dfa2612bae795a9d80c2ad3d480661f3.ppt
- Количество слайдов: 15

West Nile Source: H. Gelderblom, RKI First international proficiency study on West Nile virus molecular detection. ROBERT KOCH INSTITUT Matthias Niedrig, Sonja Linke Christian Drosten ENIVD 06/06 3/19/2018 Hervé Zeller

Distribution of disease vectors and WN virus in Europe Aedes albopictus Ochlerotatus atropalpus WN Ochlerotatus japonicus WN WN source: Snow & Ramsdale, Biologist (2002) 49 (2) Hubalek & Halauzka; EID, (1999) 5 (5) ENIVD 05/06 3/19/2018 WN WN
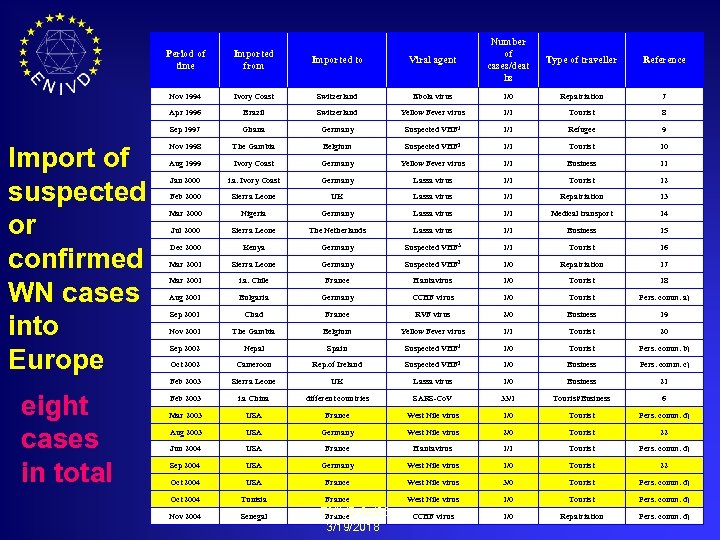
Viral agent Number of cases/deat hs Type of traveller Reference Switzerland Ebola virus 1/0 Repatriation 7 Brazil Switzerland Yellow Fever virus 1/1 Tourist 8 Ghana Germany Suspected VHF 1 1/1 Refugee 9 VHF 2 1/1 Tourist 10 Period of time Imported to Nov 1994 Ivory Coast Apr 1996 Sep 1997 Import of suspected or confirmed WN cases into Europe Imported from Nov 1998 The Gambia Belgium Suspected Aug 1999 Ivory Coast Germany Yellow Fever virus 1/1 Business 11 Jan 2000 i. a. Ivory Coast Germany Lassa virus 1/1 Tourist 12 Feb 2000 Sierra Leone UK Lassa virus 1/1 Repatriation 13 Mar 2000 Nigeria Germany Lassa virus 1/1 Medical transport 14 Jul 2000 Sierra Leone The Netherlands Lassa virus 1/1 Business 15 VHF 3 1/1 Tourist 16 Kenya Germany Suspected Mar 2001 Sierra Leone Germany Suspected VHF 2 1/0 Repatriation 17 Mar 2001 i. a. Chile France Hantavirus 1/0 Tourist 18 Aug 2001 Bulgaria Germany CCHF virus 1/0 Tourist Pers. comm. a) Sep 2001 Chad France RVF virus 2/0 Business 19 Nov 2001 The Gambia Belgium Yellow Fever virus 1/1 Tourist 20 Sep 2002 Nepal Spain Suspected VHF 1 1/0 Tourist Pers. comm. b) Oct 2002 Cameroon Rep. of Ireland Suspected VHF 2 1/0 Business Pers. comm. c) Feb 2003 eight cases in total Dec 2000 Sierra Leone UK Lassa virus 1/0 Business 21 Feb 2003 i. a China different countries SARS-Co. V 33/1 Tourist/Business 6 Mar 2003 USA France West Nile virus 1/0 Tourist Pers. comm. d) Aug 2003 USA Germany West Nile virus 2/0 Tourist 22 Jun 2004 USA France Hantavirus 1/1 Tourist Pers. comm. d) Sep 2004 USA Germany West Nile virus 1/0 Tourist 22 Oct 2004 USA France West Nile virus 3/0 Tourist Pers. comm. d) Oct 2004 Tunisia France West Nile virus 1/0 Tourist Pers. comm. d) Nov 2004 Senegal CCHF virus 1/0 Repatriation Pers. comm. d) ENIVD 05/06 France 3/19/2018

