ac3e6042e4ff9ab9584bd6c23f7a8d1c.ppt
- Количество слайдов: 17
 West Nile Virus Update on Assay Development George J. Dawson, Ph. D. Infectious Diseases: Core R & D Abbott Laboratories
West Nile Virus Update on Assay Development George J. Dawson, Ph. D. Infectious Diseases: Core R & D Abbott Laboratories
 West Nile Virus Strategies n Determine marker profile n n n The overlap between RNA, antigen, and Ig. M detection Markers associated with acute, symptomatic infection Develop prototype Ig. M class antibody test for detection of antibodies to WNV n Recombinant proteins have been obtained from two laboratories n EIA’s are being evaluated for Ig. M detection Primer pairs have been selected for in-house RT-PCR n n (Material Transfer Agreement’s executed) Evaluate utility of Ig. M test n n n Screening blood donors Reinstatement of WNV RNA positive donors Diagnosis of acute infection
West Nile Virus Strategies n Determine marker profile n n n The overlap between RNA, antigen, and Ig. M detection Markers associated with acute, symptomatic infection Develop prototype Ig. M class antibody test for detection of antibodies to WNV n Recombinant proteins have been obtained from two laboratories n EIA’s are being evaluated for Ig. M detection Primer pairs have been selected for in-house RT-PCR n n (Material Transfer Agreement’s executed) Evaluate utility of Ig. M test n n n Screening blood donors Reinstatement of WNV RNA positive donors Diagnosis of acute infection
