be28708ac2d3b1665cb13210847e099a.ppt
- Количество слайдов: 49
 Welcome Ask The Experts March 24 -27, 2007 New Orleans, LA
Welcome Ask The Experts March 24 -27, 2007 New Orleans, LA
 Year in Review 2006 Christopher P. Cannon, MD Senior Investigator, TIMI Study Group Cardiovascular Division Brigham and Women's Hospital Associate Professor of Medicine Harvard Medical School Boston, MA C. Michael Gibson, MS, MD Associate Professor of Medicine Harvard Medical School Chief of Clinical Research Cardiology Division Beth Israel Deaconess Medical Center Boston, MA
Year in Review 2006 Christopher P. Cannon, MD Senior Investigator, TIMI Study Group Cardiovascular Division Brigham and Women's Hospital Associate Professor of Medicine Harvard Medical School Boston, MA C. Michael Gibson, MS, MD Associate Professor of Medicine Harvard Medical School Chief of Clinical Research Cardiology Division Beth Israel Deaconess Medical Center Boston, MA
 Top Ten Clinical Trials of the Year
Top Ten Clinical Trials of the Year
 BASKET LATE Trial Basel Stent Cost-effectiveness Trial-Late Thrombotic Events (BASKET LATE) Trial Presented at The American College of Cardiology Scientific Session 2006 Presented by Dr. Matthias E. Pfisterer
BASKET LATE Trial Basel Stent Cost-effectiveness Trial-Late Thrombotic Events (BASKET LATE) Trial Presented at The American College of Cardiology Scientific Session 2006 Presented by Dr. Matthias E. Pfisterer
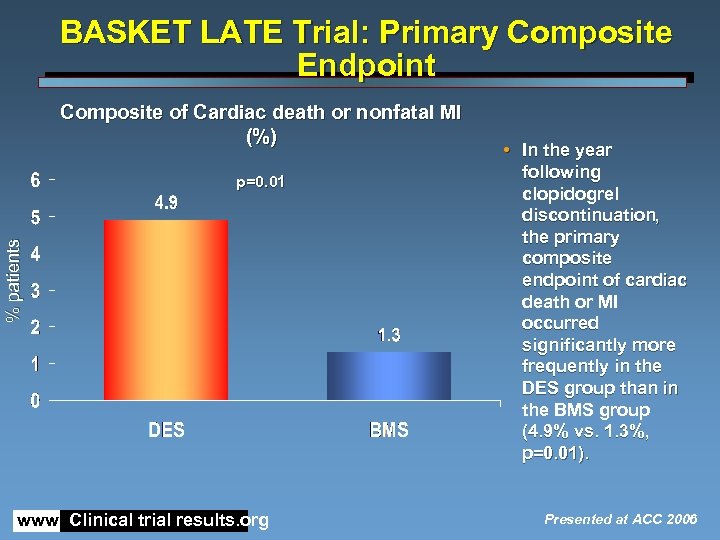 BASKET LATE Trial: Primary Composite Endpoint Composite of Cardiac death or nonfatal MI (%) % patients p=0. 01 www. Clinical trial results. org • In the year following clopidogrel discontinuation, the primary composite endpoint of cardiac death or MI occurred significantly more frequently in the DES group than in the BMS group (4. 9% vs. 1. 3%, p=0. 01). Presented at ACC 2006
BASKET LATE Trial: Primary Composite Endpoint Composite of Cardiac death or nonfatal MI (%) % patients p=0. 01 www. Clinical trial results. org • In the year following clopidogrel discontinuation, the primary composite endpoint of cardiac death or MI occurred significantly more frequently in the DES group than in the BMS group (4. 9% vs. 1. 3%, p=0. 01). Presented at ACC 2006
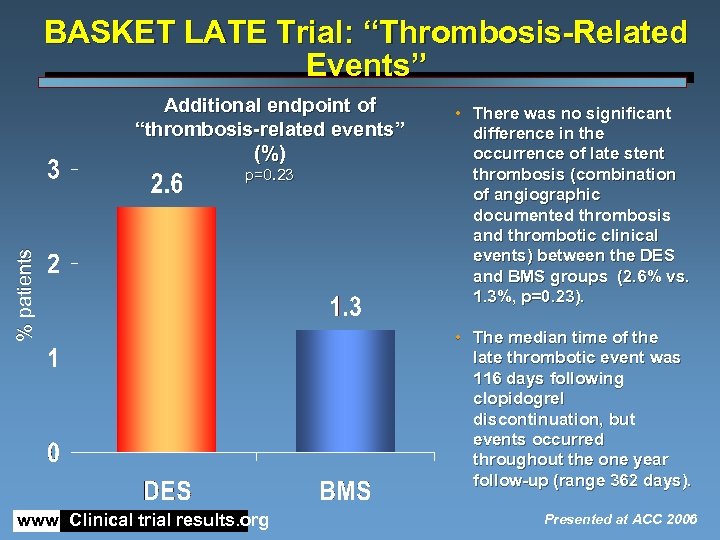 BASKET LATE Trial: “Thrombosis-Related Events” Additional endpoint of “thrombosis-related events” (%) % patients p=0. 23 www. Clinical trial results. org • There was no significant difference in the occurrence of late stent thrombosis (combination of angiographic documented thrombosis and thrombotic clinical events) between the DES and BMS groups (2. 6% vs. 1. 3%, p=0. 23). • The median time of the late thrombotic event was 116 days following clopidogrel discontinuation, but events occurred throughout the one year follow-up (range 362 days). Presented at ACC 2006
BASKET LATE Trial: “Thrombosis-Related Events” Additional endpoint of “thrombosis-related events” (%) % patients p=0. 23 www. Clinical trial results. org • There was no significant difference in the occurrence of late stent thrombosis (combination of angiographic documented thrombosis and thrombotic clinical events) between the DES and BMS groups (2. 6% vs. 1. 3%, p=0. 23). • The median time of the late thrombotic event was 116 days following clopidogrel discontinuation, but events occurred throughout the one year follow-up (range 362 days). Presented at ACC 2006
 Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) Deepak L. Bhatt M. D. , Keith A. A. Fox M. B. Ch. B. , Werner Hacke M. D. , Peter B. Berger M. D. , Henry R. Black M. D. , William E. Boden M. D. , Patrice Cacoub M. D. , Eric A. Cohen M. D. , Mark A. Creager M. D. , J. Donald Easton M. D. , Marcus D. Flather M. D. , Steven M. Haffner M. D. , Christian W. Hamm M. D. , Graeme J. Hankey M. D. , S. Claiborne Johnston M. D. , Koon-Hou Mak M. D. , Jean-Louis Mas M. D. , Gilles Montalescot M. D. , Ph. D. , Thomas A. Pearson M. D. , P. Gabriel Steg M. D. , Steven R. Steinhubl M. D. , Michael A. Weber M. D. , Danielle M. Brennan M. S. , Liz Fabry-Ribaudo M. S. N. , R. N. , Joan Booth R. N. , Eric J. Topol M. D. , on behalf of the CHARISMA Investigators The Cleveland Clinic Foundation
Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) Deepak L. Bhatt M. D. , Keith A. A. Fox M. B. Ch. B. , Werner Hacke M. D. , Peter B. Berger M. D. , Henry R. Black M. D. , William E. Boden M. D. , Patrice Cacoub M. D. , Eric A. Cohen M. D. , Mark A. Creager M. D. , J. Donald Easton M. D. , Marcus D. Flather M. D. , Steven M. Haffner M. D. , Christian W. Hamm M. D. , Graeme J. Hankey M. D. , S. Claiborne Johnston M. D. , Koon-Hou Mak M. D. , Jean-Louis Mas M. D. , Gilles Montalescot M. D. , Ph. D. , Thomas A. Pearson M. D. , P. Gabriel Steg M. D. , Steven R. Steinhubl M. D. , Michael A. Weber M. D. , Danielle M. Brennan M. S. , Liz Fabry-Ribaudo M. S. N. , R. N. , Joan Booth R. N. , Eric J. Topol M. D. , on behalf of the CHARISMA Investigators The Cleveland Clinic Foundation
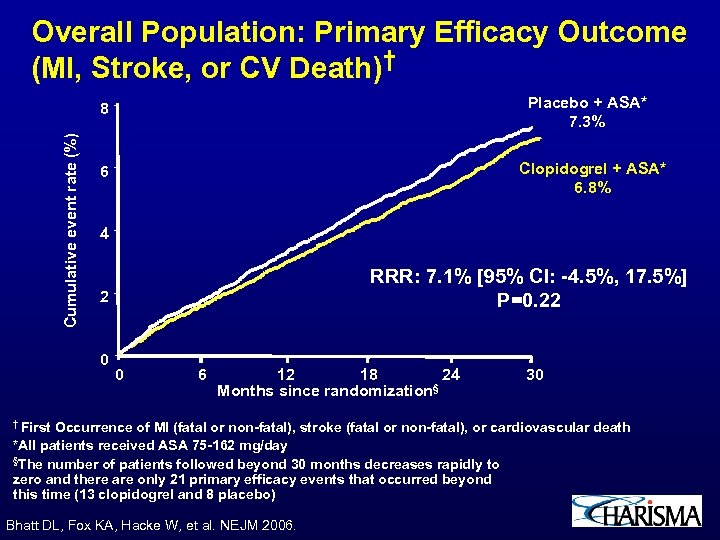 Overall Population: Primary Efficacy Outcome (MI, Stroke, or CV Death)† Placebo + ASA* 7. 3% Cumulative event rate (%) 8 Clopidogrel + ASA* 6. 8% 6 4 RRR: 7. 1% [95% CI: -4. 5%, 17. 5%] P=0. 22 2 0 0 6 12 18 24 § Months since randomization † First 30 Occurrence of MI (fatal or non-fatal), stroke (fatal or non-fatal), or cardiovascular death *All patients received ASA 75 -162 mg/day §The number of patients followed beyond 30 months decreases rapidly to zero and there are only 21 primary efficacy events that occurred beyond this time (13 clopidogrel and 8 placebo) Bhatt DL, Fox KA, Hacke W, et al. NEJM 2006.
