725e352a2b5e0579611b6024bb3b98ca.ppt
- Количество слайдов: 63
 Welcome to ITRC’s Internet Training ITRC Technical and Regulatory Guidance Document: “Enhanced In Situ Bioremediation of Chlorinated Solvents in Ground Water” Sponsored by the ITRC, the EPA-TIO and the RTDF 1 www. itrcweb. org
Welcome to ITRC’s Internet Training ITRC Technical and Regulatory Guidance Document: “Enhanced In Situ Bioremediation of Chlorinated Solvents in Ground Water” Sponsored by the ITRC, the EPA-TIO and the RTDF 1 www. itrcweb. org
 ITRC – Shaping the Future of Regulatory Acceptance ITRC Internet Training Courses ITRC Membership é Natural Attenuation é EISB (Enhanced In Situ States Bioremediation) é Permeable Reactive Barriers (basic and advanced) é Diffusion Samplers é Phytotechnologies Federal é ISCO (In Situ Chemical Oxidation) Partners é Constructed Treatment Wetlands é Small Arms Firing Range Characterization and Remediation Sponsors é Systematic Approach to In Situ Bioremediation 2 www. itrcweb. org ITRC Member State Industry, Academia, Consultants, Citizen Stakeholders
ITRC – Shaping the Future of Regulatory Acceptance ITRC Internet Training Courses ITRC Membership é Natural Attenuation é EISB (Enhanced In Situ States Bioremediation) é Permeable Reactive Barriers (basic and advanced) é Diffusion Samplers é Phytotechnologies Federal é ISCO (In Situ Chemical Oxidation) Partners é Constructed Treatment Wetlands é Small Arms Firing Range Characterization and Remediation Sponsors é Systematic Approach to In Situ Bioremediation 2 www. itrcweb. org ITRC Member State Industry, Academia, Consultants, Citizen Stakeholders
 Enhanced In Situ Bioremediation of Solvents in Ground Water EISB Presentation Overview ü Regulatory Issues ü Chemical Terminology ü Microbial Processes / Degradation Pathways ü Questions & Answers ü Amendments & Delivery ü Field Pilots / Technical Requirements ü Benefits / Rules of Thumb ü Questions & Answers ü Links to additional resources ü Your feedback 3 Logistical Reminders ü Phone Audience l Keep phone on mute l * 6 to mute your phone and again to un-mute l Do NOT put call on hold ü Simulcast Audience l Use at top of each slide to submit questions
Enhanced In Situ Bioremediation of Solvents in Ground Water EISB Presentation Overview ü Regulatory Issues ü Chemical Terminology ü Microbial Processes / Degradation Pathways ü Questions & Answers ü Amendments & Delivery ü Field Pilots / Technical Requirements ü Benefits / Rules of Thumb ü Questions & Answers ü Links to additional resources ü Your feedback 3 Logistical Reminders ü Phone Audience l Keep phone on mute l * 6 to mute your phone and again to un-mute l Do NOT put call on hold ü Simulcast Audience l Use at top of each slide to submit questions
 Today’s Presenters é Ron Buchanan - Instructor l Principal Consultant, Dupont Ø ron. j. buchanan@usa. dupont. com é Guy Tomassoni – Instructor l Environmental Protection Specialist, EPA Office of Solid Waste / Corrective Actions Ø Tomassoni. guy@epa. gov é Mary Yelken - Moderator l 4 Environmental Programs Advisor, WGA/ITRC Ø myelken@westgov. org
Today’s Presenters é Ron Buchanan - Instructor l Principal Consultant, Dupont Ø ron. j. buchanan@usa. dupont. com é Guy Tomassoni – Instructor l Environmental Protection Specialist, EPA Office of Solid Waste / Corrective Actions Ø Tomassoni. guy@epa. gov é Mary Yelken - Moderator l 4 Environmental Programs Advisor, WGA/ITRC Ø myelken@westgov. org
 What is EISB? é Engineered technique for optimizing subsurface conditions (hydrogeological, geochemical, microbial) to biodegrade contaminants in situ é Includes injecting substrate and nutrients (i. e. , amendments) é Creates in situ conditions conducive to microbes é May include extracting and recirculating amended groundwater é Establishes/accelerates contaminant biodegradation in situ 5