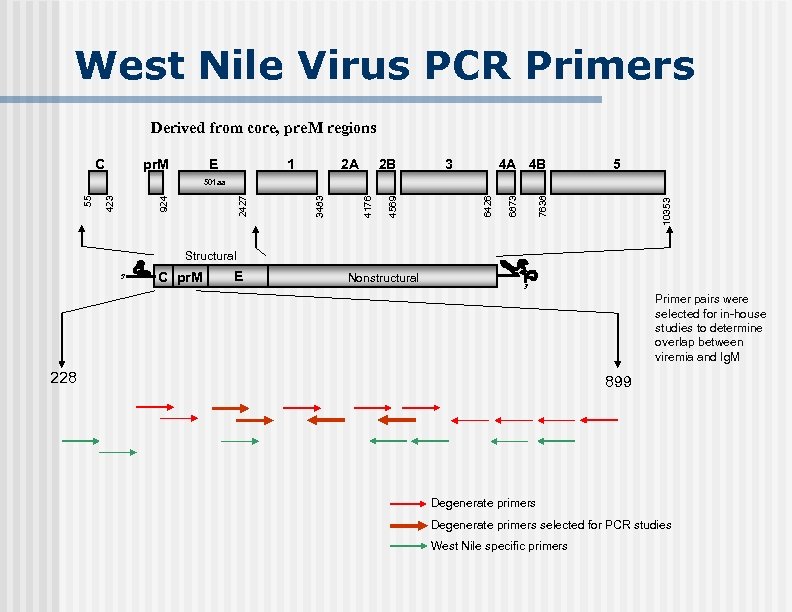 West Nile Virus PCR Primers Derived from core, pre. M regions C pr. M E 1 2 A 2 B 3 4 A 4 B 5 10353 7638 6873 6426 4569 4176 3483 2427 924 423 55 501 aa Structural 5' C pr. M E Nonstructural 3' Primer pairs were selected for in-house studies to determine overlap between viremia and Ig. M 228 899 Degenerate primers selected for PCR studies West Nile specific primers
West Nile Virus PCR Primers Derived from core, pre. M regions C pr. M E 1 2 A 2 B 3 4 A 4 B 5 10353 7638 6873 6426 4569 4176 3483 2427 924 423 55 501 aa Structural 5' C pr. M E Nonstructural 3' Primer pairs were selected for in-house studies to determine overlap between viremia and Ig. M 228 899 Degenerate primers selected for PCR studies West Nile specific primers
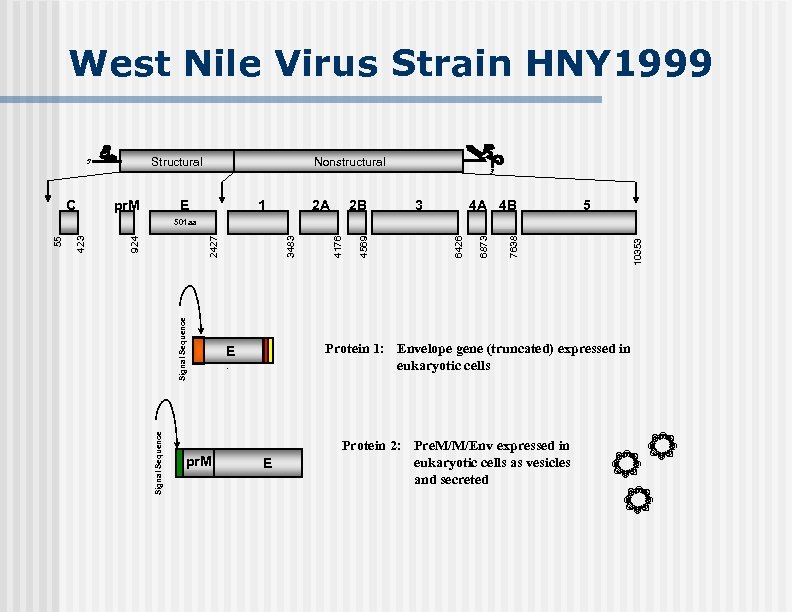 West Nile Virus Strain HNY 1999 Structural 5' C pr. M Nonstructural E 1 2 A 2 B 3' 3 4 A 4 B 5 pr. M Protein 1: Envelope gene (truncated) expressed in eukaryotic cells E. E Protein 2: Pre. M/M/Env expressed in eukaryotic cells as vesicles and secreted 10353 7638 6873 6426 4569 4176 3483 2427 Signal Sequence 924 423 55 501 aa
West Nile Virus Strain HNY 1999 Structural 5' C pr. M Nonstructural E 1 2 A 2 B 3' 3 4 A 4 B 5 pr. M Protein 1: Envelope gene (truncated) expressed in eukaryotic cells E. E Protein 2: Pre. M/M/Env expressed in eukaryotic cells as vesicles and secreted 10353 7638 6873 6426 4569 4176 3483 2427 Signal Sequence 924 423 55 501 aa
