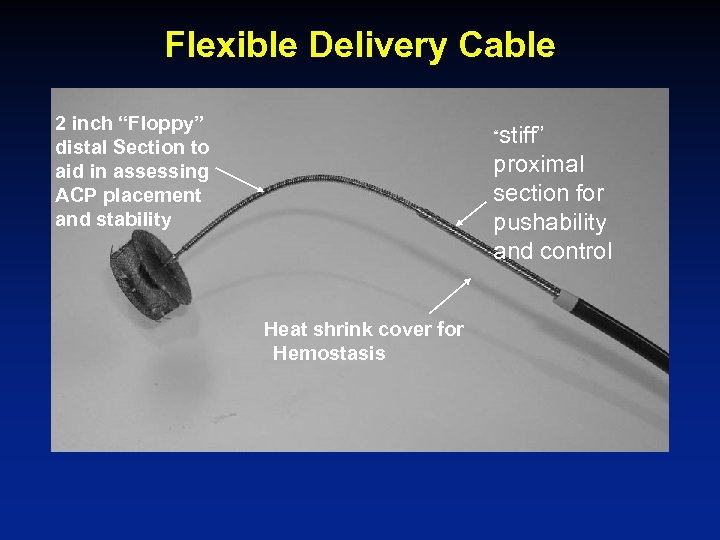 Flexible Delivery Cable 2 inch “Floppy” distal Section to aid in assessing ACP placement and stability “stiff” proximal section for pushability and control Heat shrink cover for Hemostasis
Flexible Delivery Cable 2 inch “Floppy” distal Section to aid in assessing ACP placement and stability “stiff” proximal section for pushability and control Heat shrink cover for Hemostasis
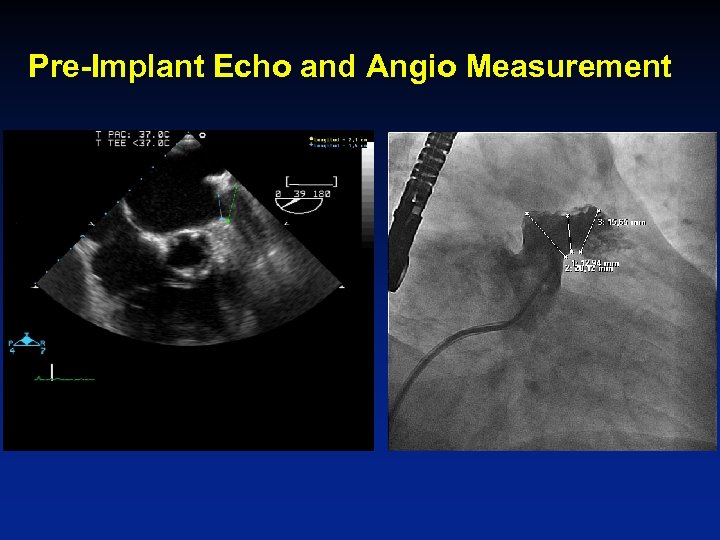 Pre-Implant Echo and Angio Measurement
Pre-Implant Echo and Angio Measurement
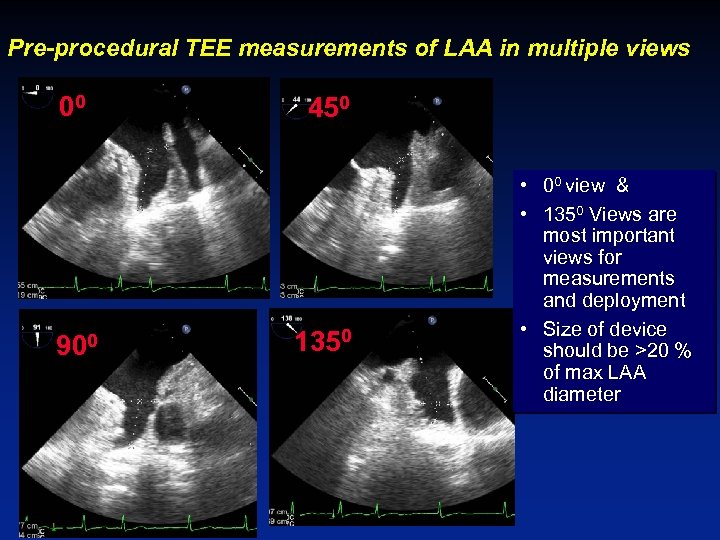 Pre-procedural TEE measurements of LAA in multiple views 00 900 450 1350 • 00 view & • 1350 Views are most important views for measurements and deployment • Size of device should be >20 % of max LAA diameter
Pre-procedural TEE measurements of LAA in multiple views 00 900 450 1350 • 00 view & • 1350 Views are most important views for measurements and deployment • Size of device should be >20 % of max LAA diameter