What is EISB? é Engineered technique for optimizing subsurface conditions (hydrogeological, geochemical, microbial) to biodegrade contaminants in situ é Includes injecting substrate and nutrients (i. e. , amendments) é Creates in situ conditions conducive to microbes é May include extracting and recirculating amended groundwater é Establishes/accelerates contaminant biodegradation in situ 5
 Regulatory Issues éEISB is a complex regulatory process lregulatory authorities lregulatory status of contaminated groundwater lregulation status of amendments 6
Regulatory Issues éEISB is a complex regulatory process lregulatory authorities lregulatory status of contaminated groundwater lregulation status of amendments 6
 Regulatory Authorities é Regulatory Authority/Authorities l RCRA (Subtitle C) or CERCLA (Section 104 or 106) l Safe Drinking Water Act - 40 CFR Section 144 Underground Injection Control Program l State Cleanup programs 7
Regulatory Authorities é Regulatory Authority/Authorities l RCRA (Subtitle C) or CERCLA (Section 104 or 106) l Safe Drinking Water Act - 40 CFR Section 144 Underground Injection Control Program l State Cleanup programs 7
 Regulatory Status of Contaminated Ground Water é Contaminated media, including ground water, may “contain” hazardous wastes and therefore be subject to regulation under RCRA subtitle C (contained-in policy) é If ground water contains HW we must understand application of; l l l 8 Land Disposal Restrictions (LDRs) Minimum Technological Standards (MTRs) Permitting
Regulatory Status of Contaminated Ground Water é Contaminated media, including ground water, may “contain” hazardous wastes and therefore be subject to regulation under RCRA subtitle C (contained-in policy) é If ground water contains HW we must understand application of; l l l 8 Land Disposal Restrictions (LDRs) Minimum Technological Standards (MTRs) Permitting
 Application of RCRA Subtitle C (continued) é Land Disposal Restrictions l RCRA Section 3020(b) clarification (EPA Guidance Memorandum “Applicability RCRA Section 3020 to In. Situ Treatment of Ground Water, Dec. 27, 2000”) é Treatment standards for waste waters é Site-specific treatment variance (if applicable) é Management that does not constitute “placement” l 9 e. g. , some NPDES permitting
Application of RCRA Subtitle C (continued) é Land Disposal Restrictions l RCRA Section 3020(b) clarification (EPA Guidance Memorandum “Applicability RCRA Section 3020 to In. Situ Treatment of Ground Water, Dec. 27, 2000”) é Treatment standards for waste waters é Site-specific treatment variance (if applicable) é Management that does not constitute “placement” l 9 e. g. , some NPDES permitting
 Application of RCRA Subtitle C (Cont’d) é Minimum Technological Standards l l different standards apply to different types of units consider “temporary units” é Permitting l 10 EPA, under CERCLA, and many states, under their own programs, exempt from administrative permitting requirements on-site cleanup provided substantive standards are achieved
Application of RCRA Subtitle C (Cont’d) é Minimum Technological Standards l l different standards apply to different types of units consider “temporary units” é Permitting l 10 EPA, under CERCLA, and many states, under their own programs, exempt from administrative permitting requirements on-site cleanup provided substantive standards are achieved
 Application of Underground Injection Control Programs é Class IV waived under 40 CFR Part 144. 13 & must obtain waiver in each case l NJDEP intent to issue “General NJPDES Permit” where only amendments are added l é Class V Wells Federal Performance Standards l State Standards l 11
Application of Underground Injection Control Programs é Class IV waived under 40 CFR Part 144. 13 & must obtain waiver in each case l NJDEP intent to issue “General NJPDES Permit” where only amendments are added l é Class V Wells Federal Performance Standards l State Standards l 11