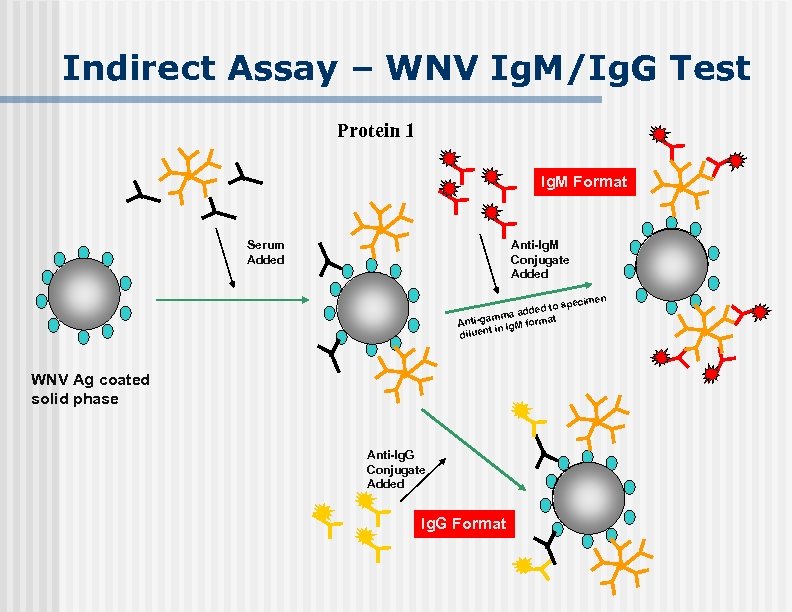 Indirect Assay – WNV Ig. M/Ig. G Test Protein 1 Ig. M Format Serum Added Anti-Ig. M Conjugate Added imen spec ded to ma ad am rmat Anti-g Ig. M fo ent in dilu WNV Ag coated solid phase Anti-Ig. G Conjugate Added Ig. G Format
Indirect Assay – WNV Ig. M/Ig. G Test Protein 1 Ig. M Format Serum Added Anti-Ig. M Conjugate Added imen spec ded to ma ad am rmat Anti-g Ig. M fo ent in dilu WNV Ag coated solid phase Anti-Ig. G Conjugate Added Ig. G Format
 Ig. M Capture EIA Protein 2 Serum Added Anti-Ig. M Coated Solid Phase Antigen Added Conjugate Added
Ig. M Capture EIA Protein 2 Serum Added Anti-Ig. M Coated Solid Phase Antigen Added Conjugate Added
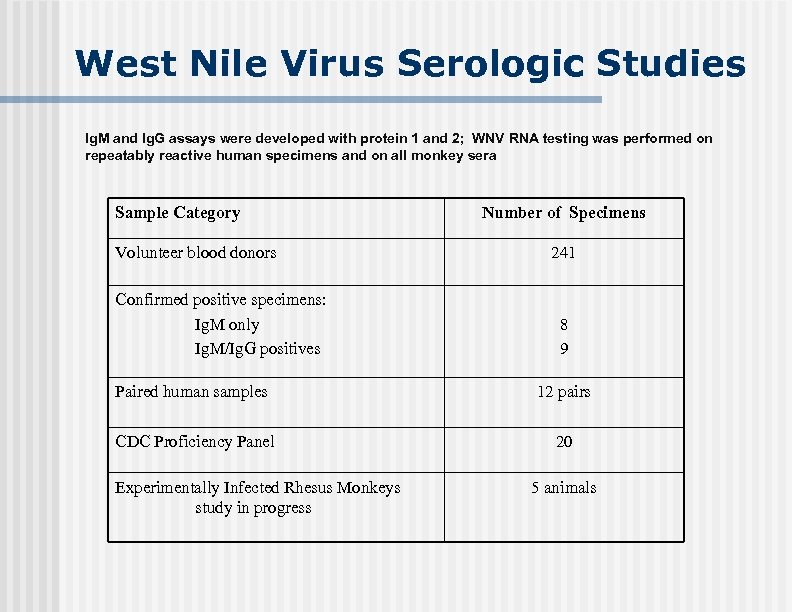 West Nile Virus Serologic Studies Ig. M and Ig. G assays were developed with protein 1 and 2; WNV RNA testing was performed on repeatably reactive human specimens and on all monkey sera Sample Category Volunteer blood donors Confirmed positive specimens: Ig. M only Ig. M/Ig. G positives Number of Specimens 241 8 9 Paired human samples 12 pairs CDC Proficiency Panel 20 Experimentally Infected Rhesus Monkeys study in progress 5 animals
West Nile Virus Serologic Studies Ig. M and Ig. G assays were developed with protein 1 and 2; WNV RNA testing was performed on repeatably reactive human specimens and on all monkey sera Sample Category Volunteer blood donors Confirmed positive specimens: Ig. M only Ig. M/Ig. G positives Number of Specimens 241 8 9 Paired human samples 12 pairs CDC Proficiency Panel 20 Experimentally Infected Rhesus Monkeys study in progress 5 animals