Overall Population: Primary Efficacy Outcome (MI, Stroke, or CV Death)† Placebo + ASA* 7. 3% Cumulative event rate (%) 8 Clopidogrel + ASA* 6. 8% 6 4 RRR: 7. 1% [95% CI: -4. 5%, 17. 5%] P=0. 22 2 0 0 6 12 18 24 § Months since randomization † First 30 Occurrence of MI (fatal or non-fatal), stroke (fatal or non-fatal), or cardiovascular death *All patients received ASA 75 -162 mg/day §The number of patients followed beyond 30 months decreases rapidly to zero and there are only 21 primary efficacy events that occurred beyond this time (13 clopidogrel and 8 placebo) Bhatt DL, Fox KA, Hacke W, et al. NEJM 2006.
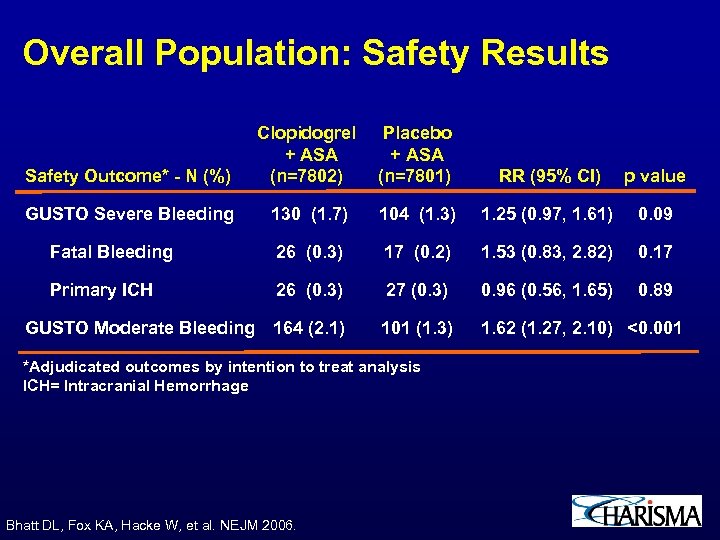 Overall Population: Safety Results Safety Outcome* - N (%) Clopidogrel + ASA (n=7802) Placebo + ASA (n=7801) RR (95% CI) p value GUSTO Severe Bleeding 130 (1. 7) 104 (1. 3) 1. 25 (0. 97, 1. 61) 0. 09 Fatal Bleeding 26 (0. 3) 17 (0. 2) 1. 53 (0. 83, 2. 82) 0. 17 Primary ICH 26 (0. 3) 27 (0. 3) 0. 96 (0. 56, 1. 65) 0. 89 GUSTO Moderate Bleeding 164 (2. 1) 101 (1. 3) 1. 62 (1. 27, 2. 10) <0. 001 *Adjudicated outcomes by intention to treat analysis ICH= Intracranial Hemorrhage Bhatt DL, Fox KA, Hacke W, et al. NEJM 2006.
Overall Population: Safety Results Safety Outcome* - N (%) Clopidogrel + ASA (n=7802) Placebo + ASA (n=7801) RR (95% CI) p value GUSTO Severe Bleeding 130 (1. 7) 104 (1. 3) 1. 25 (0. 97, 1. 61) 0. 09 Fatal Bleeding 26 (0. 3) 17 (0. 2) 1. 53 (0. 83, 2. 82) 0. 17 Primary ICH 26 (0. 3) 27 (0. 3) 0. 96 (0. 56, 1. 65) 0. 89 GUSTO Moderate Bleeding 164 (2. 1) 101 (1. 3) 1. 62 (1. 27, 2. 10) <0. 001 *Adjudicated outcomes by intention to treat analysis ICH= Intracranial Hemorrhage Bhatt DL, Fox KA, Hacke W, et al. NEJM 2006.
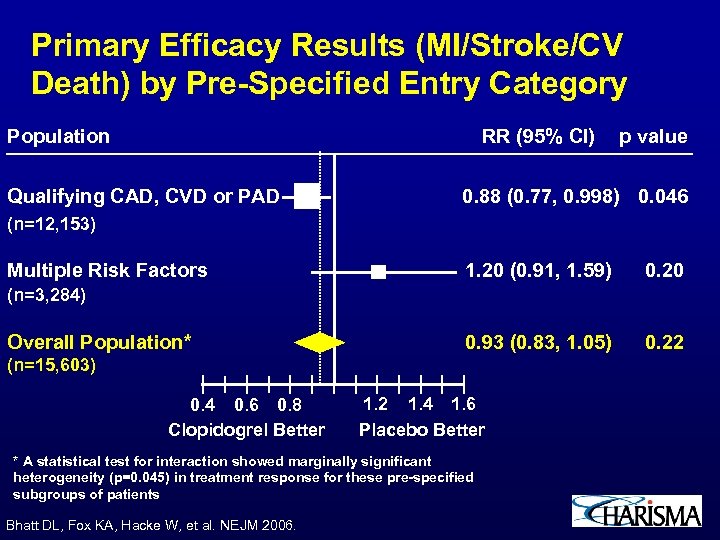 Primary Efficacy Results (MI/Stroke/CV Death) by Pre-Specified Entry Category Population RR (95% CI) Qualifying CAD, CVD or PAD p value 0. 88 (0. 77, 0. 998) 0. 046 (n=12, 153) Multiple Risk Factors 1. 20 (0. 91, 1. 59) 0. 20 0. 93 (0. 83, 1. 05) 0. 22 (n=3, 284) Overall Population* (n=15, 603) 0. 4 0. 6 0. 8 Clopidogrel Better 1. 2 1. 4 1. 6 Placebo Better * A statistical test for interaction showed marginally significant heterogeneity (p=0. 045) in treatment response for these pre-specified subgroups of patients Bhatt DL, Fox KA, Hacke W, et al. NEJM 2006.
Primary Efficacy Results (MI/Stroke/CV Death) by Pre-Specified Entry Category Population RR (95% CI) Qualifying CAD, CVD or PAD p value 0. 88 (0. 77, 0. 998) 0. 046 (n=12, 153) Multiple Risk Factors 1. 20 (0. 91, 1. 59) 0. 20 0. 93 (0. 83, 1. 05) 0. 22 (n=3, 284) Overall Population* (n=15, 603) 0. 4 0. 6 0. 8 Clopidogrel Better 1. 2 1. 4 1. 6 Placebo Better * A statistical test for interaction showed marginally significant heterogeneity (p=0. 045) in treatment response for these pre-specified subgroups of patients Bhatt DL, Fox KA, Hacke W, et al. NEJM 2006.
