672d94df5caab1f8228f75de7b346430.ppt
- Количество слайдов: 19
Welcome to Dong. Bang FTL CORYRIGHT (C) DONGBANG FUTURE TECH & LIFE CO. , LTD. ALL RIGHTS RESERVED. CUSTOMER CENTER 82 -2 -741 -7516
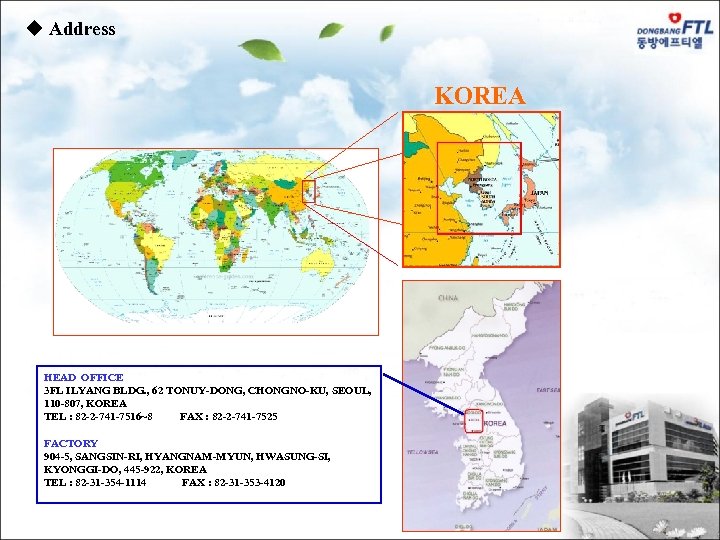
u Address KOREA HEAD OFFICE 3 FL ILYANG BLDG. , 62 TONUY-DONG, CHONGNO-KU, SEOUL, 110 -807, KOREA TEL : 82 -2 -741 -7516~8 FAX : 82 -2 -741 -7525 FACTORY 904 -5, SANGSIN-RI, HYANGNAM-MYUN, HWASUNG-SI, KYONGGI-DO, 445 -922, KOREA TEL : 82 -31 -354 -1114 FAX : 82 -31 -353 -4120
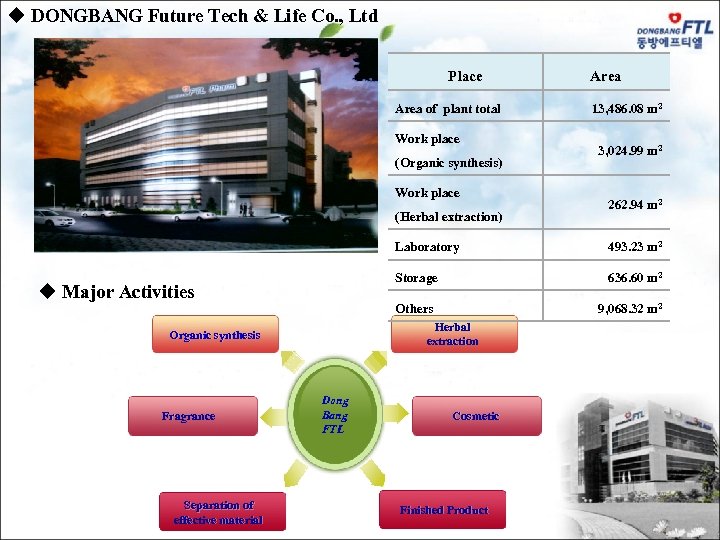
u DONGBANG Future Tech & Life Co. , Ltd Place Area of plant total Work place (Organic synthesis) Work place (Herbal extraction) Area 13, 486. 08 m 2 3, 024. 99 m 2 262. 94 m 2 Laboratory Storage u Major Activities Separation of effective material 636. 60 m 2 Others 9, 068. 32 m 2 Herbal extraction Organic synthesis Fragrance 493. 23 m 2 Dong Bang FTL Cosmetic Finished Product
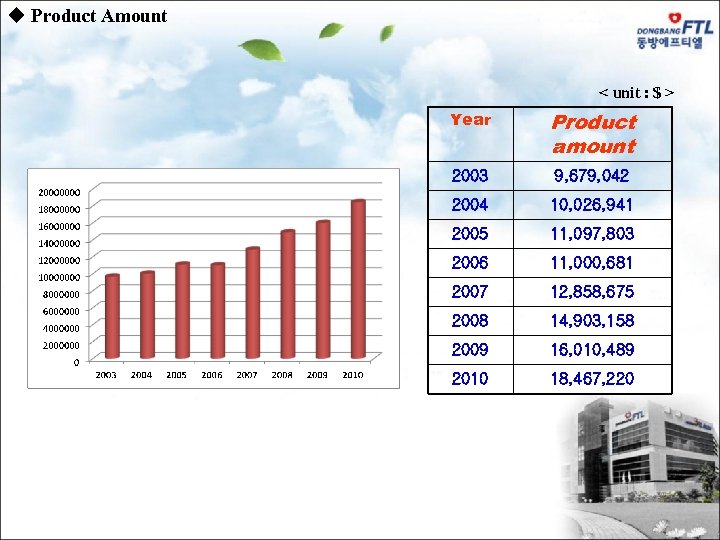
u Product Amount < unit : $ > Year Product amount 2003 9, 679, 042 2004 10, 026, 941 2005 11, 097, 803 2006 11, 000, 681 2007 12, 858, 675 2008 14, 903, 158 2009 16, 010, 489 2010 18, 467, 220
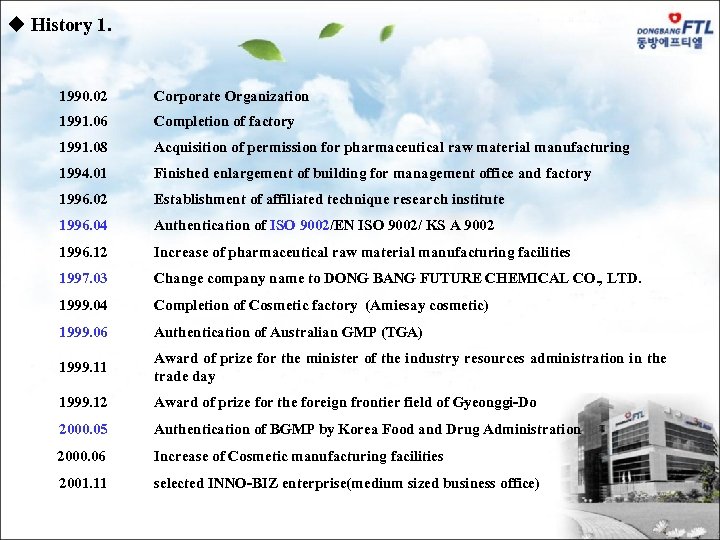
u History 1. 1990. 02 Corporate Organization 1991. 06 Completion of factory 1991. 08 Acquisition of permission for pharmaceutical raw material manufacturing 1994. 01 Finished enlargement of building for management office and factory 1996. 