 West Nile Virus Serologic Studies n Rationale for Assay Format Selection(s) n The original data demonstrating the utility of Protein 1 for WNV diagnostics utilized an indirect assay format • n The original data demonstrating the utility of Protein 2 for WNV diagnostics utilized an Ig. M Capture assay format • n Data generated in-house for Protein 1 with an Ig. M Capture format was not adequate In-house studies indicated that performance of Protein 2 in an indirect assay format was not adequate Additional studies are planned
West Nile Virus Serologic Studies n Rationale for Assay Format Selection(s) n The original data demonstrating the utility of Protein 1 for WNV diagnostics utilized an indirect assay format • n The original data demonstrating the utility of Protein 2 for WNV diagnostics utilized an Ig. M Capture assay format • n Data generated in-house for Protein 1 with an Ig. M Capture format was not adequate In-house studies indicated that performance of Protein 2 in an indirect assay format was not adequate Additional studies are planned
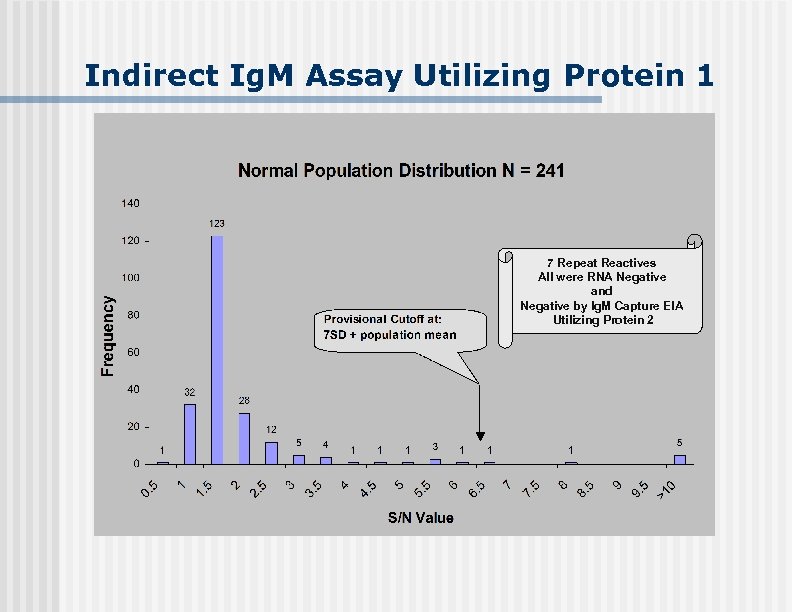 Indirect Ig. M Assay Utilizing Protein 1 7 Repeat Reactives All were RNA Negative and Negative by Ig. M Capture EIA Utilizing Protein 2
Indirect Ig. M Assay Utilizing Protein 1 7 Repeat Reactives All were RNA Negative and Negative by Ig. M Capture EIA Utilizing Protein 2