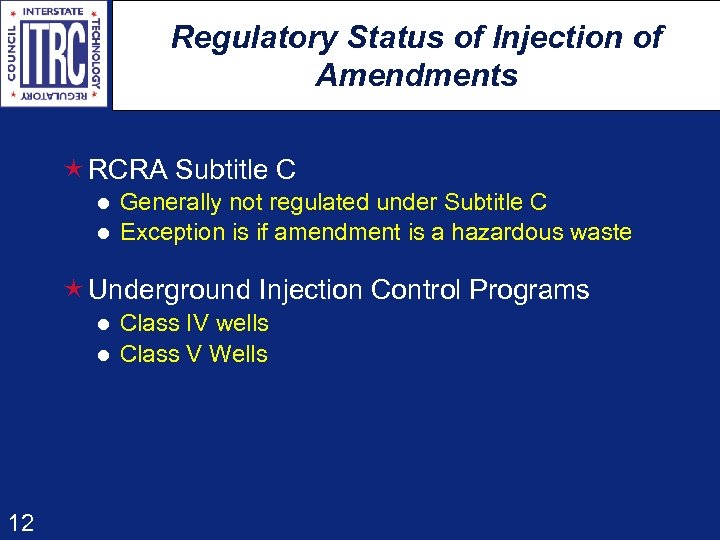 Regulatory Status of Injection of Amendments é RCRA Subtitle C l l Generally not regulated under Subtitle C Exception is if amendment is a hazardous waste é Underground Injection Control Programs l l 12 Class IV wells Class V Wells
Regulatory Status of Injection of Amendments é RCRA Subtitle C l l Generally not regulated under Subtitle C Exception is if amendment is a hazardous waste é Underground Injection Control Programs l l 12 Class IV wells Class V Wells
 Regulatory Issues Summary é Regulatory authorities é Regulatory status of contaminated ground water é Regulation of injection of amendments 13
Regulatory Issues Summary é Regulatory authorities é Regulatory status of contaminated ground water é Regulation of injection of amendments 13
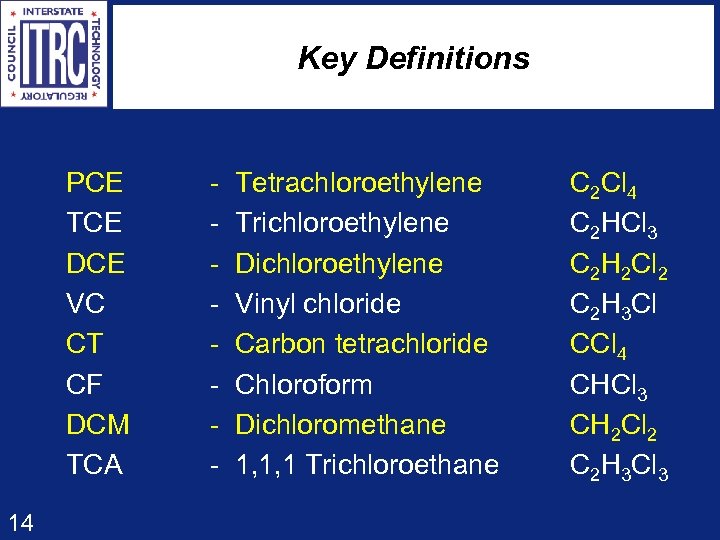 Key Definitions PCE TCE DCE VC CT CF DCM TCA 14 - Tetrachloroethylene - Trichloroethylene - Dichloroethylene - Vinyl chloride - Carbon tetrachloride - Chloroform - Dichloromethane - 1, 1, 1 Trichloroethane C 2 Cl 4 C 2 HCl 3 C 2 H 2 Cl 2 C 2 H 3 Cl CCl 4 CHCl 3 CH 2 Cl 2 C 2 H 3 Cl 3
Key Definitions PCE TCE DCE VC CT CF DCM TCA 14 - Tetrachloroethylene - Trichloroethylene - Dichloroethylene - Vinyl chloride - Carbon tetrachloride - Chloroform - Dichloromethane - 1, 1, 1 Trichloroethane C 2 Cl 4 C 2 HCl 3 C 2 H 2 Cl 2 C 2 H 3 Cl CCl 4 CHCl 3 CH 2 Cl 2 C 2 H 3 Cl 3
 Preferred Electron Donors/Acceptors é Electron Donors: small, simple molecules like sugars, organic acids, alcohols, alkanes, aromatics; man-made organic compounds, and natural organic carbon can be used. é Electron Acceptors: Oxygen, nitrate, Mn(IV), Fe(III), chlorinated solvents, sulfate, and CO 2 15
Preferred Electron Donors/Acceptors é Electron Donors: small, simple molecules like sugars, organic acids, alcohols, alkanes, aromatics; man-made organic compounds, and natural organic carbon can be used. é Electron Acceptors: Oxygen, nitrate, Mn(IV), Fe(III), chlorinated solvents, sulfate, and CO 2 15
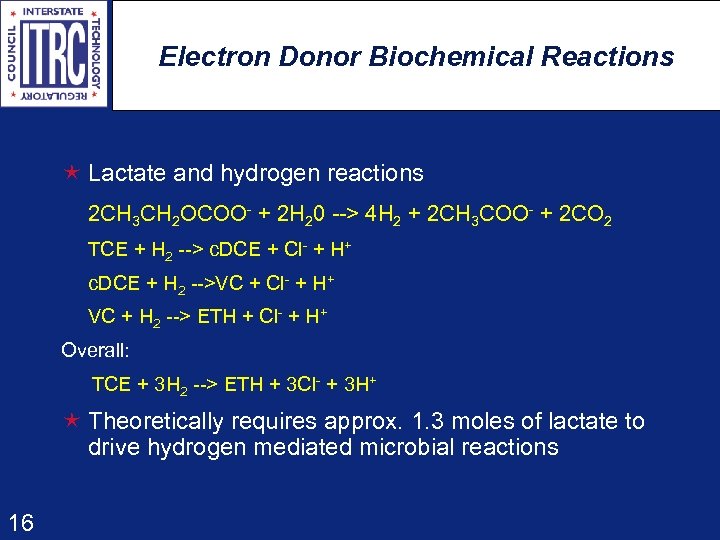 Electron Donor Biochemical Reactions é Lactate and hydrogen reactions 2 CH 3 CH 2 OCOO- + 2 H 20 --> 4 H 2 + 2 CH 3 COO- + 2 CO 2 TCE + H 2 --> c. DCE + Cl- + H+ c. DCE + H 2 -->VC + Cl- + H+ VC + H 2 --> ETH + Cl- + H+ Overall: TCE + 3 H 2 --> ETH + 3 Cl - + 3 H+ é Theoretically requires approx. 1. 3 moles of lactate to drive hydrogen mediated microbial reactions 16
Electron Donor Biochemical Reactions é Lactate and hydrogen reactions 2 CH 3 CH 2 OCOO- + 2 H 20 --> 4 H 2 + 2 CH 3 COO- + 2 CO 2 TCE + H 2 --> c. DCE + Cl- + H+ c. DCE + H 2 -->VC + Cl- + H+ VC + H 2 --> ETH + Cl- + H+ Overall: TCE + 3 H 2 --> ETH + 3 Cl - + 3 H+ é Theoretically requires approx. 1. 3 moles of lactate to drive hydrogen mediated microbial reactions 16
 Degradation Processes éThree Major Degradation Mechanisms 1) Reductive Anaerobic Dechlorination 2) Aerobic Cometabolism 3) Oxidation - reactions may be used for the destruction of vinyl chloride, dichloromethane, 1, 2 DCA, 1, 2 DCE 17
Degradation Processes éThree Major Degradation Mechanisms 1) Reductive Anaerobic Dechlorination 2) Aerobic Cometabolism 3) Oxidation - reactions may be used for the destruction of vinyl chloride, dichloromethane, 1, 2 DCA, 1, 2 DCE 17
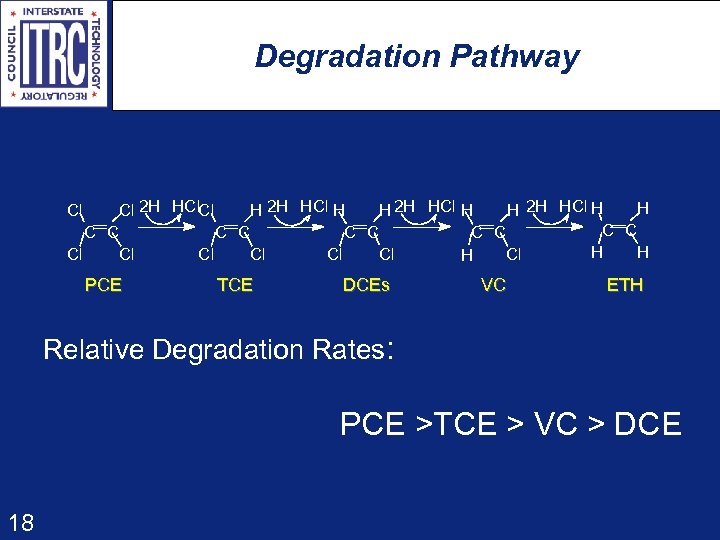 Degradation Pathway H Cl 2 H HCl. Cl H 2 H HCl H C C C C C H H Cl Cl H Cl PCE TCE DCEs VC ETH Relative Degradation Rates: PCE >TCE > VC > DCE 18
Degradation Pathway H Cl 2 H HCl. Cl H 2 H HCl H C C C C C H H Cl Cl H Cl PCE TCE DCEs VC ETH Relative Degradation Rates: PCE >TCE > VC > DCE 18
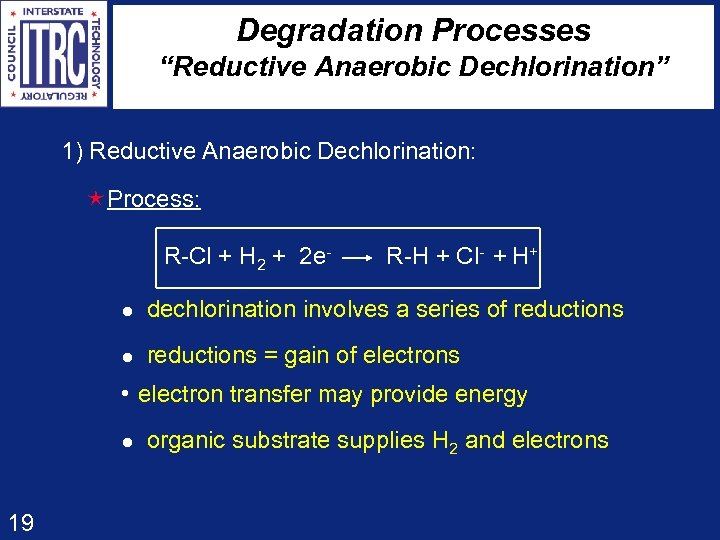 Degradation Processes “Reductive Anaerobic Dechlorination” 1) Reductive Anaerobic Dechlorination: éProcess: R-Cl + H 2 + 2 e- R-H + Cl- + H+ l dechlorination involves a series of reductions l reductions = gain of electrons l electron transfer may provide energy l organic substrate supplies H 2 and electrons 19