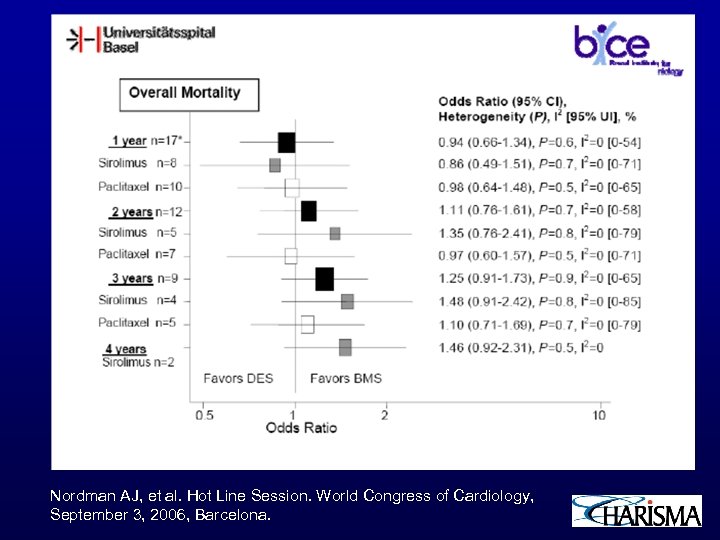 Nordman AJ, et al. Hot Line Session. World Congress of Cardiology, September 3, 2006, Barcelona.
Nordman AJ, et al. Hot Line Session. World Congress of Cardiology, September 3, 2006, Barcelona.
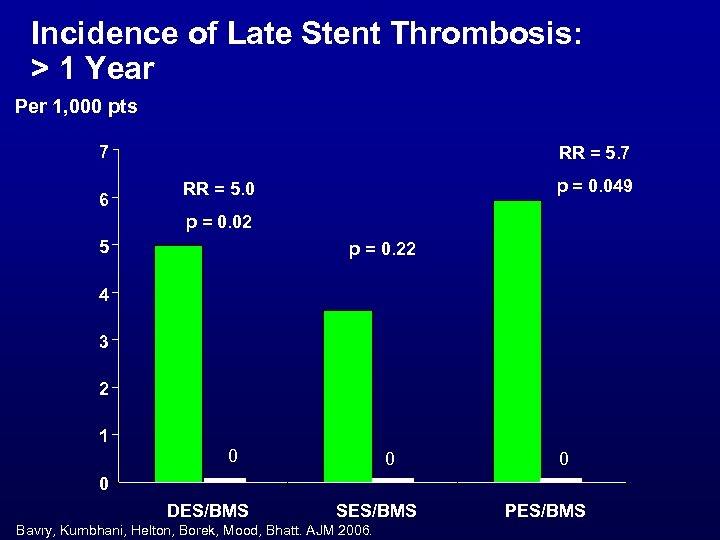 Incidence of Late Stent Thrombosis: > 1 Year Per 1, 000 pts 7 6 RR = 5. 7 p = 0. 049 RR = 5. 0 p = 0. 02 5 p = 0. 22 4 3 2 1 0 0 DES/BMS SES/BMS Bavry, Kumbhani, Helton, Borek, Mood, Bhatt. AJM 2006. PES/BMS
Incidence of Late Stent Thrombosis: > 1 Year Per 1, 000 pts 7 6 RR = 5. 7 p = 0. 049 RR = 5. 0 p = 0. 02 5 p = 0. 22 4 3 2 1 0 0 DES/BMS SES/BMS Bavry, Kumbhani, Helton, Borek, Mood, Bhatt. AJM 2006. PES/BMS

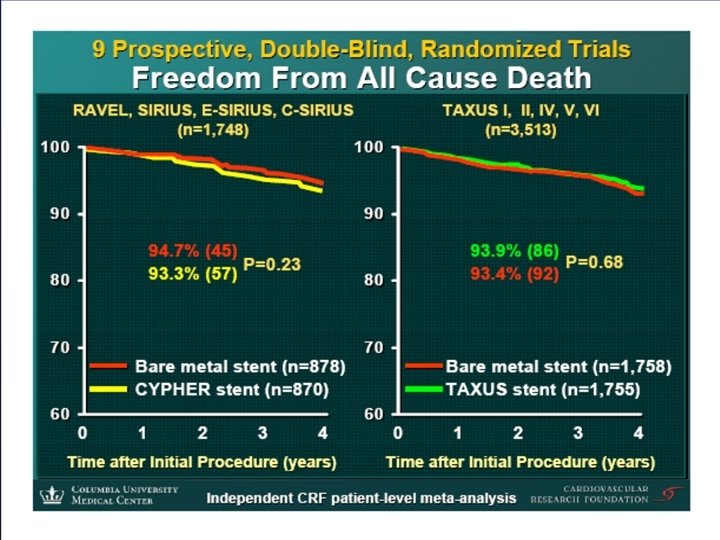
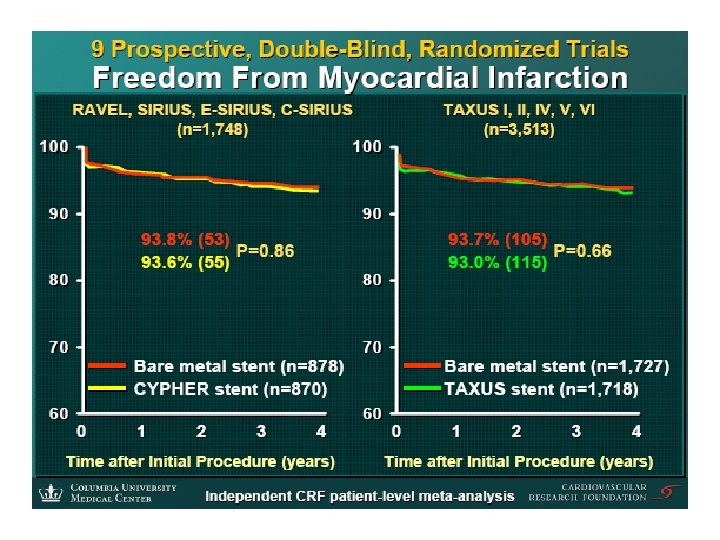
 ISAR-REACT 2 Trial Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT 2) Trial Presented at The American College of Cardiology Scientific Session 2006 Presented by Dr. Adnan Kastrati
ISAR-REACT 2 Trial Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT 2) Trial Presented at The American College of Cardiology Scientific Session 2006 Presented by Dr. Adnan Kastrati
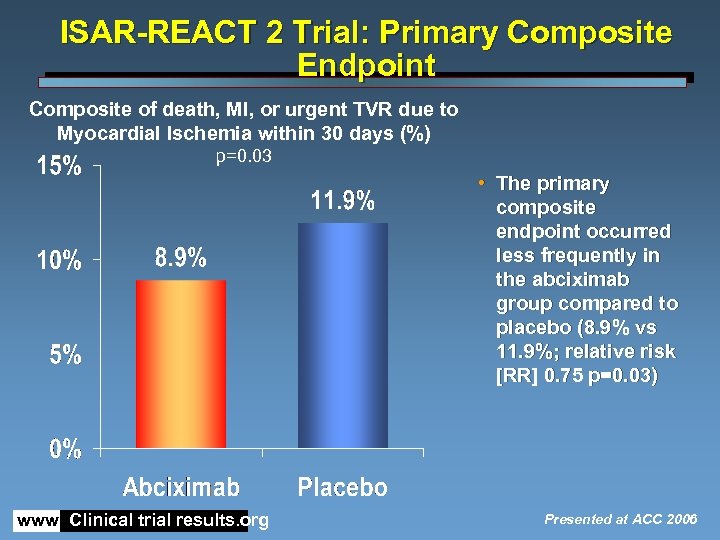 ISAR-REACT 2 Trial: Primary Composite Endpoint Composite of death, MI, or urgent TVR due to Myocardial Ischemia within 30 days (%) p=0. 03 • The primary composite endpoint occurred less frequently in the abciximab group compared to placebo (8. 9% vs 11. 9%; relative risk [RR] 0. 75 p=0. 03) www. Clinical trial results. org Presented at ACC 2006
ISAR-REACT 2 Trial: Primary Composite Endpoint Composite of death, MI, or urgent TVR due to Myocardial Ischemia within 30 days (%) p=0. 03 • The primary composite endpoint occurred less frequently in the abciximab group compared to placebo (8. 9% vs 11. 9%; relative risk [RR] 0. 75 p=0. 03) www. Clinical trial results. org Presented at ACC 2006
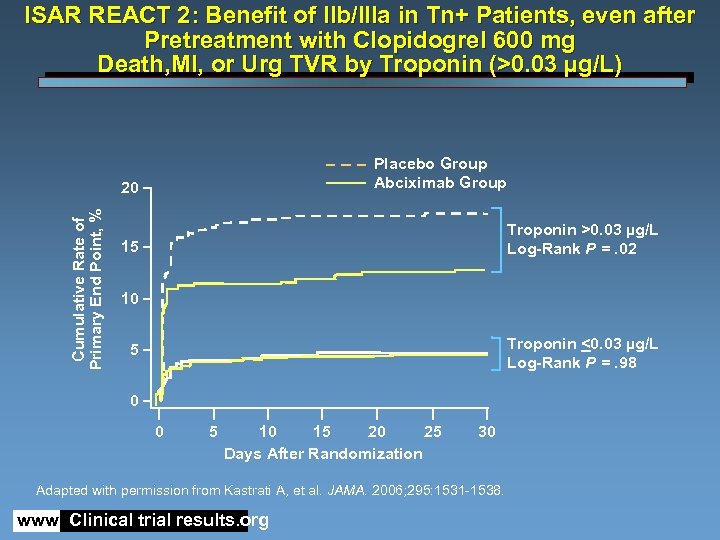 ISAR REACT 2: Benefit of IIb/IIIa in Tn+ Patients, even after Pretreatment with Clopidogrel 600 mg Death, MI, or Urg TVR by Troponin (>0. 03 µg/L) Placebo Group Abciximab Group Cumulative Rate of Primary End Point, % 20 Troponin >0. 03 µg/L Log-Rank P =. 02 15 10 Troponin <0. 03 µg/L Log-Rank P =. 98 5 0 0 5 10 15 20 25 Days After Randomization 30 Adapted with permission from Kastrati A, et al. JAMA. 2006; 295: 1531 -1538. www. Clinical trial results. org