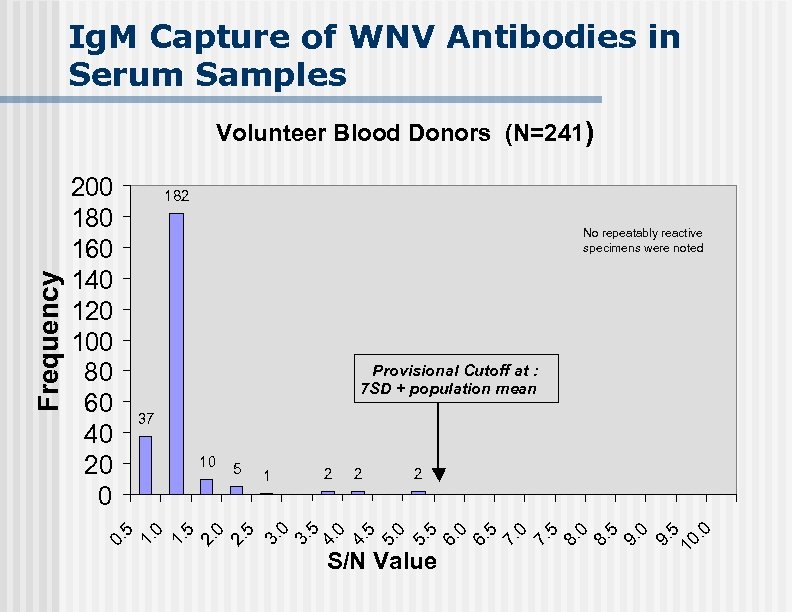 Ig. M Capture of WNV Antibodies in Serum Samples 200 180 160 140 120 100 80 60 40 20 0 182 No repeatably reactive specimens were noted Provisional Cutoff at : 7 SD + population mean 37 2 2 2 0 3. 5 4. 0 4. 5 5. 0 5. 5 6. 0 6. 5 7. 0 7. 5 8. 0 8. 5 9. 0 9. 5 10. 0 1 3. 2. 5 5 0 2. 5 1. 0 10 5 0. Frequency Volunteer Blood Donors (N=241) S/N Value
Ig. M Capture of WNV Antibodies in Serum Samples 200 180 160 140 120 100 80 60 40 20 0 182 No repeatably reactive specimens were noted Provisional Cutoff at : 7 SD + population mean 37 2 2 2 0 3. 5 4. 0 4. 5 5. 0 5. 5 6. 0 6. 5 7. 0 7. 5 8. 0 8. 5 9. 0 9. 5 10. 0 1 3. 2. 5 5 0 2. 5 1. 0 10 5 0. Frequency Volunteer Blood Donors (N=241) S/N Value
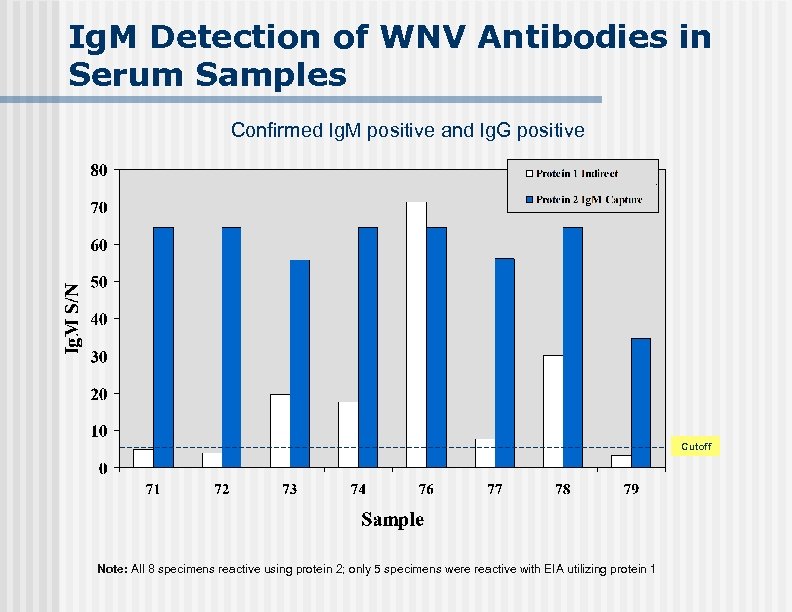 Ig. M Detection of WNV Antibodies in Serum Samples Confirmed Ig. M positive and Ig. G positive. Cutoff Note: All 8 specimens reactive using protein 2; only 5 specimens were reactive with EIA utilizing protein 1
Ig. M Detection of WNV Antibodies in Serum Samples Confirmed Ig. M positive and Ig. G positive. Cutoff Note: All 8 specimens reactive using protein 2; only 5 specimens were reactive with EIA utilizing protein 1
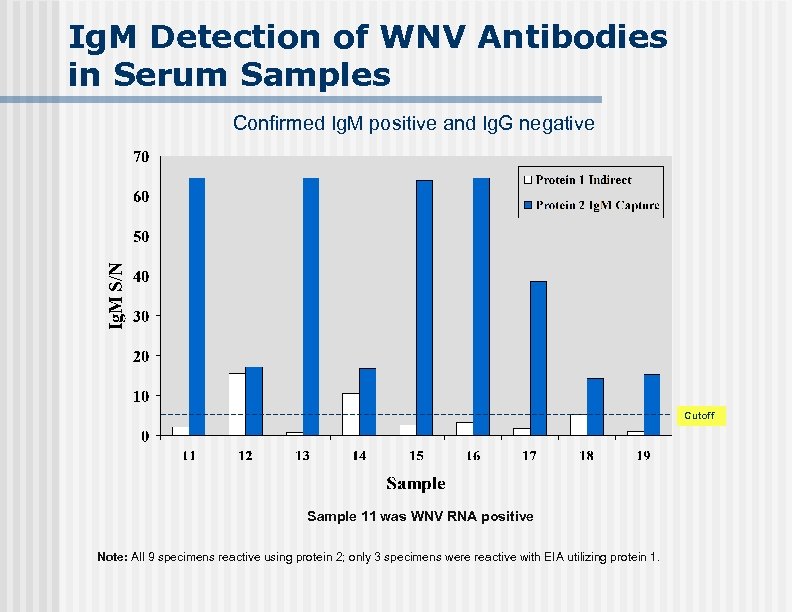 Ig. M Detection of WNV Antibodies in Serum Samples Confirmed Ig. M positive and Ig. G negative Cutoff Sample 11 was WNV RNA positive Note: All 9 specimens reactive using protein 2; only 3 specimens were reactive with EIA utilizing protein 1.