02 Establishment of affiliated technique research institute 1996. 04 Authentication of ISO 9002/EN ISO 9002/ KS A 9002 1996. 12 Increase of pharmaceutical raw material manufacturing facilities 1997. 03 Change company name to DONG BANG FUTURE CHEMICAL CO. , LTD. 1999. 04 Completion of Cosmetic factory (Amiesay cosmetic) 1999. 06 Authentication of Australian GMP (TGA) 1999. 11 Award of prize for the minister of the industry resources administration in the trade day 1999. 12 Award of prize for the foreign frontier field of Gyeonggi-Do 2000. 05 Authentication of BGMP by Korea Food and Drug Administration 2000. 06 Increase of Cosmetic manufacturing facilities 2001. 11 selected INNO-BIZ enterprise(medium sized business office)
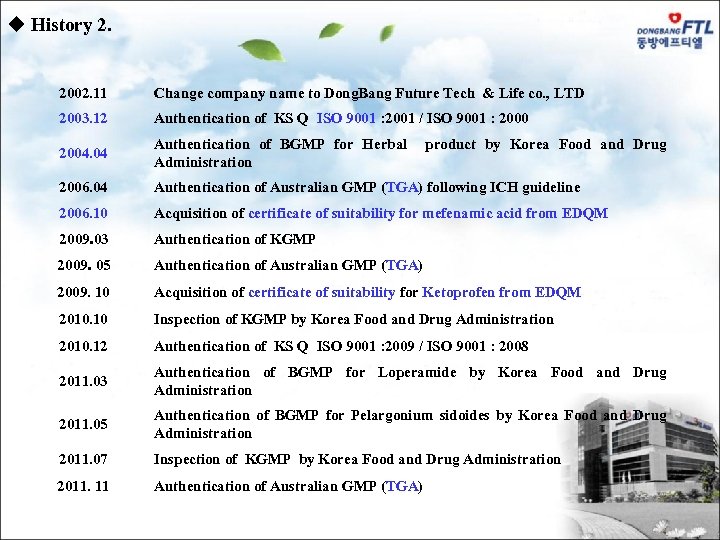
u History 2. 2002. 11 Change company name to Dong. Bang Future Tech & Life co. , LTD 2003. 12 Authentication of KS Q ISO 9001 : 2001 / ISO 9001 : 2000 2004. 04 Authentication of BGMP for Herbal product by Korea Food and Drug Administration 2006. 04 Authentication of Australian GMP (TGA) following ICH guideline 2006. 10 Acquisition of certificate of suitability for mefenamic acid from EDQM 2009. 03 Authentication of KGMP 2009. 05 Authentication of Australian GMP (TGA) 2009. 10 Acquisition of certificate of suitability for Ketoprofen from EDQM 2010. 10 Inspection of KGMP by Korea Food and Drug Administration 2010. 