Preparation of PCR samples for the External Quality Assurance (EQA) ü cell culture supernatants of infected ü ü cells with the respective viruses heat treatment for 1 h at 56°C and gamma irradiation of 30 k. Gy check for infectivity by cell cultivation dilute with human plasma, aliquots of 100 µl are frozen at – 70°C before freeze-drying for 12 hours samples were distributed by normal mail service ENIVD 05/06 3/19/2018
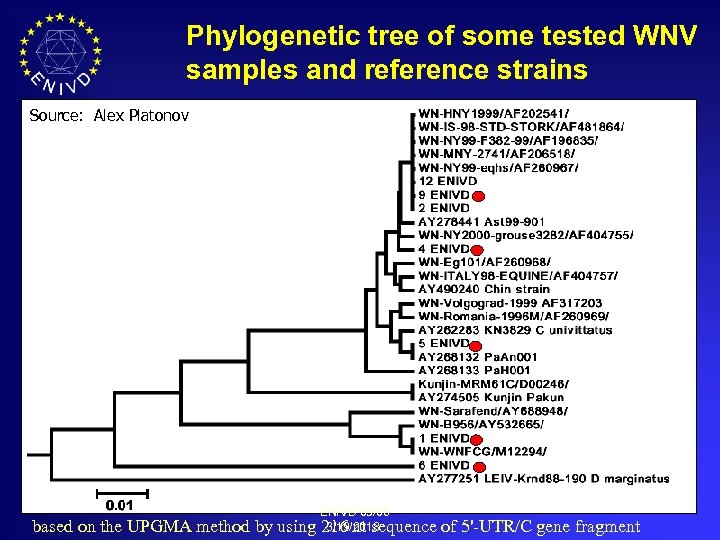
Phylogenetic tree of some tested WNV samples and reference strains Source: Alex Platonov based on the UPGMA method by ENIVD 05/06 3/19/2018 using 216 nt sequence of 5'-UTR/C gene fragment
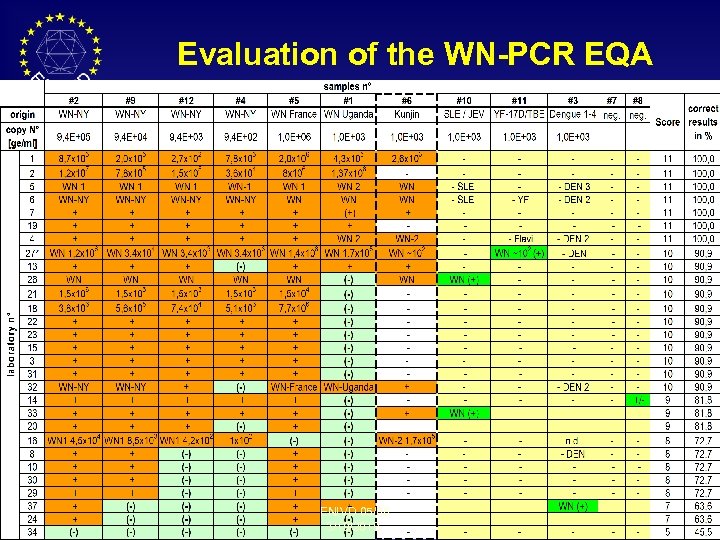
Evaluation of the WN-PCR EQA ENIVD 05/06 3/19/2018
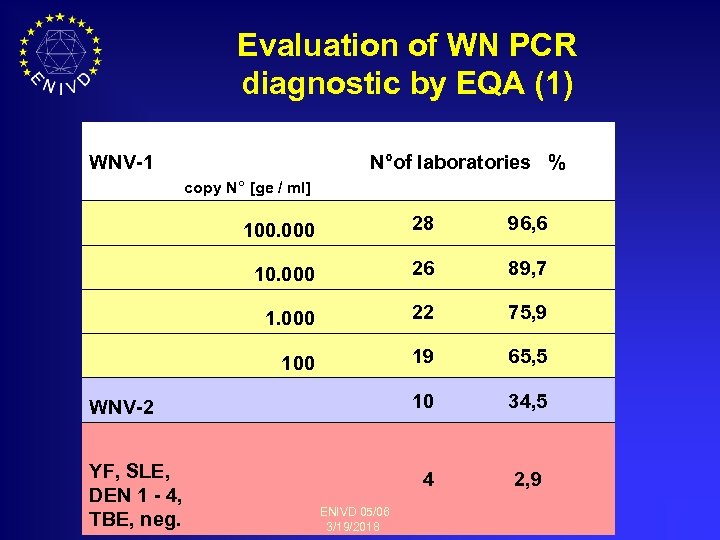