Ig. M Detection of WNV Antibodies in Serum Samples Confirmed Ig. M positive and Ig. G negative Cutoff Sample 11 was WNV RNA positive Note: All 9 specimens reactive using protein 2; only 3 specimens were reactive with EIA utilizing protein 1.
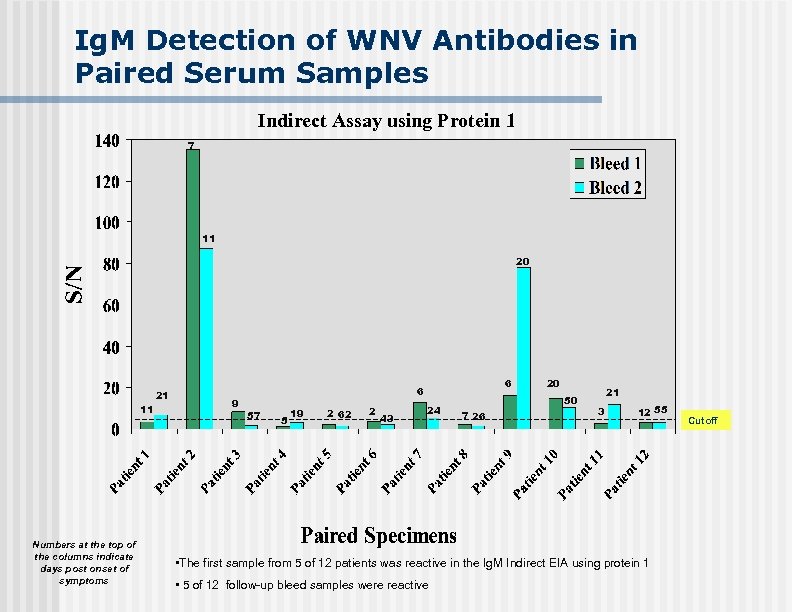 Ig. M Detection of WNV Antibodies in Paired Serum Samples Indirect Assay using Protein 1 7 11 20 21 11 Numbers at the top of the columns indicate days post onset of symptoms 9 6 6 57 5 19 2 62 2 43 24 20 50 7 26 21 3 12 55 • The first sample from 5 of 12 patients was reactive in the Ig. M Indirect EIA using protein 1 • 5 of 12 follow-up bleed samples were reactive Cutoff
Ig. M Detection of WNV Antibodies in Paired Serum Samples Indirect Assay using Protein 1 7 11 20 21 11 Numbers at the top of the columns indicate days post onset of symptoms 9 6 6 57 5 19 2 62 2 43 24 20 50 7 26 21 3 12 55 • The first sample from 5 of 12 patients was reactive in the Ig. M Indirect EIA using protein 1 • 5 of 12 follow-up bleed samples were reactive Cutoff
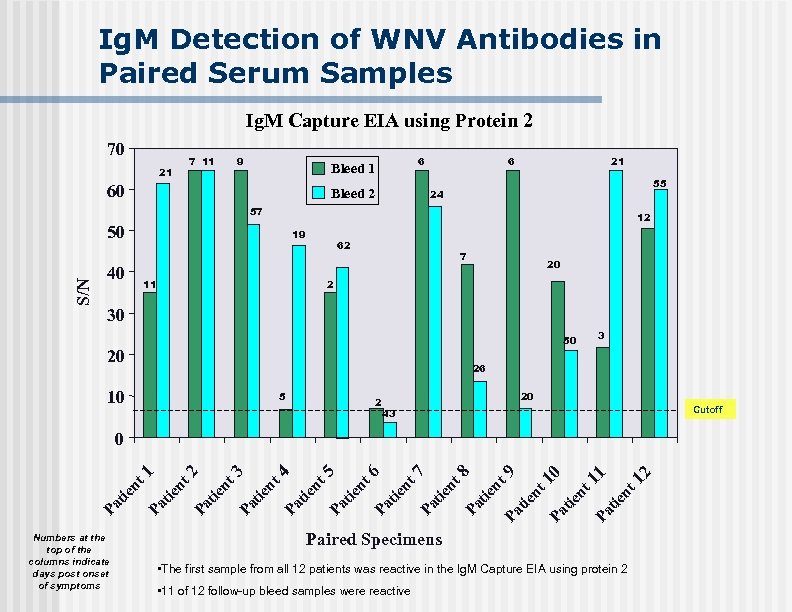 Ig. M Detection of WNV Antibodies in Paired Serum Samples Ig. M Capture EIA using Protein 2 70 7 11 21 9 6 Bleed 1 60 Bleed 2 6 21 55 24 57 12 S/N 50 19 40 62 11 7 20 2 30 50 20 3 26 10 5 2 20 Cutoff 43 Numbers at the top of the columns indicate days post onset of symptoms 2 10 Pa tie nt 11 Pa tie nt 1 nt 9 Pa tie nt 8 Pa tie nt 7 Pa tie nt 6 Pa nt tie Pa tie nt 5 4 Pa tie nt 3 Pa tie nt 2 Pa nt tie Pa Pa tie nt 1 0 Paired Specimens • The first sample from all 12 patients was reactive in the Ig. M Capture EIA using protein 2 • 11 of 12 follow-up bleed samples were reactive