Degradation Processes “Reductive Anaerobic Dechlorination” 1) Reductive Anaerobic Dechlorination: éProcess: R-Cl + H 2 + 2 e- R-H + Cl- + H+ l dechlorination involves a series of reductions l reductions = gain of electrons l electron transfer may provide energy l organic substrate supplies H 2 and electrons 19
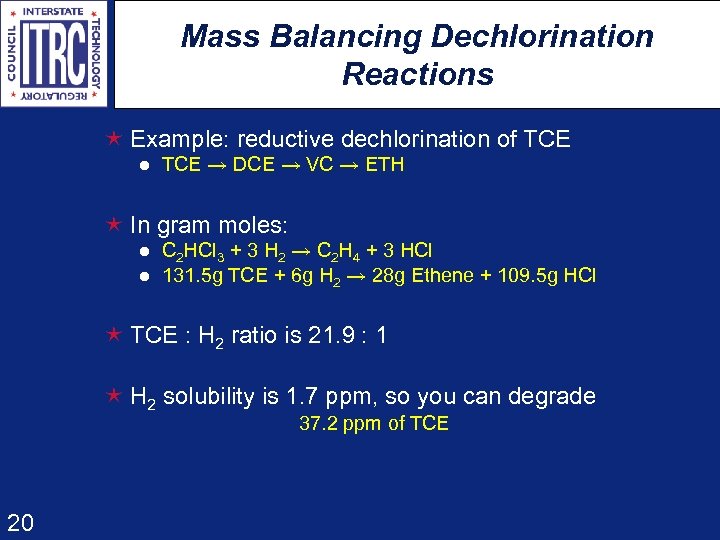 Mass Balancing Dechlorination Reactions é Example: reductive dechlorination of TCE l TCE DCE VC ETH é In gram moles: l l C 2 HCl 3 + 3 H 2 C 2 H 4 + 3 HCl 131. 5 g TCE + 6 g H 2 28 g Ethene + 109. 5 g HCl é TCE : H 2 ratio is 21. 9 : 1 é H 2 solubility is 1. 7 ppm, so you can degrade 37. 2 ppm of TCE 20
Mass Balancing Dechlorination Reactions é Example: reductive dechlorination of TCE l TCE DCE VC ETH é In gram moles: l l C 2 HCl 3 + 3 H 2 C 2 H 4 + 3 HCl 131. 5 g TCE + 6 g H 2 28 g Ethene + 109. 5 g HCl é TCE : H 2 ratio is 21. 9 : 1 é H 2 solubility is 1. 7 ppm, so you can degrade 37. 2 ppm of TCE 20
 Degradation Processes “Reductive Anaerobic Dechlorination” é Potential Problems: l Low hydraulic conductivity zones l biofouling l over abundance of electron acceptors 21
Degradation Processes “Reductive Anaerobic Dechlorination” é Potential Problems: l Low hydraulic conductivity zones l biofouling l over abundance of electron acceptors 21
 Degradation Processes “Aerobic Cometabolism” 2) Aerobic Cometabolism: l highly chlorinated compounds such as PCE and CCl 4 do not appear to be susceptible to aerobic cometabolic degradation l non-specific oxygenase enzymes help metabolize substrate(s) l bacteria incidentally oxidize chlorinated compounds l process usually requires oxygen addition for aerobic bacteria l process usually requires a substrate such as methane, phenol, or toluene 22
Degradation Processes “Aerobic Cometabolism” 2) Aerobic Cometabolism: l highly chlorinated compounds such as PCE and CCl 4 do not appear to be susceptible to aerobic cometabolic degradation l non-specific oxygenase enzymes help metabolize substrate(s) l bacteria incidentally oxidize chlorinated compounds l process usually requires oxygen addition for aerobic bacteria l process usually requires a substrate such as methane, phenol, or toluene 22
 Cometabolic Degradation é A fortuitous aerobic reaction carried out by enzymes designed to metabolize a different compound the bacteria normally grows on. é Bacteria are presumed to gain nothing from the reaction and in fact, may be harmed by intermediates that are formed 23
Cometabolic Degradation é A fortuitous aerobic reaction carried out by enzymes designed to metabolize a different compound the bacteria normally grows on. é Bacteria are presumed to gain nothing from the reaction and in fact, may be harmed by intermediates that are formed 23
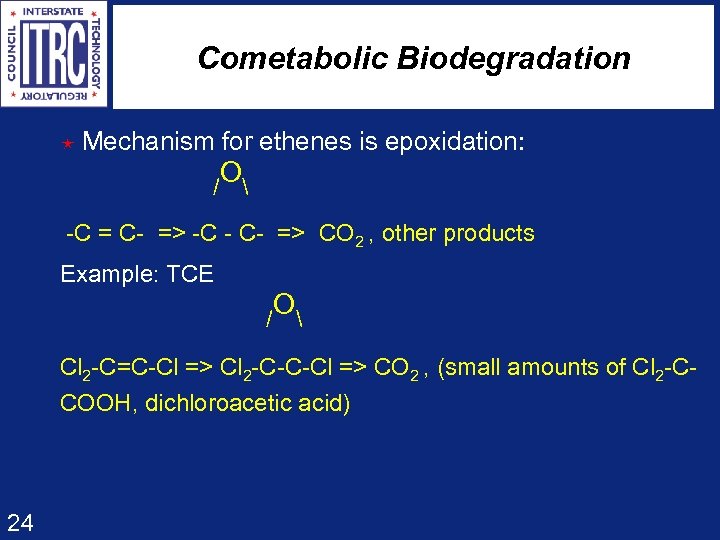 Cometabolic Biodegradation é Mechanism for ethenes is epoxidation: O / -C = C- => -C - C- => CO 2 , other products Example: TCE O / Cl 2 -C=C-Cl => Cl 2 -C-C-Cl => CO 2 , (small amounts of Cl 2 -CCOOH, dichloroacetic acid) 24
Cometabolic Biodegradation é Mechanism for ethenes is epoxidation: O / -C = C- => -C - C- => CO 2 , other products Example: TCE O / Cl 2 -C=C-Cl => Cl 2 -C-C-Cl => CO 2 , (small amounts of Cl 2 -CCOOH, dichloroacetic acid) 24
 Cometabolic Degradation é Compounds documented in the literature supporting aerobic cometabolic reactions: l l simple aromatic ring compounds: toluene, phenol (Nelson et. al. , 1986) l 25 methane (Wilson and Wilson, 1985), other shortchained alkanes like ethane, propane (Wackett et. al. , 1989), NH 4+ (Arciero et. al. , 1989)
Cometabolic Degradation é Compounds documented in the literature supporting aerobic cometabolic reactions: l l simple aromatic ring compounds: toluene, phenol (Nelson et. al. , 1986) l 25 methane (Wilson and Wilson, 1985), other shortchained alkanes like ethane, propane (Wackett et. al. , 1989), NH 4+ (Arciero et. al. , 1989)