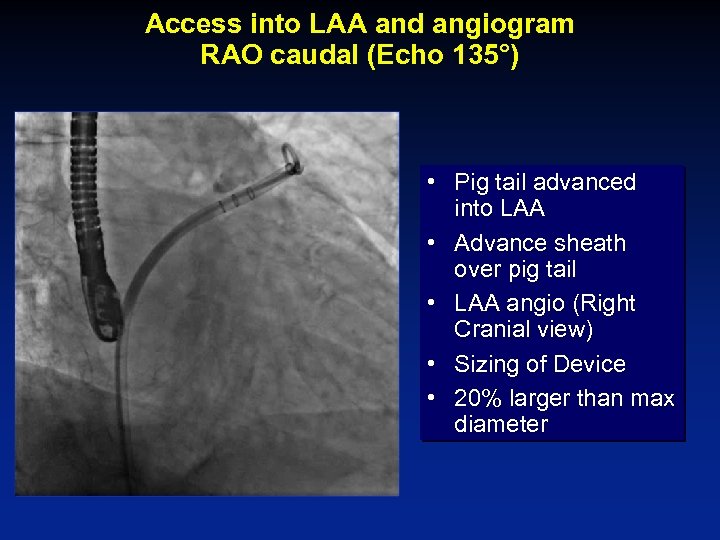 Access into LAA and angiogram RAO caudal (Echo 135°) • Pig tail advanced into LAA • Advance sheath over pig tail • LAA angio (Right Cranial view) • Sizing of Device • 20% larger than max diameter
Access into LAA and angiogram RAO caudal (Echo 135°) • Pig tail advanced into LAA • Advance sheath over pig tail • LAA angio (Right Cranial view) • Sizing of Device • 20% larger than max diameter
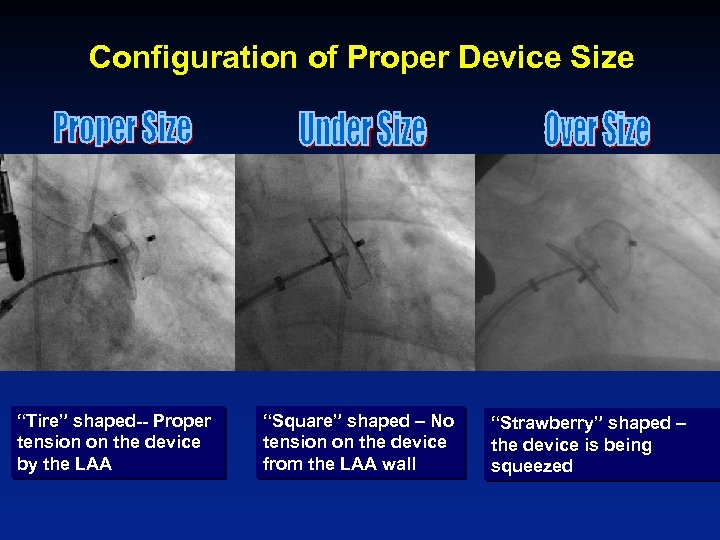 Configuration of Proper Device Size “Tire” shaped-- Proper tension on the device by the LAA “Square” shaped – No tension on the device from the LAA wall “Strawberry” shaped – the device is being squeezed
Configuration of Proper Device Size “Tire” shaped-- Proper tension on the device by the LAA “Square” shaped – No tension on the device from the LAA wall “Strawberry” shaped – the device is being squeezed
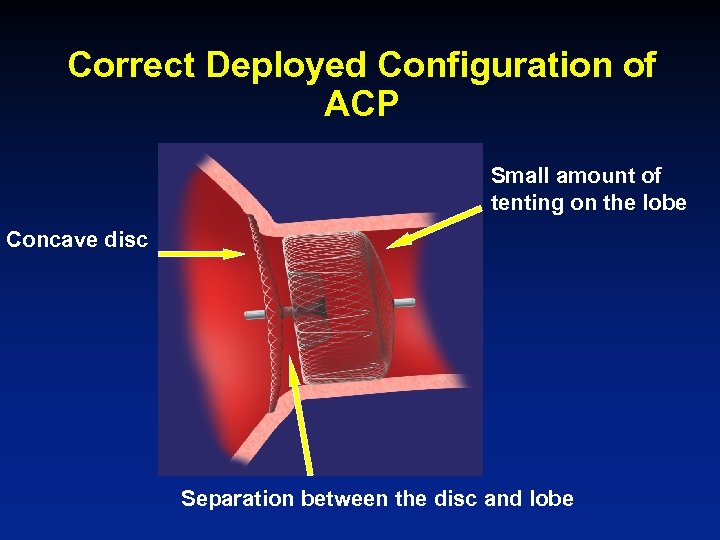 Correct Deployed Configuration of ACP Small amount of tenting on the lobe Concave disc Separation between the disc and lobe