Ig. M Detection of WNV Antibodies in Paired Serum Samples Ig. M Capture EIA using Protein 2 70 7 11 21 9 6 Bleed 1 60 Bleed 2 6 21 55 24 57 12 S/N 50 19 40 62 11 7 20 2 30 50 20 3 26 10 5 2 20 Cutoff 43 Numbers at the top of the columns indicate days post onset of symptoms 2 10 Pa tie nt 11 Pa tie nt 1 nt 9 Pa tie nt 8 Pa tie nt 7 Pa tie nt 6 Pa nt tie Pa tie nt 5 4 Pa tie nt 3 Pa tie nt 2 Pa nt tie Pa Pa tie nt 1 0 Paired Specimens • The first sample from all 12 patients was reactive in the Ig. M Capture EIA using protein 2 • 11 of 12 follow-up bleed samples were reactive
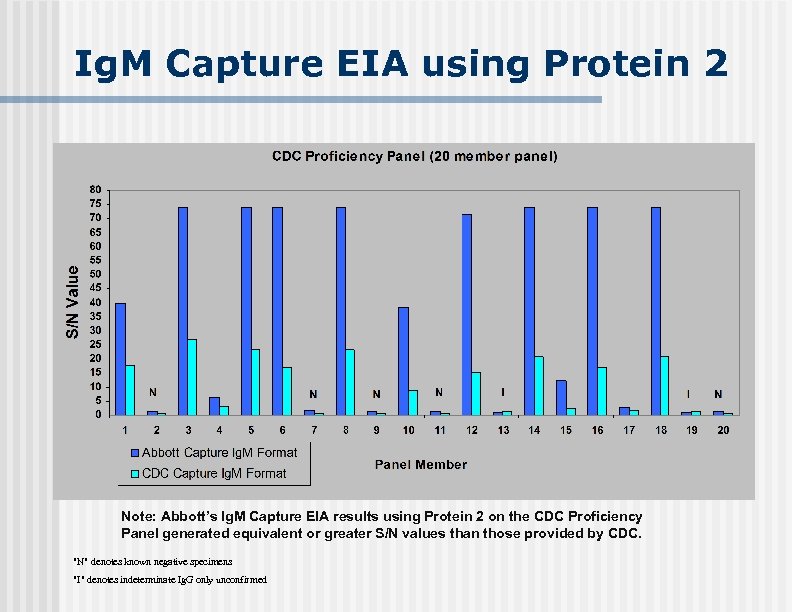 Ig. M Capture EIA using Protein 2 Ig. G Reactivity + - + + + - + I + + + Note: Abbott’s Ig. M Capture EIA results using Protein 2 on the CDC Proficiency Panel generated equivalent or greater S/N values than those provided by CDC. "N" denotes known negative specimens "I" denotes indeterminate Ig. G only unconfirmed I -
Ig. M Capture EIA using Protein 2 Ig. G Reactivity + - + + + - + I + + + Note: Abbott’s Ig. M Capture EIA results using Protein 2 on the CDC Proficiency Panel generated equivalent or greater S/N values than those provided by CDC. "N" denotes known negative specimens "I" denotes indeterminate Ig. G only unconfirmed I -
 SUMMARY n Protein 2 detected more acute phase WNV samples than Protein 1 n n n Data suggest that conformational epitopes present in Protein 2 are important for early Ig. M detection The cell line expressing Protein 2 is being scaled-up in-house Preliminary evaluation of Ig. M testing indicates: n n For blood screening – additional studies are required The Ig. M response was robust in most symptomatic individuals • Ig. M in conjunction with Ig. G could be part of the donor reinstatement algorithm • For diagnostics, Ig. M is the preferred method of detection n Prototype assay formats are being investigated n n EIA’s on polystyrene beads Microparticle based assays on Ax. SYM and IMx