 Mass Balancing Cometabolic Reactions é Example: co-oxidation of TCE l 2 C 2 HCl 3 + 21 O 2 + 2 C 7 H 8 --> 6 HCl + 18 CO 2 + 6 H 2 O é In gram moles: l 263 g TCE + 672 g O 2 + 184 g C 7 H 8 ----> 219 g HCl + 792 g CO 2 + 108 g H 2 O é TCE to O 2 ratio is 0. 39 : 1 é Water with 10 ppm dissolved oxygen can degrade a max of 3. 9 ppm TCE 26
Mass Balancing Cometabolic Reactions é Example: co-oxidation of TCE l 2 C 2 HCl 3 + 21 O 2 + 2 C 7 H 8 --> 6 HCl + 18 CO 2 + 6 H 2 O é In gram moles: l 263 g TCE + 672 g O 2 + 184 g C 7 H 8 ----> 219 g HCl + 792 g CO 2 + 108 g H 2 O é TCE to O 2 ratio is 0. 39 : 1 é Water with 10 ppm dissolved oxygen can degrade a max of 3. 9 ppm TCE 26
 Degradation Processes “Aerobic Cometabolism (Cont’d)” é Potential Problems: l process does not work on highly chlorinated compounds l cost of maintaining aerobic conditions l intermediates may be toxic to bacteria (i. e. . epoxide) l substrate amendments maybe RCRA regulated compounds 27
Degradation Processes “Aerobic Cometabolism (Cont’d)” é Potential Problems: l process does not work on highly chlorinated compounds l cost of maintaining aerobic conditions l intermediates may be toxic to bacteria (i. e. . epoxide) l substrate amendments maybe RCRA regulated compounds 27
 Degradation Processes “Oxidation” 3) Oxidation: l used on dichloromethane (DCM), vinyl chloride, chloroethane, and chloromethane é Process: l oxidizing agent (amendment) / reducing agent (contaminant) l l 28 elemental oxygen and or hydroxyl radical replace chloride oxidizing agent supplied directly or indirectly (i. e. O 2, H 2 O 2)
Degradation Processes “Oxidation” 3) Oxidation: l used on dichloromethane (DCM), vinyl chloride, chloroethane, and chloromethane é Process: l oxidizing agent (amendment) / reducing agent (contaminant) l l 28 elemental oxygen and or hydroxyl radical replace chloride oxidizing agent supplied directly or indirectly (i. e. O 2, H 2 O 2)
 Direct Aerobic Biodegradation é Aerobic bacteria “grow” on the compound by using it as their carbon and energy source (electron donor). é Process is rapid, compound almost always degraded to CO 2, degradation intermediates may be formed. 29
Direct Aerobic Biodegradation é Aerobic bacteria “grow” on the compound by using it as their carbon and energy source (electron donor). é Process is rapid, compound almost always degraded to CO 2, degradation intermediates may be formed. 29
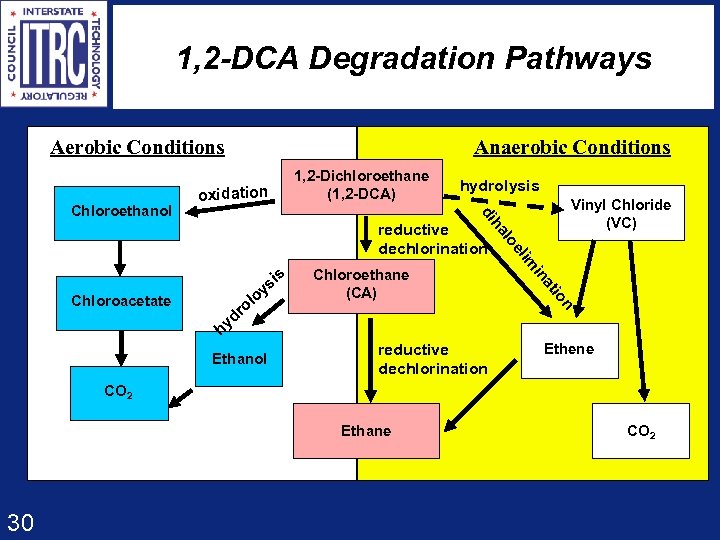 1, 2 -DCA Degradation Pathways Aerobic Conditions 1, 2 -Dichloroethane (1, 2 -DCA) oxidation hydrolysis Vinyl Chloride (VC) di Chloroethanol Anaerobic Conditions io n Ethanol at r yd h in Chloroethane (CA) im is el Chloroacetate s oy ol lo ha reductive dechlorination Ethene CO 2 Ethane 30 CO 2
1, 2 -DCA Degradation Pathways Aerobic Conditions 1, 2 -Dichloroethane (1, 2 -DCA) oxidation hydrolysis Vinyl Chloride (VC) di Chloroethanol Anaerobic Conditions io n Ethanol at r yd h in Chloroethane (CA) im is el Chloroacetate s oy ol lo ha reductive dechlorination Ethene CO 2 Ethane 30 CO 2
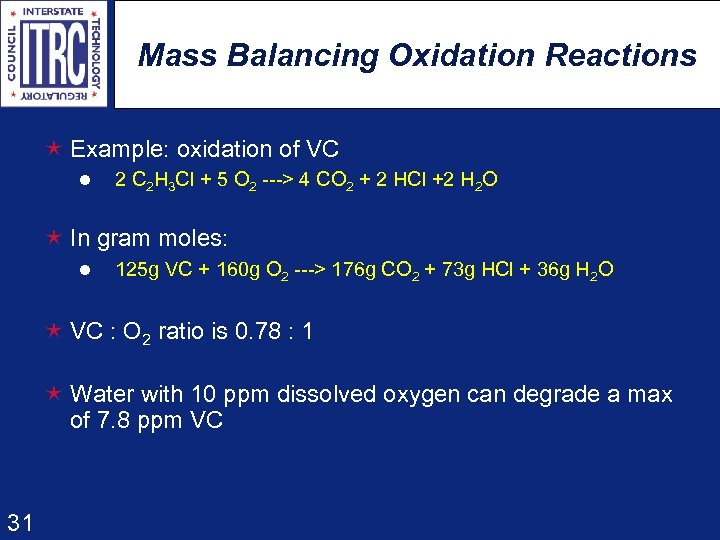 Mass Balancing Oxidation Reactions é Example: oxidation of VC l 2 C 2 H 3 Cl + 5 O 2 ---> 4 CO 2 + 2 HCl +2 H 2 O é In gram moles: l 125 g VC + 160 g O 2 ---> 176 g CO 2 + 73 g HCl + 36 g H 2 O é VC : O 2 ratio is 0. 78 : 1 é Water with 10 ppm dissolved oxygen can degrade a max of 7. 8 ppm VC 31
Mass Balancing Oxidation Reactions é Example: oxidation of VC l 2 C 2 H 3 Cl + 5 O 2 ---> 4 CO 2 + 2 HCl +2 H 2 O é In gram moles: l 125 g VC + 160 g O 2 ---> 176 g CO 2 + 73 g HCl + 36 g H 2 O é VC : O 2 ratio is 0. 78 : 1 é Water with 10 ppm dissolved oxygen can degrade a max of 7. 8 ppm VC 31
 Degradation Processes “Oxidation (cont’d)” é Potential Problems: l l Oxygen transport in ground water l 32 low hydraulic conductivity high levels of naturally occurring organic carbon
Degradation Processes “Oxidation (cont’d)” é Potential Problems: l l Oxygen transport in ground water l 32 low hydraulic conductivity high levels of naturally occurring organic carbon
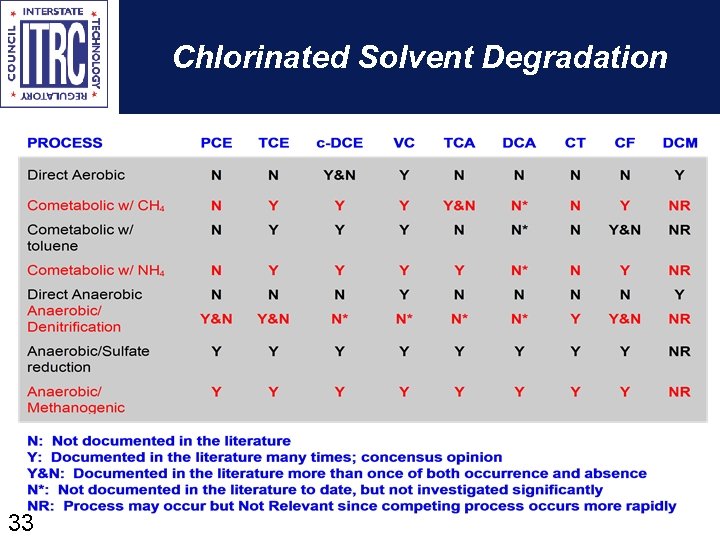 Chlorinated Solvent Degradation 33