ISAR REACT 2: Benefit of IIb/IIIa in Tn+ Patients, even after Pretreatment with Clopidogrel 600 mg Death, MI, or Urg TVR by Troponin (>0. 03 µg/L) Placebo Group Abciximab Group Cumulative Rate of Primary End Point, % 20 Troponin >0. 03 µg/L Log-Rank P =. 02 15 10 Troponin <0. 03 µg/L Log-Rank P =. 98 5 0 0 5 10 15 20 25 Days After Randomization 30 Adapted with permission from Kastrati A, et al. JAMA. 2006; 295: 1531 -1538. www. Clinical trial results. org
 Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Ex. TRACT-TIMI 25 ACC 2006 Atlanta, GA Disclosure Statement: Dr. Antman received research grant support via the Brigham and Women’s Hospital from sanofi-aventis
Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Ex. TRACT-TIMI 25 ACC 2006 Atlanta, GA Disclosure Statement: Dr. Antman received research grant support via the Brigham and Women’s Hospital from sanofi-aventis
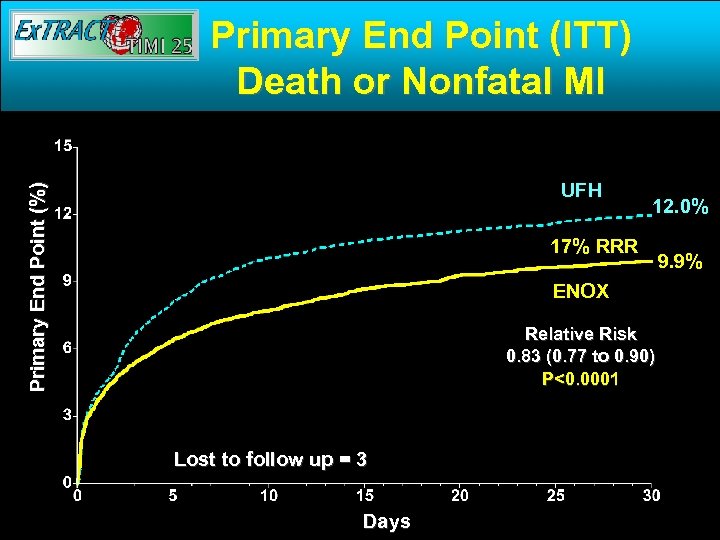 Primary End Point (ITT) Death or Nonfatal MI Primary End Point (%) UFH 12. 0% 17% RRR ENOX Relative Risk 0. 83 (0. 77 to 0. 90) P<0. 0001 Lost to follow up = 3 Days 9. 9%
Primary End Point (ITT) Death or Nonfatal MI Primary End Point (%) UFH 12. 0% 17% RRR ENOX Relative Risk 0. 83 (0. 77 to 0. 90) P<0. 0001 Lost to follow up = 3 Days 9. 9%
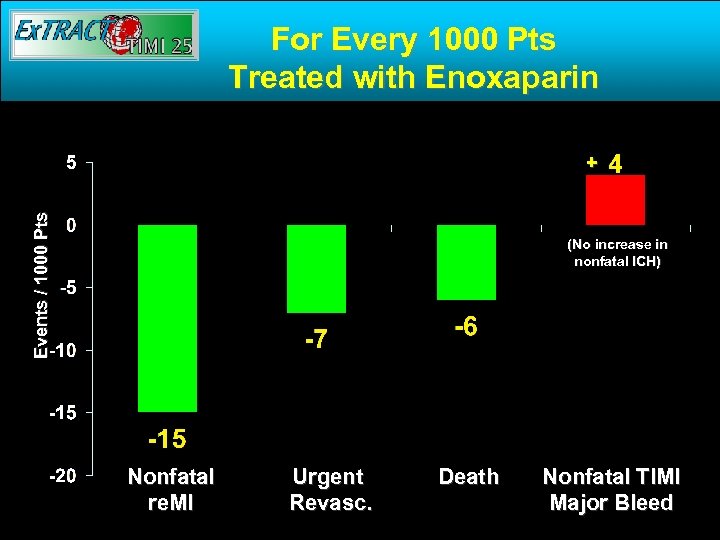 For Every 1000 Pts Treated with Enoxaparin Events / 1000 Pts + (No increase in nonfatal ICH) Nonfatal re. MI Urgent Revasc. Death Nonfatal TIMI Major Bleed
For Every 1000 Pts Treated with Enoxaparin Events / 1000 Pts + (No increase in nonfatal ICH) Nonfatal re. MI Urgent Revasc. Death Nonfatal TIMI Major Bleed

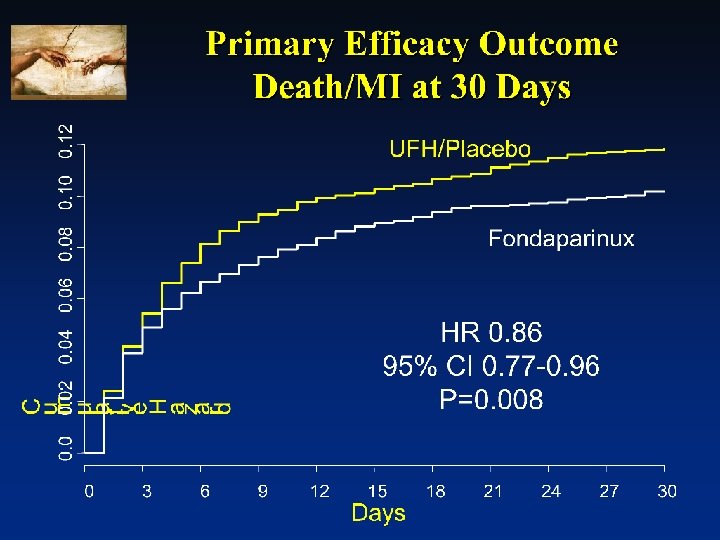
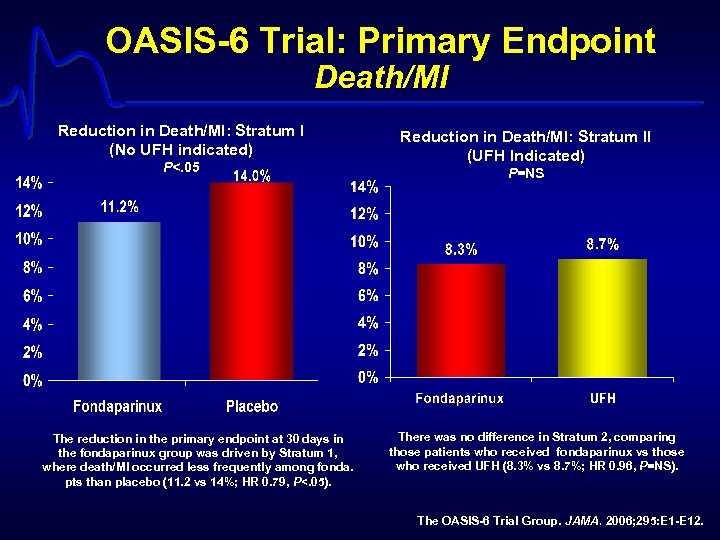 OASIS-6 Trial: Primary Endpoint Death/MI Reduction in Death/MI: Stratum I (No UFH indicated) P<. 05 Reduction in Death/MI: Stratum II (UFH Indicated) P=NS p=0. 97 The reduction in the primary endpoint at 30 days in the fondaparinux group was driven by Stratum 1, where death/MI occurred less frequently among fonda. pts than placebo (11. 2 vs 14%; HR 0. 79, P<. 05). There was no difference in Stratum 2, comparing those patients who received fondaparinux vs those who received UFH (8. 3% vs 8. 7%; HR 0. 96, P=NS). The OASIS-6 Trial Group. JAMA. 2006; 295: E 1 -E 12.