12 Authentication of KS Q ISO 9001 : 2009 / ISO 9001 : 2008 2011. 03 Authentication of BGMP for Loperamide by Korea Food and Drug Administration 2011. 05 Authentication of BGMP for Pelargonium sidoides by Korea Food and Drug Administration 2011. 07 Inspection of KGMP by Korea Food and Drug Administration 2011. 11 Authentication of Australian GMP (TGA)

u Company Certificate 1. TGA Authentication BGMP Authentication

u Company Certificate 2. ISO Authentication

u Company Certificate 3. Mefenamic acid COS-1 Mefenamic acid COS-2 Mefenamic acid COS-3

u Company Certificate 4. Ketoprofen COS-1 Ketoprofen COS-2
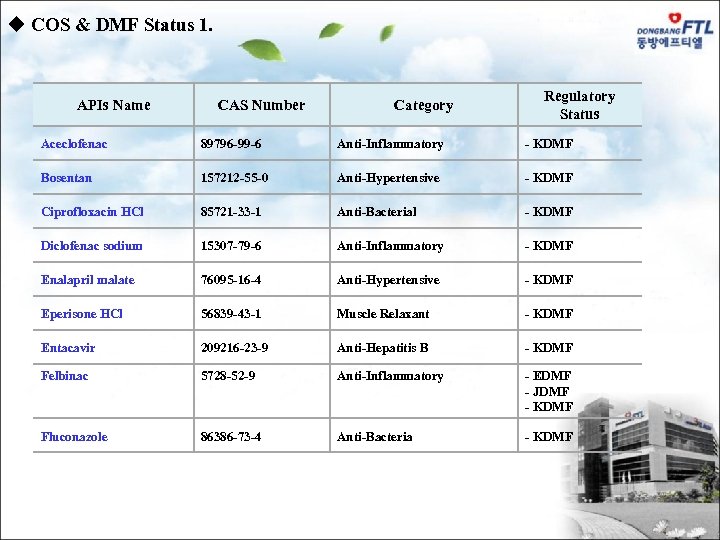
u COS & DMF Status 1. APIs Name CAS Number Category Regulatory Status Aceclofenac 89796 -99 -6 Anti-Inflammatory - KDMF Bosentan 157212 -55 -0 Anti-Hypertensive - KDMF Ciprofloxacin HCl 85721 -33 -1 Anti-Bacterial - KDMF Diclofenac sodium 15307 -79 -6 Anti-Inflammatory - KDMF Enalapril malate 76095 -16 -4 Anti-Hypertensive - KDMF Eperisone HCl 56839 -43 -1 Muscle Relaxant - KDMF Entacavir 209216 -23 -9 Anti-Hepatitis B - KDMF Felbinac 5728 -52 -9 Anti-Inflammatory - EDMF - JDMF - KDMF Fluconazole 86386 -73 -4 Anti-Bacteria - KDMF
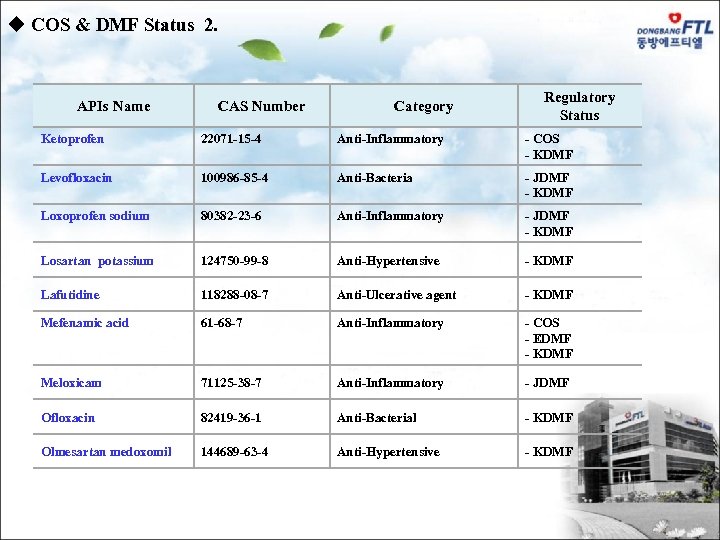