Evaluation of WN PCR diagnostic by EQA (1) WNV-1 N°of laboratories % copy N° [ge / ml] 100. 000 28 96, 6 10. 000 26 89, 7 1. 000 22 75, 9 100 19 65, 5 10 34, 5 4 2, 9 WNV-2 YF, SLE, DEN 1 - 4, TBE, neg. ENIVD 05/06 3/19/2018
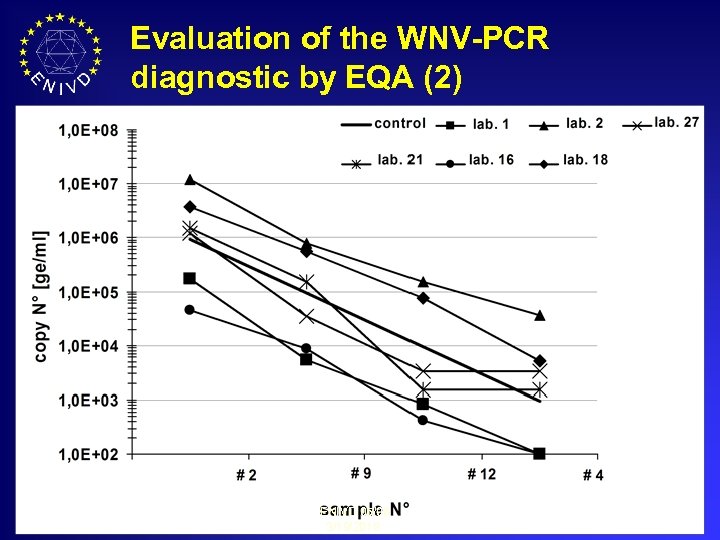
Evaluation of the WNV-PCR diagnostic by EQA (2) ENIVD 05/06 3/19/2018
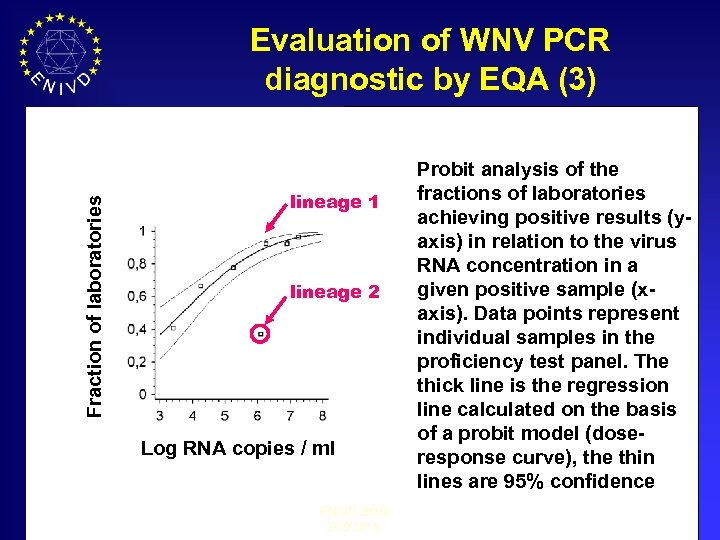
Fraction of laboratories Evaluation of WNV PCR diagnostic by EQA (3) lineage 1 lineage 2 Log RNA copies / ml ENIVD 05/06 3/19/2018 Probit analysis of the fractions of laboratories achieving positive results (yaxis) in relation to the virus RNA concentration in a given positive sample (xaxis). Data points represent individual samples in the proficiency test panel. The thick line is the regression line calculated on the basis of a probit model (doseresponse curve), the thin lines are 95% confidence