OASIS-6 Trial: Primary Endpoint Death/MI Reduction in Death/MI: Stratum I (No UFH indicated) P<. 05 Reduction in Death/MI: Stratum II (UFH Indicated) P=NS p=0. 97 The reduction in the primary endpoint at 30 days in the fondaparinux group was driven by Stratum 1, where death/MI occurred less frequently among fonda. pts than placebo (11. 2 vs 14%; HR 0. 79, P<. 05). There was no difference in Stratum 2, comparing those patients who received fondaparinux vs those who received UFH (8. 3% vs 8. 7%; HR 0. 96, P=NS). The OASIS-6 Trial Group. JAMA. 2006; 295: E 1 -E 12.
 ASSENT- 4 PCI Trial The Assessment of the Safety and Efficacy of a New Treatment Strategy for Acute Myocardial Infarction (ASSENT-4 PCI) Trial Presented at The European Society of Cardiology Hot Line Session 2005 Presented by Dr. Frans Van de Werf
ASSENT- 4 PCI Trial The Assessment of the Safety and Efficacy of a New Treatment Strategy for Acute Myocardial Infarction (ASSENT-4 PCI) Trial Presented at The European Society of Cardiology Hot Line Session 2005 Presented by Dr. Frans Van de Werf
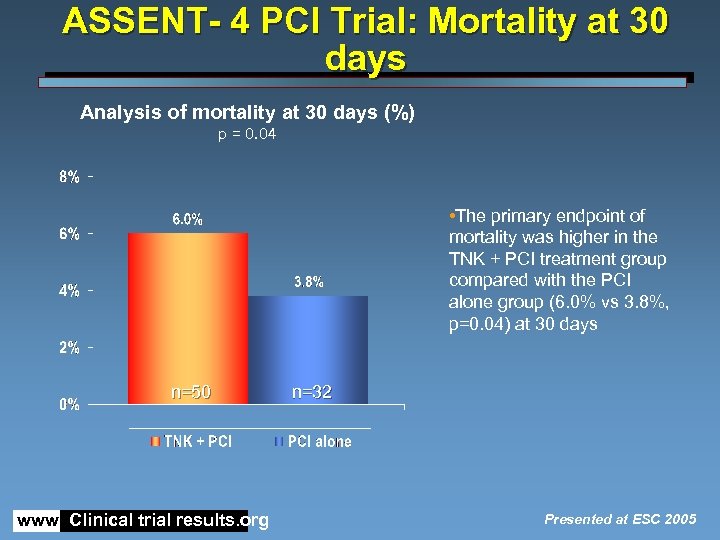 ASSENT- 4 PCI Trial: Mortality at 30 days Analysis of mortality at 30 days (%) p = 0. 04 • The primary endpoint of mortality was higher in the TNK + PCI treatment group compared with the PCI alone group (6. 0% vs 3. 8%, p=0. 04) at 30 days n=50 www. Clinical trial results. org n=32 Presented at ESC 2005
ASSENT- 4 PCI Trial: Mortality at 30 days Analysis of mortality at 30 days (%) p = 0. 04 • The primary endpoint of mortality was higher in the TNK + PCI treatment group compared with the PCI alone group (6. 0% vs 3. 8%, p=0. 04) at 30 days n=50 www. Clinical trial results. org n=32 Presented at ESC 2005
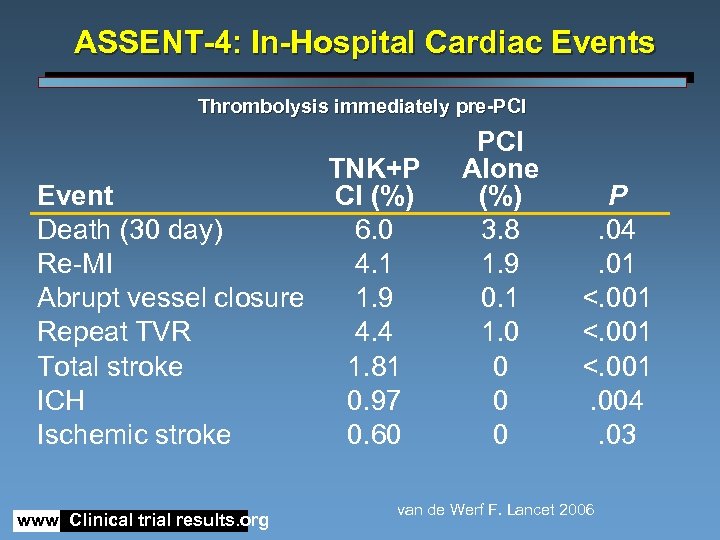 ASSENT-4: In-Hospital Cardiac Events Thrombolysis immediately pre-PCI TNK+P Event CI (%) Death (30 day) 6. 0 Re-MI 4. 1 Abrupt vessel closure 1. 9 Repeat TVR 4. 4 Total stroke 1. 81 ICH 0. 97 Ischemic stroke 0. 60 www. Clinical trial results. org PCI Alone (%) 3. 8 1. 9 0. 1 1. 0 0 P. 04. 01 <. 001. 004. 03 van de Werf F. Lancet 2006
ASSENT-4: In-Hospital Cardiac Events Thrombolysis immediately pre-PCI TNK+P Event CI (%) Death (30 day) 6. 0 Re-MI 4. 1 Abrupt vessel closure 1. 9 Repeat TVR 4. 4 Total stroke 1. 81 ICH 0. 97 Ischemic stroke 0. 60 www. Clinical trial results. org PCI Alone (%) 3. 8 1. 9 0. 1 1. 0 0 P. 04. 01 <. 001. 004. 03 van de Werf F. Lancet 2006
 Prospective, Randomized Comparison of Heparin Plus IIb/IIIa Inhibition and Bivalirudin With or Without IIb/IIIa Inhibition in Patients with Acute Coronary Syndromes Gregg W. Stone MD for the ACUITY Investigators
Prospective, Randomized Comparison of Heparin Plus IIb/IIIa Inhibition and Bivalirudin With or Without IIb/IIIa Inhibition in Patients with Acute Coronary Syndromes Gregg W. Stone MD for the ACUITY Investigators
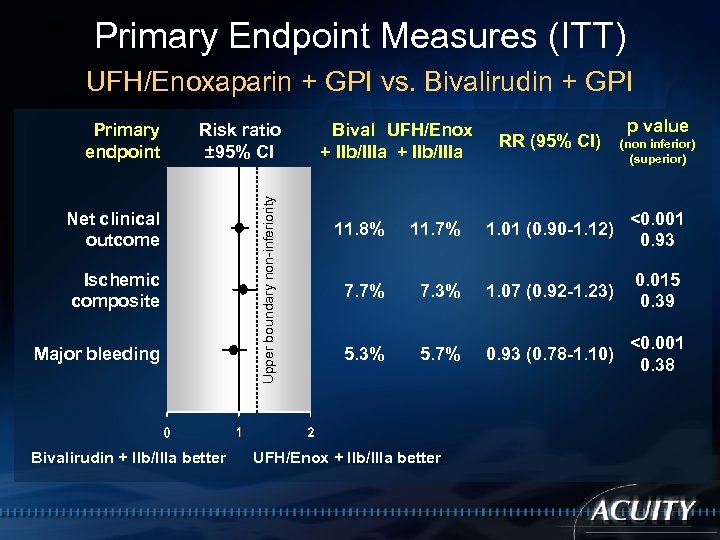 Primary Endpoint Measures (ITT) UFH/Enoxaparin + GPI vs. Bivalirudin + GPI Risk ratio ± 95% CI Net clinical outcome Ischemic composite Major bleeding Bivalirudin + IIb/IIIa better Upper boundary non-inferiority Primary endpoint Bival UFH/Enox + IIb/IIIa RR (95% CI) p value (non inferior) (superior) 11. 8% 11. 7% 1. 01 (0. 90 -1. 12) <0. 001 0. 93 7. 7% 7. 3% 1. 07 (0. 92 -1. 23) 0. 015 0. 39 5. 3% 5. 7% 0. 93 (0. 78 -1. 10) <0. 001 0. 38 UFH/Enox + IIb/IIIa better