Chlorinated Solvent Degradation 33
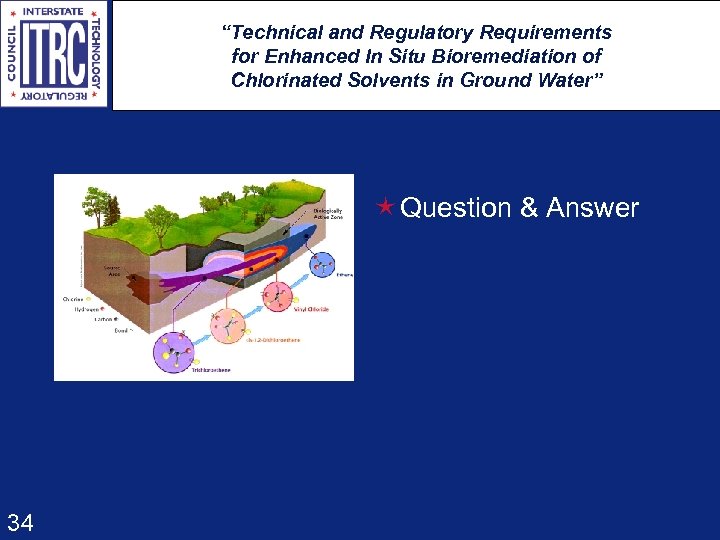 “Technical and Regulatory Requirements for Enhanced In Situ Bioremediation of Chlorinated Solvents in Ground Water” é Question & Answer 34
“Technical and Regulatory Requirements for Enhanced In Situ Bioremediation of Chlorinated Solvents in Ground Water” é Question & Answer 34
 Amendment Delivery Systems éEffective EISB requires delivery of amendments to the targeted portion of the plume A) Dual Well or Trench Recirculation l extraction and reinjection of groundwater l effective mixing of amendments and water l forms a treatment zone B) Injection Only Systems l gravity or forced injection l lack of hydraulic containment 35
Amendment Delivery Systems éEffective EISB requires delivery of amendments to the targeted portion of the plume A) Dual Well or Trench Recirculation l extraction and reinjection of groundwater l effective mixing of amendments and water l forms a treatment zone B) Injection Only Systems l gravity or forced injection l lack of hydraulic containment 35
 Delivery Systems (Cont’d) C) Gas Injection Systems l injection of vapor phase amendment(s) D) Passive Systems l no forced injection or recirculation l amendments added within or in the path of a plume 36
Delivery Systems (Cont’d) C) Gas Injection Systems l injection of vapor phase amendment(s) D) Passive Systems l no forced injection or recirculation l amendments added within or in the path of a plume 36
 Amendments for Microbial Growth A) Substrate l growth source and electron donor l vary from site to site l example: Na lactate B) Nutrients l ground water analysis for needed inorganics l monitoring of concentration levels l example: P, N 37
Amendments for Microbial Growth A) Substrate l growth source and electron donor l vary from site to site l example: Na lactate B) Nutrients l ground water analysis for needed inorganics l monitoring of concentration levels l example: P, N 37
 Amendments for Microbial Growth (cont’d) C) Electron Acceptors l associated with aerobic cometabolism or direct oxidation l O 2, H 2 O 2 D) Bioaugmentation l introduction of non-native bacteria l duration l short lived, outcompeted easily by indigenous microbes 38
Amendments for Microbial Growth (cont’d) C) Electron Acceptors l associated with aerobic cometabolism or direct oxidation l O 2, H 2 O 2 D) Bioaugmentation l introduction of non-native bacteria l duration l short lived, outcompeted easily by indigenous microbes 38
 When is EISB Appropriate? é To reduce chlorinated solvent levels below regulatory requirements é As a polishing step é When reduction-oxidation (redox) potential is low 39
When is EISB Appropriate? é To reduce chlorinated solvent levels below regulatory requirements é As a polishing step é When reduction-oxidation (redox) potential is low 39
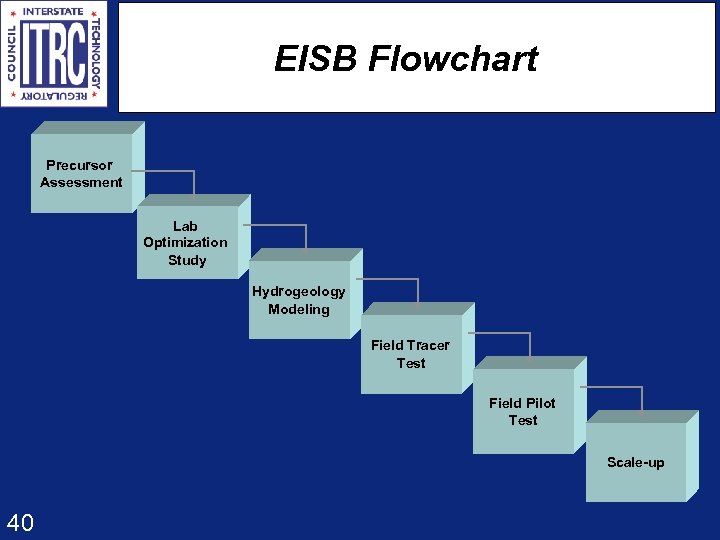 EISB Flowchart Precursor Assessment Lab Optimization Study Hydrogeology Modeling Field Tracer Test Field Pilot Test Scale-up 40
EISB Flowchart Precursor Assessment Lab Optimization Study Hydrogeology Modeling Field Tracer Test Field Pilot Test Scale-up 40
 Technical Requirements A) Site Assessment l review of previous site data l development of site characterization work plans l hydrogeologic and geochemical characterization 41
Technical Requirements A) Site Assessment l review of previous site data l development of site characterization work plans l hydrogeologic and geochemical characterization 41
 Technical Requirements (cont’d) A) Site Assessment (cont’d) l source area characterization l plume characterization l conceptual model and site evaluation 42
Technical Requirements (cont’d) A) Site Assessment (cont’d) l source area characterization l plume characterization l conceptual model and site evaluation 42