Results WNV PCR EQA l Does the PCR method used has an influence on the quality of the results? n= 28; 15 real time PCR, 11 RT-PCR, 2 commercial There is no significant correlation between the PCR method and the quality of the results. l Do other factors influence the PCR results ? genome preparation: n= 28, 19 Qiagen, 5 Roche, 4 other There is no significant correlation between the genome preparation method and the quality of the results. ENIVD 05/06 3/19/2018
- Conclusions - WNV PCR EQA l using cell culture supernatants, diluting samples with human plasma and freeze-drying before shipping is useful for sample preparation - gamma-irradiation is a suitable method for inactivation of samples l 7 laboratories showed excellent results (sensitivity of 100%), whereas 10 laboratories that were less sensitive need to improve their methods l PCR methods for detection of WNV should also detect lineage 2 which is now present in Europe. l 4 laboratories create false positive results ENIVD 05/06 3/19/2018
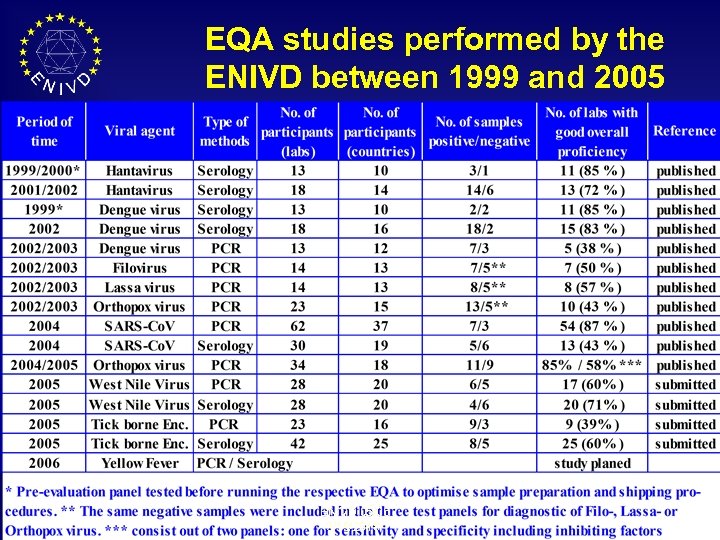
EQA studies performed by the ENIVD between 1999 and 2005 ENIVD 05/06 3/19/2018

What‘s coming next ENIVD 05/06 3/19/2018

Acknowledgement: H. Bin, Central Virol. Laboratory, Sheba Medical Center, Tel Hashomer, Israel D. J. Gubler, CDC, Fort Collins, USA A. Platonov, Central Research Institute of Epidemiology, Moscow, Russia H. Zeller, Jean Mérieux-INSERM P 4 laboratory, Institut Pasteur, Lyon, France C. Drosten, Bernhard Nocht Institut, Hamburg, Germany Stephan W. Aberle, Franz Heinz, Medical University of Vienna, Austria Parts of this project were supported by the European Network for diagnostics of "Imported" viral Diseases (ENIVD) presently funded by DG-SANCO of the European Community under the program „AIDS and other communicable diseases“ ENIVD 05/06 3/19/2018

European Network for diagnostics of "Imported" viral Diseases (ENIVD) for further information: www. enivd. de www. enivd. net www. enivd. org ENIVD 06/06 3/19/2018
dfa2612bae795a9d80c2ad3d480661f3.ppt