Primary Endpoint Measures (ITT) UFH/Enoxaparin + GPI vs. Bivalirudin + GPI Risk ratio ± 95% CI Net clinical outcome Ischemic composite Major bleeding Bivalirudin + IIb/IIIa better Upper boundary non-inferiority Primary endpoint Bival UFH/Enox + IIb/IIIa RR (95% CI) p value (non inferior) (superior) 11. 8% 11. 7% 1. 01 (0. 90 -1. 12) <0. 001 0. 93 7. 7% 7. 3% 1. 07 (0. 92 -1. 23) 0. 015 0. 39 5. 3% 5. 7% 0. 93 (0. 78 -1. 10) <0. 001 0. 38 UFH/Enox + IIb/IIIa better
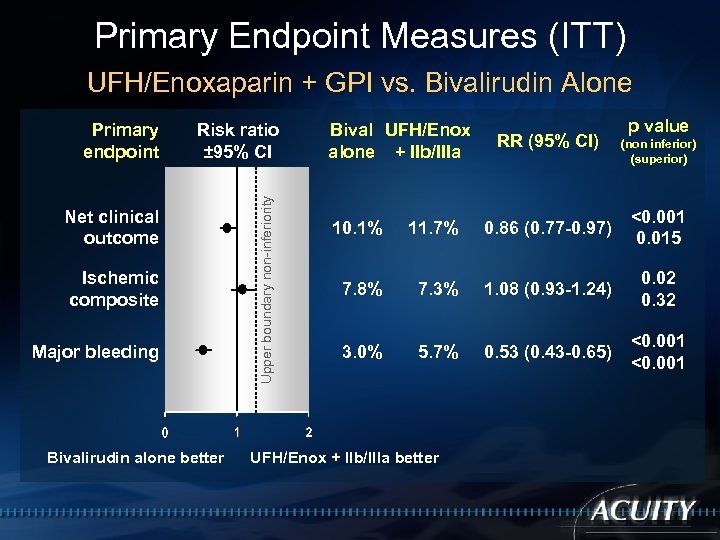 Primary Endpoint Measures (ITT) UFH/Enoxaparin + GPI vs. Bivalirudin Alone Risk ratio ± 95% CI Net clinical outcome Ischemic composite Major bleeding Bivalirudin alone better Upper boundary non-inferiority Primary endpoint Bival UFH/Enox alone + IIb/IIIa RR (95% CI) p value (non inferior) (superior) 10. 1% 11. 7% 0. 86 (0. 77 -0. 97) <0. 001 0. 015 7. 8% 7. 3% 1. 08 (0. 93 -1. 24) 0. 02 0. 32 3. 0% 5. 7% 0. 53 (0. 43 -0. 65) <0. 001 UFH/Enox + IIb/IIIa better
Primary Endpoint Measures (ITT) UFH/Enoxaparin + GPI vs. Bivalirudin Alone Risk ratio ± 95% CI Net clinical outcome Ischemic composite Major bleeding Bivalirudin alone better Upper boundary non-inferiority Primary endpoint Bival UFH/Enox alone + IIb/IIIa RR (95% CI) p value (non inferior) (superior) 10. 1% 11. 7% 0. 86 (0. 77 -0. 97) <0. 001 0. 015 7. 8% 7. 3% 1. 08 (0. 93 -1. 24) 0. 02 0. 32 3. 0% 5. 7% 0. 53 (0. 43 -0. 65) <0. 001 UFH/Enox + IIb/IIIa better
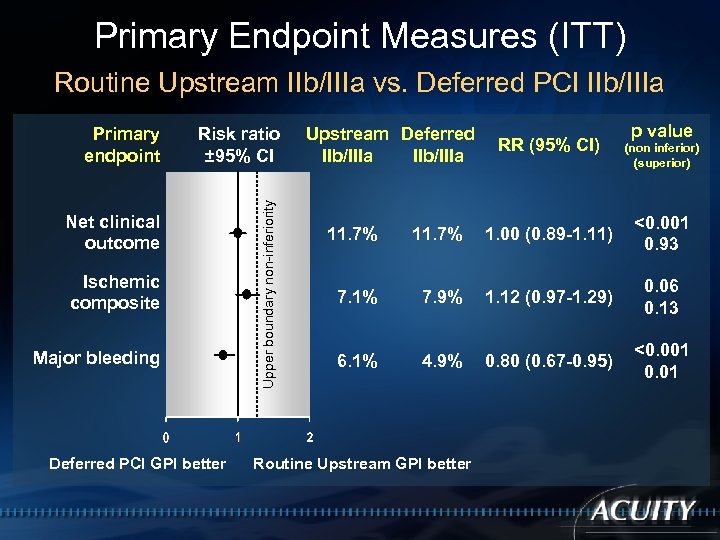 Primary Endpoint Measures (ITT) Routine Upstream IIb/IIIa vs. Deferred PCI IIb/IIIa Risk ratio ± 95% CI Net clinical outcome Ischemic composite Major bleeding Deferred PCI GPI better Upper boundary non-inferiority Primary endpoint Upstream Deferred IIb/IIIa RR (95% CI) p value (non inferior) (superior) 11. 7% 1. 00 (0. 89 -1. 11) <0. 001 0. 93 7. 1% 7. 9% 1. 12 (0. 97 -1. 29) 0. 06 0. 13 6. 1% 4. 9% 0. 80 (0. 67 -0. 95) <0. 001 0. 01 Routine Upstream GPI better
Primary Endpoint Measures (ITT) Routine Upstream IIb/IIIa vs. Deferred PCI IIb/IIIa Risk ratio ± 95% CI Net clinical outcome Ischemic composite Major bleeding Deferred PCI GPI better Upper boundary non-inferiority Primary endpoint Upstream Deferred IIb/IIIa RR (95% CI) p value (non inferior) (superior) 11. 7% 1. 00 (0. 89 -1. 11) <0. 001 0. 93 7. 1% 7. 9% 1. 12 (0. 97 -1. 29) 0. 06 0. 13 6. 1% 4. 9% 0. 80 (0. 67 -0. 95) <0. 001 0. 01 Routine Upstream GPI better
 DREAM Diabetes REduction Assessment with ramipril and rosiglitazone Medication
DREAM Diabetes REduction Assessment with ramipril and rosiglitazone Medication
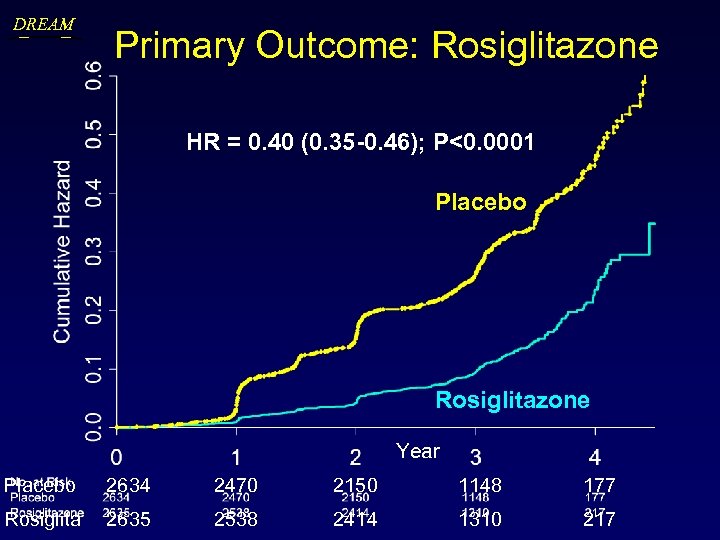 DREAM Primary Outcome: Rosiglitazone HR = 0. 40 (0. 35 -0. 46); P<0. 0001 Placebo Rosiglitazone Year Placebo 2634 2470 2150 1148 177 Rosiglita 2635 2538 2414 1310 217
DREAM Primary Outcome: Rosiglitazone HR = 0. 40 (0. 35 -0. 46); P<0. 0001 Placebo Rosiglitazone Year Placebo 2634 2470 2150 1148 177 Rosiglita 2635 2538 2414 1310 217
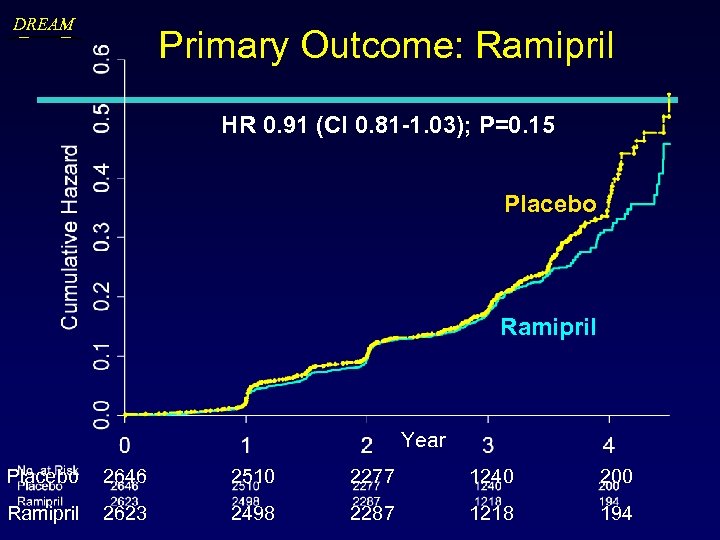 DREAM Primary Outcome: Ramipril HR 0. 91 (CI 0. 81 -1. 03); P=0. 15 Placebo Ramipril Year Placebo 2646 2510 2277 1240 200 Ramipril 2623 2498 2287 1218 194
DREAM Primary Outcome: Ramipril HR 0. 91 (CI 0. 81 -1. 03); P=0. 15 Placebo Ramipril Year Placebo 2646 2510 2277 1240 200 Ramipril 2623 2498 2287 1218 194