u COS & DMF Status 2. APIs Name CAS Number Category Regulatory Status Ketoprofen 22071 -15 -4 Anti-Inflammatory - COS - KDMF Levofloxacin 100986 -85 -4 Anti-Bacteria - JDMF - KDMF Loxoprofen sodium 80382 -23 -6 Anti-Inflammatory - JDMF - KDMF Losartan potassium 124750 -99 -8 Anti-Hypertensive - KDMF Lafutidine 118288 -08 -7 Anti-Ulcerative agent - KDMF Mefenamic acid 61 -68 -7 Anti-Inflammatory - COS - EDMF - KDMF Meloxicam 71125 -38 -7 Anti-Inflammatory - JDMF Ofloxacin 82419 -36 -1 Anti-Bacterial - KDMF Olmesartan medoxomil 144689 -63 -4 Anti-Hypertensive - KDMF
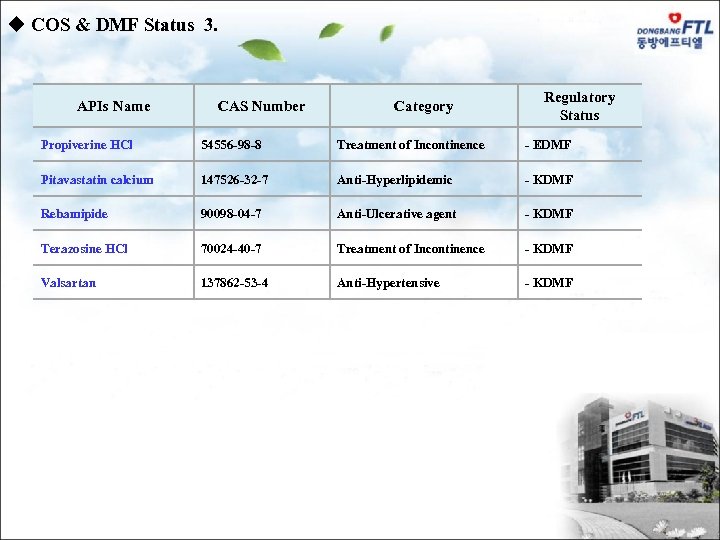
u COS & DMF Status 3. APIs Name CAS Number Category Regulatory Status Propiverine HCl 54556 -98 -8 Treatment of Incontinence - EDMF Pitavastatin calcium 147526 -32 -7 Anti-Hyperlipidemic - KDMF Rebamipide 90098 -04 -7 Anti-Ulcerative agent - KDMF Terazosine HCl 70024 -40 -7 Treatment of Incontinence - KDMF Valsartan 137862 -53 -4 Anti-Hypertensive - KDMF
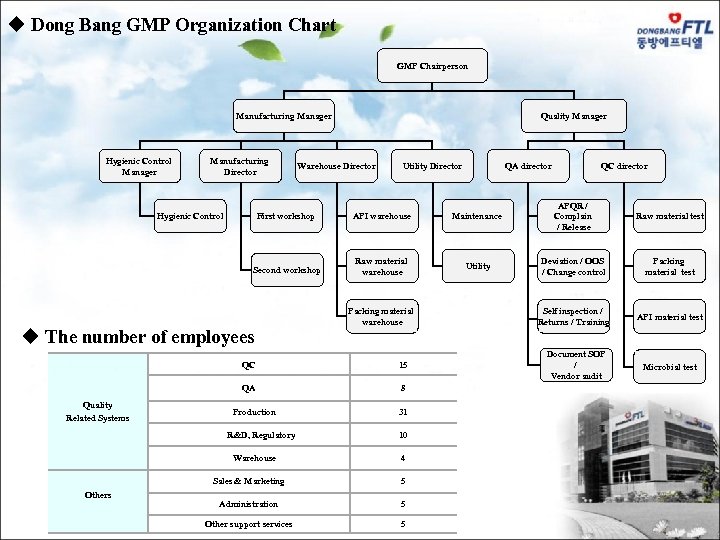
u Dong Bang GMP Organization Chart GMP Chairperson Quality Manager Manufacturing Manager Hygienic Control Manager Manufacturing Director Warehouse Director Utility Director QA director QC director First workshop API warehouse Maintenance APQR / Complain / Release Raw material test Second workshop Raw material warehouse Utility Deviation / OOS / Change control Packing material test Self inspection / Returns / Training API material test Document SOP / Vendor audit Microbial test Hygienic Control u The number of employees Packing material warehouse QC QA Production 31 10 Warehouse 4 Sales & Marketing Others 8 R&D, Regulatory Quality Related Systems 15 5 Administration 5 Other support services 5