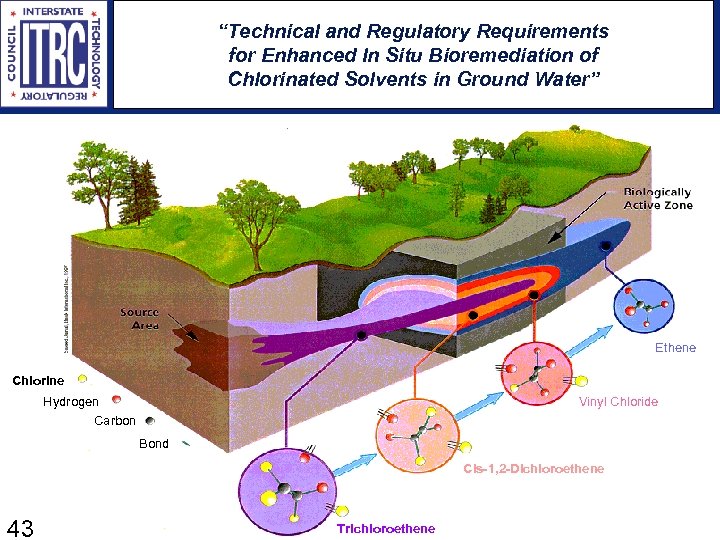 “Technical and Regulatory Requirements for Enhanced In Situ Bioremediation of Chlorinated Solvents in Ground Water” Not it Ethene Chlorine Hydrogen Carbon Vinyl Chloride Bond Cis-1, 2 -Dichloroethene 43 43 Trichloroethene
“Technical and Regulatory Requirements for Enhanced In Situ Bioremediation of Chlorinated Solvents in Ground Water” Not it Ethene Chlorine Hydrogen Carbon Vinyl Chloride Bond Cis-1, 2 -Dichloroethene 43 43 Trichloroethene
 Technical Requirements (cont’d) B) Laboratory Treatability Test Phase l laboratory treatability studies l analysis l evaluating laboratory treatability results 44
Technical Requirements (cont’d) B) Laboratory Treatability Test Phase l laboratory treatability studies l analysis l evaluating laboratory treatability results 44
 Technical Requirements (cont’d) B) Laboratory Treatability Test Phase cont’d) l Anaerobic Laboratory Treatability Studies Cookson, John T. - Bioremediation Engineering, Design and Application, 1995, Mc. Graw-Hill Harkness (et al. , 1998) - Stimulation of complete reductive dechlorination of TCE in Strother Soil: Microcosm and column studies. ITRC In Situ Bioremediation Team (1998) - Technical and Regulatory Requirements for Enhanced In Situ Bioremediation of Chlorinated Solvents in Ground Water 45
Technical Requirements (cont’d) B) Laboratory Treatability Test Phase cont’d) l Anaerobic Laboratory Treatability Studies Cookson, John T. - Bioremediation Engineering, Design and Application, 1995, Mc. Graw-Hill Harkness (et al. , 1998) - Stimulation of complete reductive dechlorination of TCE in Strother Soil: Microcosm and column studies. ITRC In Situ Bioremediation Team (1998) - Technical and Regulatory Requirements for Enhanced In Situ Bioremediation of Chlorinated Solvents in Ground Water 45
 Technical Requirements (cont’d) C) Field Pilot Test Phase l permitting and regulatory acceptance l preliminary site selection l focused hydrogeologic study 46
Technical Requirements (cont’d) C) Field Pilot Test Phase l permitting and regulatory acceptance l preliminary site selection l focused hydrogeologic study 46
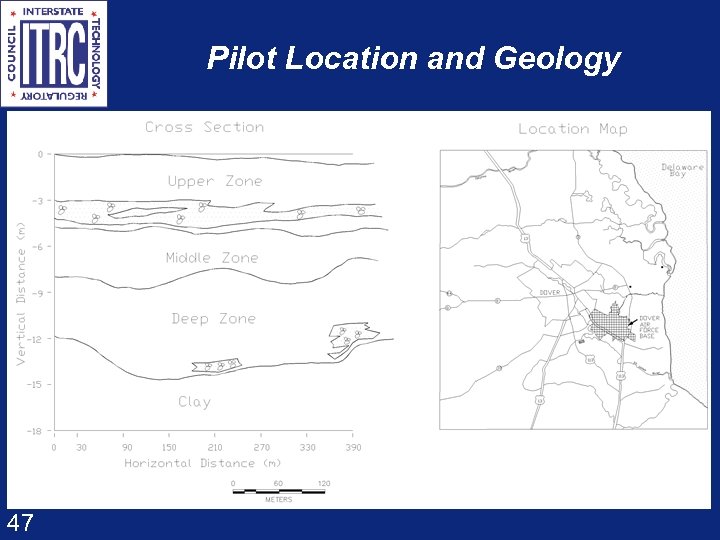 Pilot Location and Geology 47
Pilot Location and Geology 47
 Pilot Study Plume Configuration 48
Pilot Study Plume Configuration 48
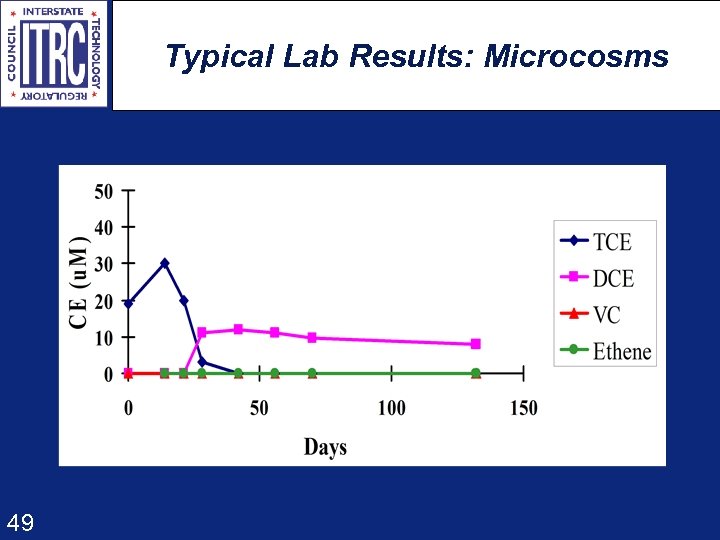 Typical Lab Results: Microcosms 49
Typical Lab Results: Microcosms 49
 Hydrogeologic Modeling “EISB Recirculation System” 50
Hydrogeologic Modeling “EISB Recirculation System” 50
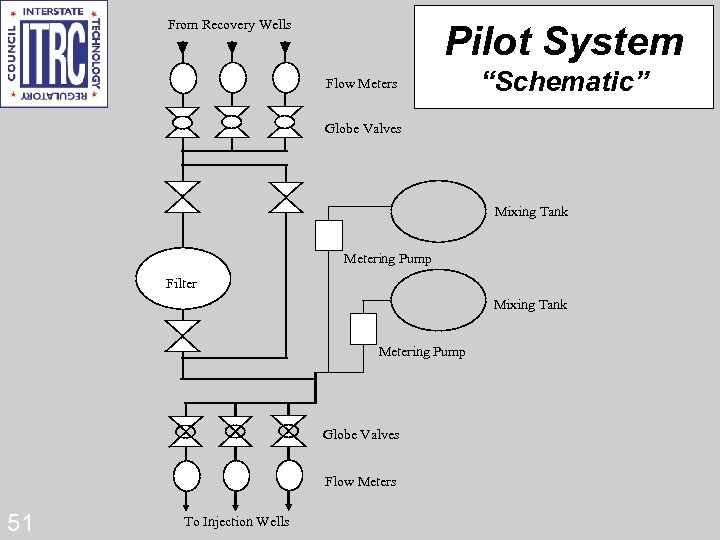 From Recovery Wells Pilot System Flow Meters “Schematic” Globe Valves Mixing Tank Metering Pump Filter Mixing Tank Metering Pump Globe Valves Flow Meters 51 To Injection Wells
From Recovery Wells Pilot System Flow Meters “Schematic” Globe Valves Mixing Tank Metering Pump Filter Mixing Tank Metering Pump Globe Valves Flow Meters 51 To Injection Wells
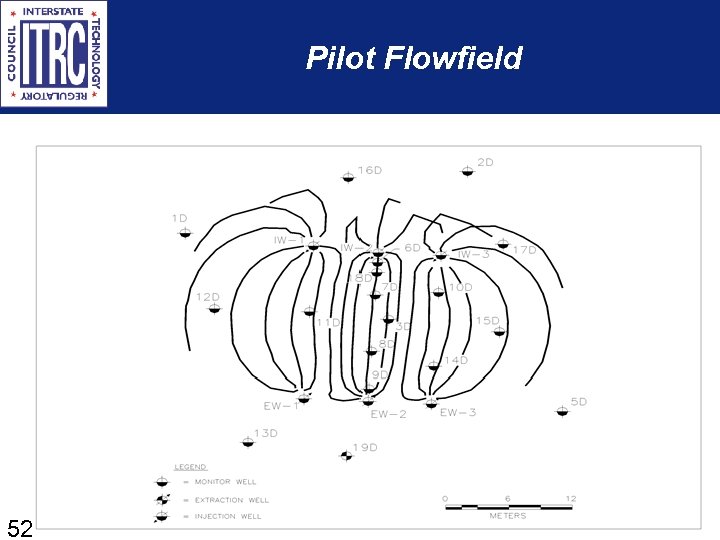 Pilot Flowfield 52 52
Pilot Flowfield 52 52
 Pilot Piping 53
Pilot Piping 53
 Amendments & Metering Pumps 54
Amendments & Metering Pumps 54
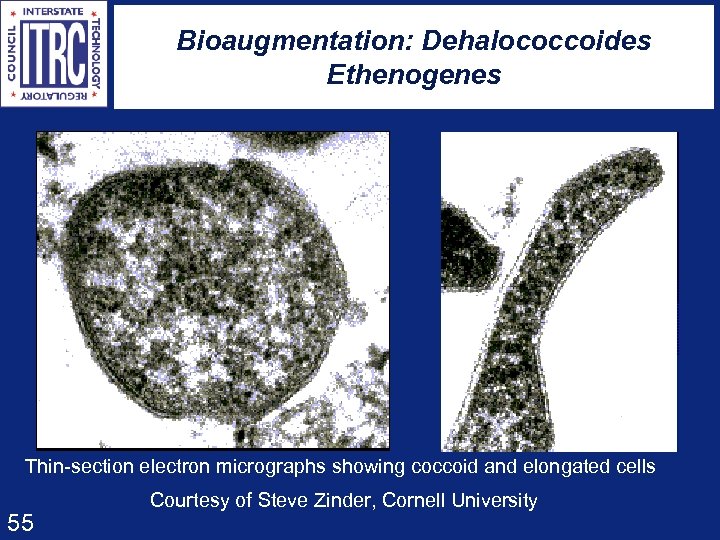 Bioaugmentation: Dehalococcoides Ethenogenes Thin-section electron micrographs showing coccoid and elongated cells 55 Courtesy of Steve Zinder, Cornell University
Bioaugmentation: Dehalococcoides Ethenogenes Thin-section electron micrographs showing coccoid and elongated cells 55 Courtesy of Steve Zinder, Cornell University
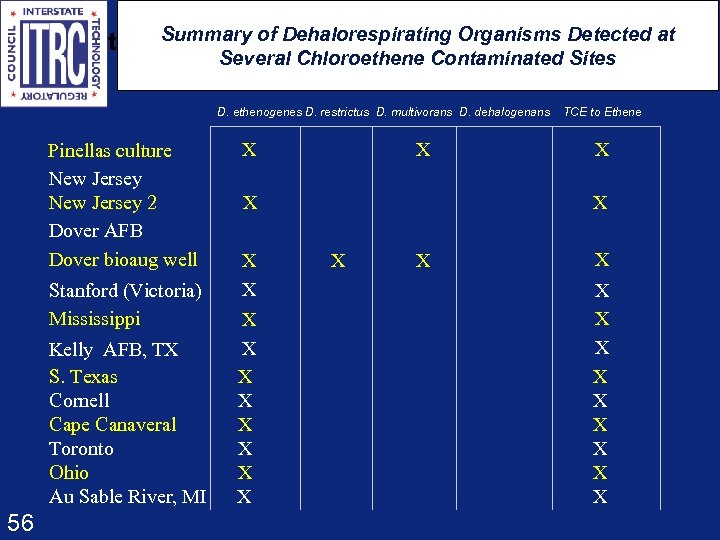 t Summary of Dehalorespirating Organisms Detected at Several Chloroethene Contaminated Sites D. ethenogenes D. restrictus D. multivorans D. dehalogenans Pinellas culture New Jersey 2 Dover AFB Dover bioaug well Stanford (Victoria) Mississippi Kelly AFB, TX S. Texas Cornell Cape Canaveral Toronto Ohio Au Sable River, MI 56 X X X X TCE to Ethene X X X X
t Summary of Dehalorespirating Organisms Detected at Several Chloroethene Contaminated Sites D. ethenogenes D. restrictus D. multivorans D. dehalogenans Pinellas culture New Jersey 2 Dover AFB Dover bioaug well Stanford (Victoria) Mississippi Kelly AFB, TX S. Texas Cornell Cape Canaveral Toronto Ohio Au Sable River, MI 56 X X X X TCE to Ethene X X X X