 Diclofenac Arthritis Long-term (MEDAL) Study Program Cardiovascular Outcomes Following Longterm Treatment with Etoricoxib vs Diclofenac in Patients with Osteoarthritis and Rheumatoid Arthritis Christopher P. Cannon, MD, Sean P. Curtis, MD, Amarjot Kaur, Ph. D, Loren Laine, MD, for the MEDAL Steering Committee
Diclofenac Arthritis Long-term (MEDAL) Study Program Cardiovascular Outcomes Following Longterm Treatment with Etoricoxib vs Diclofenac in Patients with Osteoarthritis and Rheumatoid Arthritis Christopher P. Cannon, MD, Sean P. Curtis, MD, Amarjot Kaur, Ph. D, Loren Laine, MD, for the MEDAL Steering Committee
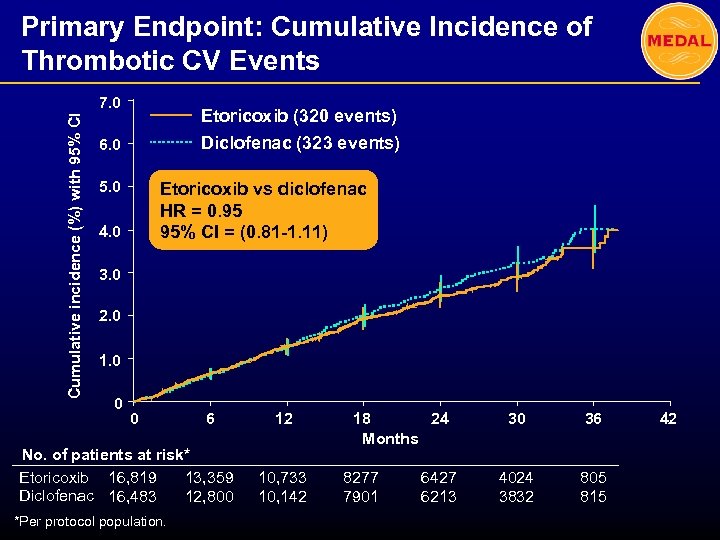 Primary Endpoint: Cumulative Incidence of Thrombotic CV Events Cumulative incidence (%) with 95% CI 7. 0 Etoricoxib (320 events) Diclofenac (323 events) 6. 0 5. 0 Etoricoxib vs diclofenac HR = 0. 95 95% CI = (0. 81 -1. 11) 4. 0 3. 0 2. 0 1. 0 0 0 6 No. of patients at risk* Etoricoxib 16, 819 13, 359 Diclofenac 16, 483 12, 800 *Per protocol population. 12 10, 733 10, 142 18 24 Months 8277 7901 6427 6213 30 36 4024 3832 805 815 42
Primary Endpoint: Cumulative Incidence of Thrombotic CV Events Cumulative incidence (%) with 95% CI 7. 0 Etoricoxib (320 events) Diclofenac (323 events) 6. 0 5. 0 Etoricoxib vs diclofenac HR = 0. 95 95% CI = (0. 81 -1. 11) 4. 0 3. 0 2. 0 1. 0 0 0 6 No. of patients at risk* Etoricoxib 16, 819 13, 359 Diclofenac 16, 483 12, 800 *Per protocol population. 12 10, 733 10, 142 18 24 Months 8277 7901 6427 6213 30 36 4024 3832 805 815 42
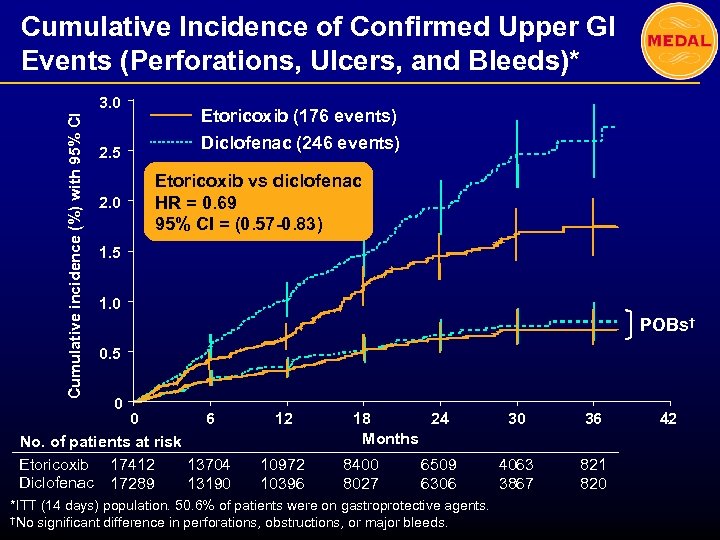 Cumulative Incidence of Confirmed Upper GI Events (Perforations, Ulcers, and Bleeds)* Cumulative incidence (%) with 95% CI 3. 0 2. 5 2. 0 Etoricoxib (176 events) Diclofenac (246 events) Etoricoxib vs diclofenac HR = 0. 69 95% CI = (0. 57 -0. 83) 1. 5 1. 0 POBs† 0. 5 0 0 6 No. of patients at risk Etoricoxib 17412 13704 Diclofenac 17289 13190 12 10972 10396 18 24 Months 8400 8027 6509 6306 *ITT (14 days) population. 50. 6% of patients were on gastroprotective agents. †No significant difference in perforations, obstructions, or major bleeds. 30 36 4063 3867 821 820 42
Cumulative Incidence of Confirmed Upper GI Events (Perforations, Ulcers, and Bleeds)* Cumulative incidence (%) with 95% CI 3. 0 2. 5 2. 0 Etoricoxib (176 events) Diclofenac (246 events) Etoricoxib vs diclofenac HR = 0. 69 95% CI = (0. 57 -0. 83) 1. 5 1. 0 POBs† 0. 5 0 0 6 No. of patients at risk Etoricoxib 17412 13704 Diclofenac 17289 13190 12 10972 10396 18 24 Months 8400 8027 6509 6306 *ITT (14 days) population. 50. 6% of patients were on gastroprotective agents. †No significant difference in perforations, obstructions, or major bleeds. 30 36 4063 3867 821 820 42
 ASTEROID The Effect of Very High-Intensity Statin Therapy on Regression of Coronary Atherosclerosis Steven E. Nissen MD Disclosure Consulting: Astra. Zeneca, Abbott, Atherogenics, Bayer, Lipid Sciences, Wyeth, Novartis, Pfizer, Sankyo, Haptogard, Hoffman-La. Roche, Kemia, Takeda, Kowa, Sanofi-Aventis, Protevia, Novo-Nordisk, Eli Lilly, Kos, Glaxo. Smith. Kline, Forbes Medi-tech, Vasogenix, Vascular Biogenics, Isis Pharma, Viron Therapeutics, Roche, and Merck–Schering Plough Lectures: Astra. Zeneca and Pfizer Clinical Trials: Astra. Zeneca, Eli Lilly, Takeda, Sankyo, Sanofi-Aventis, Pfizer, Atherogenics, and Lipid Sciences. Companies are directed to pay any honoraria directly to charity. No personal reimbursement is accepted for directing or participating in clinical trials.