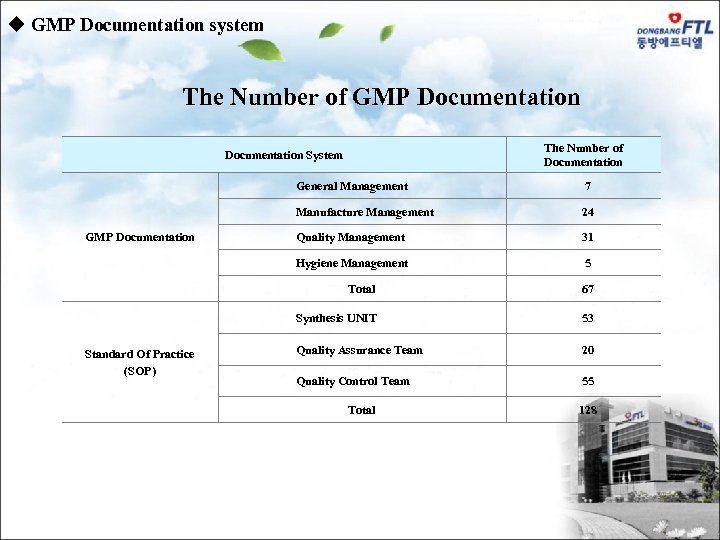
u GMP Documentation system The Number of GMP Documentation The Number of Documentation System General Management Manufacture Management 24 Quality Management 31 Hygiene Management GMP Documentation 7 5 Total Synthesis UNIT Synthesis Standard Of Practice (SOP) 67 53 Quality Assurance Team 20 Quality Control Team 55 Total 128

u Quality Equipment Particle size analyzer Fourier Transform Infrared Spectroscopy High Performance Liquid Chromatography Convection oven Total Organic Carbon Analyzer Gas Chromatography Karl fisher Muffle Furnace p. H Meter UV-VIS Spectrophotometer

u Production Facility 1) Reactor

u Production Facility 2) Dryer 3) Filter

Thank you ISO 9001 Authentication T G A Authentication B G M P Authentication K G M P Authentication DONGBANG FUTURE TECH & LIFE CO. , LTD. SEOUL, KOREA - HEAD OFFICE 3 FL ILYANG BLDG. , 62 TONUY-DONG, CHONGNO-KU, SEOUL, 110 -807, KOREA TEL : 82 -2 -741 -7516~8 FAX : 82 -2 -741 -7525 e-mail : expo 1 @ dongbangchem. co. kr - FACTORY 904 -5, SANGSIN-RI, HYANGNAM-MYUN, HWASUNG-SI, KYONGGI-DO, 445 -922, KOREA TEL : 82 -31 -354 -1114 FAX : 82 -31 -353 -4120 e-mail : qc 1 @ dongbangchem. co. kr CORYRIGHT (C) DONGBANG FUTURE TECH & LIFE CO. , LTD. ALL RIGHTS RESERVED. CUSTOMER CENTER 82 -2 -741 -7516
672d94df5caab1f8228f75de7b346430.ppt