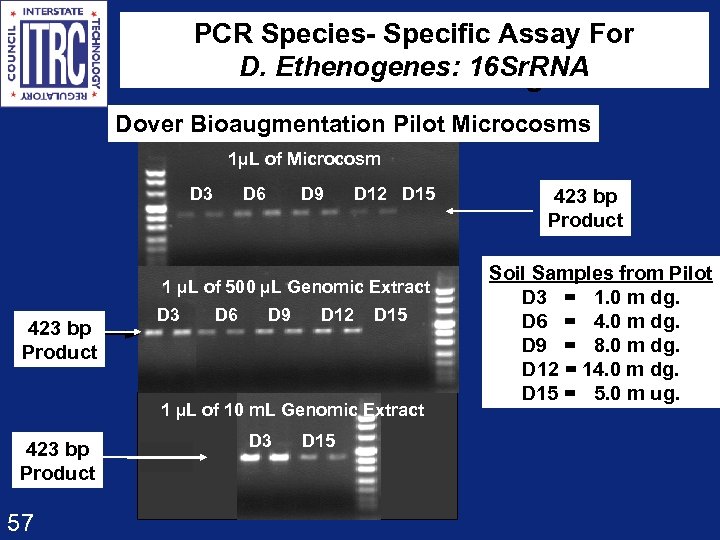 PCR Species- Specific Assay For D. Ethenogenes: 16 Sr. RNA Dehalococcoides ethenogenes Dover Bioaugmentation Pilot Microcosms 1µL of Microcosm D 3 D 6 D 9 D 12 D 15 1 µL of 500 µL Genomic Extract 423 bp Product D 3 D 6 D 9 D 12 D 15 1 µL of 10 m. L Genomic Extract 423 bp Product 57 D 3 D 15 423 bp Product Soil Samples from Pilot D 3 = 1. 0 m dg. D 6 = 4. 0 m dg. D 9 = 8. 0 m dg. D 12 = 14. 0 m dg. D 15 = 5. 0 m ug.
PCR Species- Specific Assay For D. Ethenogenes: 16 Sr. RNA Dehalococcoides ethenogenes Dover Bioaugmentation Pilot Microcosms 1µL of Microcosm D 3 D 6 D 9 D 12 D 15 1 µL of 500 µL Genomic Extract 423 bp Product D 3 D 6 D 9 D 12 D 15 1 µL of 10 m. L Genomic Extract 423 bp Product 57 D 3 D 15 423 bp Product Soil Samples from Pilot D 3 = 1. 0 m dg. D 6 = 4. 0 m dg. D 9 = 8. 0 m dg. D 12 = 14. 0 m dg. D 15 = 5. 0 m ug.
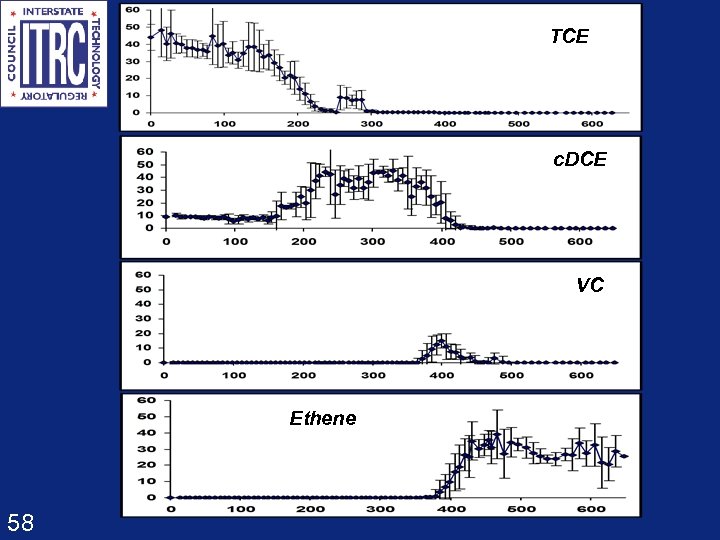 TCE c. DCE VC Ethene 58
TCE c. DCE VC Ethene 58
 Technical Requirements (cont’d) C) Field Pilot Test Phase (cont’d) é Engineering design l l l COOKSON, John T. - Bioremediation Engineering, Design and Application, 1995 Mc. Graw-Hill AFCEE - Aerobic Cometabolic In Situ Bioremediation Technology Guidance Manual and Screening Software Users Guide: Installation Restoration Program ITRC In Situ Bioremediation Team (1998) - Technical and Regulatory Requirements for Enhanced In Situ Bioremediation of Chlorinated Solvents in Ground Water é Evaluating pilot test results l 59 Ellis, D. E. , Lutz, E. J. , Odom, J. M. , Buchanan, R. J. , Bartlett, C. , Lee, M. D. , Harkness, M. , Deweerd, K. , Bioaugmentation For Accelerated In Situ Anaerobic Bioremediation, Environmental Sci. & Technology, Vol. 34, No. 11, 2000, Pg. 2254 -2260.
Technical Requirements (cont’d) C) Field Pilot Test Phase (cont’d) é Engineering design l l l COOKSON, John T. - Bioremediation Engineering, Design and Application, 1995 Mc. Graw-Hill AFCEE - Aerobic Cometabolic In Situ Bioremediation Technology Guidance Manual and Screening Software Users Guide: Installation Restoration Program ITRC In Situ Bioremediation Team (1998) - Technical and Regulatory Requirements for Enhanced In Situ Bioremediation of Chlorinated Solvents in Ground Water é Evaluating pilot test results l 59 Ellis, D. E. , Lutz, E. J. , Odom, J. M. , Buchanan, R. J. , Bartlett, C. , Lee, M. D. , Harkness, M. , Deweerd, K. , Bioaugmentation For Accelerated In Situ Anaerobic Bioremediation, Environmental Sci. & Technology, Vol. 34, No. 11, 2000, Pg. 2254 -2260.
 Benefits of the Technology é Less Expensive form of treatment é Field-Pilot Scale vs. Full Scale é Less complex design (“ the enhancement of a natural process”) é Remediate a specific and/or multiple portions of a plume é Avoid extra cost associated with treatment of created by-products 60
Benefits of the Technology é Less Expensive form of treatment é Field-Pilot Scale vs. Full Scale é Less complex design (“ the enhancement of a natural process”) é Remediate a specific and/or multiple portions of a plume é Avoid extra cost associated with treatment of created by-products 60
 Rules of Thumb é Correct Environmental Conditions (p. H, Eh, N, P) é Transforming Microbes Present é Suitable e - Donor Present 61
Rules of Thumb é Correct Environmental Conditions (p. H, Eh, N, P) é Transforming Microbes Present é Suitable e - Donor Present 61
 Wrap-up é QUESTIONS AND ANSWERS Thank you for attending this ITRC training course. 62 For more information on ITRC training opportunities visit: www. itrcweb. org
Wrap-up é QUESTIONS AND ANSWERS Thank you for attending this ITRC training course. 62 For more information on ITRC training opportunities visit: www. itrcweb. org
 Thank You! Links to Additional Resources 63
Thank You! Links to Additional Resources 63