SUMMARY n Protein 2 detected more acute phase WNV samples than Protein 1 n n n Data suggest that conformational epitopes present in Protein 2 are important for early Ig. M detection The cell line expressing Protein 2 is being scaled-up in-house Preliminary evaluation of Ig. M testing indicates: n n For blood screening – additional studies are required The Ig. M response was robust in most symptomatic individuals • Ig. M in conjunction with Ig. G could be part of the donor reinstatement algorithm • For diagnostics, Ig. M is the preferred method of detection n Prototype assay formats are being investigated n n EIA’s on polystyrene beads Microparticle based assays on Ax. SYM and IMx
 Studies Planned or In Progress n Optimization of assay n n Confirmatory strategies n n n RT-PCR on early phase specimens Ig. G test with Protein 2 Ig. M and Ig. G tests with alternate protein Plaque reduction neutralization tests ( in collaboration with CDC or state labs) External Studies n n Incubation times, sample volume Concentration of rare reagents, washing steps Processing steps for rare reagents Serologic studies on quarantined units from 2002 epidemic Donor reinstatement studies as companion to WNV NAT efforts Testing at state and/or hospital laboratories during 2003 WNV season Additional proteins are being expressed in-house to determine their potential utility
Studies Planned or In Progress n Optimization of assay n n Confirmatory strategies n n n RT-PCR on early phase specimens Ig. G test with Protein 2 Ig. M and Ig. G tests with alternate protein Plaque reduction neutralization tests ( in collaboration with CDC or state labs) External Studies n n Incubation times, sample volume Concentration of rare reagents, washing steps Processing steps for rare reagents Serologic studies on quarantined units from 2002 epidemic Donor reinstatement studies as companion to WNV NAT efforts Testing at state and/or hospital laboratories during 2003 WNV season Additional proteins are being expressed in-house to determine their potential utility