Correct Deployed Configuration of ACP Small amount of tenting on the lobe Concave disc Separation between the disc and lobe
 Figure of 8 subcutaneous suture
Figure of 8 subcutaneous suture
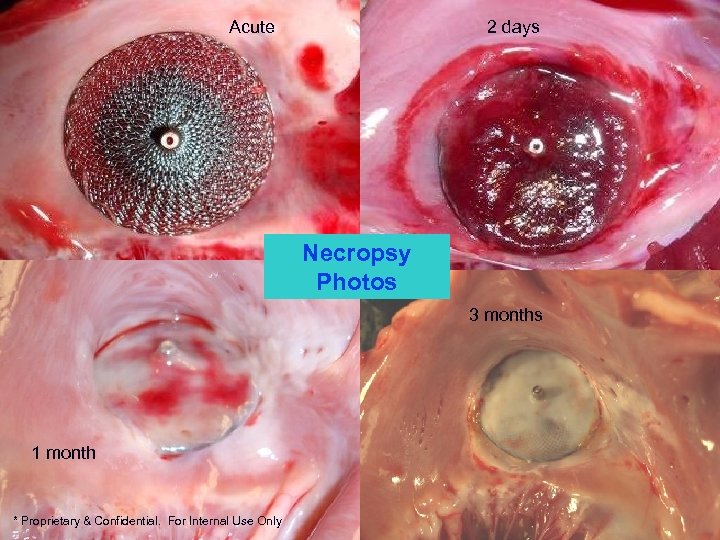 Acute 2 days Necropsy Photos 3 months 1 month * Proprietary & Confidential. For Internal Use Only
Acute 2 days Necropsy Photos 3 months 1 month * Proprietary & Confidential. For Internal Use Only
 Summary Conclusions 1. Important complications of LAA occlusion are: Cardiac tamponade Stroke Residual leak Vascular complications 2. Attention to detail at every step and proper use of imaging (Fluoro/Echo) can help prevent these complications
Summary Conclusions 1. Important complications of LAA occlusion are: Cardiac tamponade Stroke Residual leak Vascular complications 2. Attention to detail at every step and proper use of imaging (Fluoro/Echo) can help prevent these complications
 Thank you
Thank you