ASTEROID The Effect of Very High-Intensity Statin Therapy on Regression of Coronary Atherosclerosis Steven E. Nissen MD Disclosure Consulting: Astra. Zeneca, Abbott, Atherogenics, Bayer, Lipid Sciences, Wyeth, Novartis, Pfizer, Sankyo, Haptogard, Hoffman-La. Roche, Kemia, Takeda, Kowa, Sanofi-Aventis, Protevia, Novo-Nordisk, Eli Lilly, Kos, Glaxo. Smith. Kline, Forbes Medi-tech, Vasogenix, Vascular Biogenics, Isis Pharma, Viron Therapeutics, Roche, and Merck–Schering Plough Lectures: Astra. Zeneca and Pfizer Clinical Trials: Astra. Zeneca, Eli Lilly, Takeda, Sankyo, Sanofi-Aventis, Pfizer, Atherogenics, and Lipid Sciences. Companies are directed to pay any honoraria directly to charity. No personal reimbursement is accepted for directing or participating in clinical trials.
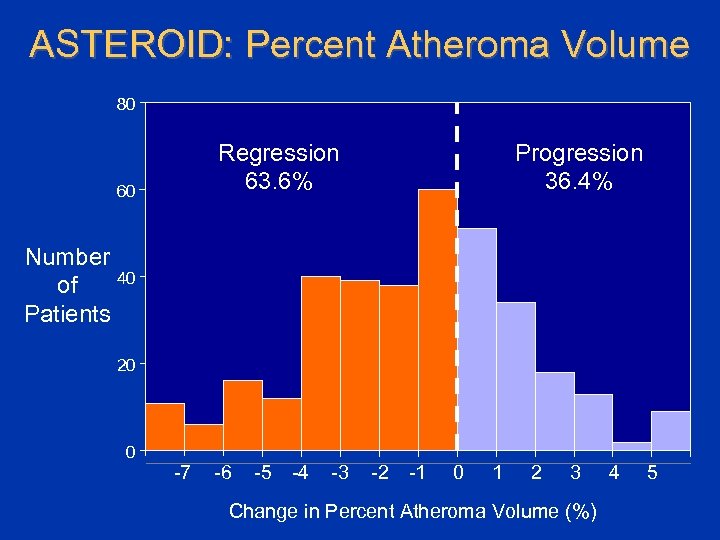 ASTEROID: Percent Atheroma Volume 80 Regression 63. 6% 60 Progression 36. 4% Number 40 of Patients 20 0 -7 -6 -5 -4 -3 -2 -1 0 1 2 3 Change in Percent Atheroma Volume (%) 4 5
ASTEROID: Percent Atheroma Volume 80 Regression 63. 6% 60 Progression 36. 4% Number 40 of Patients 20 0 -7 -6 -5 -4 -3 -2 -1 0 1 2 3 Change in Percent Atheroma Volume (%) 4 5
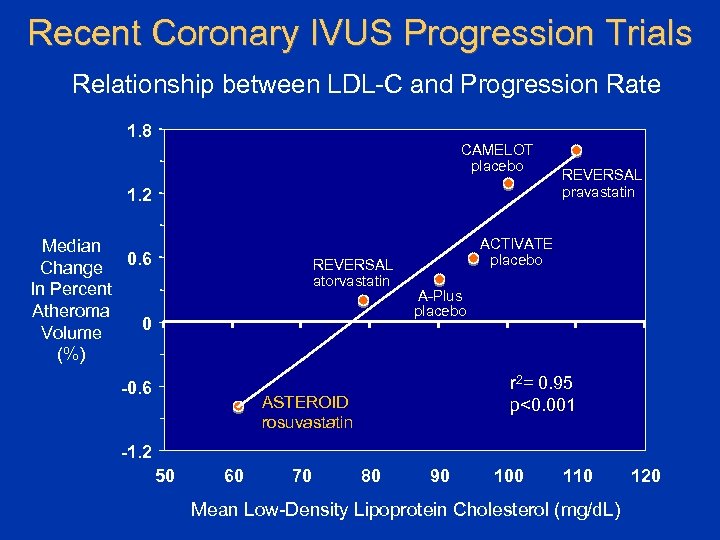 Recent Coronary IVUS Progression Trials Relationship between LDL-C and Progression Rate 1. 8 CAMELOT placebo 1. 2 Median Change 0. 6 In Percent Atheroma 0 Volume (%) REVERSAL atorvastatin -0. 6 REVERSAL pravastatin ACTIVATE placebo A-Plus placebo r 2= 0. 95 p<0. 001 ASTEROID rosuvastatin -1. 2 50 60 70 80 90 100 110 Mean Low-Density Lipoprotein Cholesterol (mg/d. L) 120
Recent Coronary IVUS Progression Trials Relationship between LDL-C and Progression Rate 1. 8 CAMELOT placebo 1. 2 Median Change 0. 6 In Percent Atheroma 0 Volume (%) REVERSAL atorvastatin -0. 6 REVERSAL pravastatin ACTIVATE placebo A-Plus placebo r 2= 0. 95 p<0. 001 ASTEROID rosuvastatin -1. 2 50 60 70 80 90 100 110 Mean Low-Density Lipoprotein Cholesterol (mg/d. L) 120
 ILLUMINATE: Torcetrapib Trial Halted ILLUMINATE trial, a randomized, double-blind evaluation of the effect of torcetrapib/atorvastatin vs atorvastatin alone on the occurrence of major cardiovascular events in 15 000 subjects with coronary heart disease or risk equivalents. The Data Safety Monitoring Board (DSMB) recommended the trial be halted due to an "imbalance of mortality and cardiovascular events. " Overall, 82 patients taking the combination of torcetrapib and atorvastatin died, compared with 51 patient deaths in the atorvastatin-alone arm. Clinical Trial Results. org
ILLUMINATE: Torcetrapib Trial Halted ILLUMINATE trial, a randomized, double-blind evaluation of the effect of torcetrapib/atorvastatin vs atorvastatin alone on the occurrence of major cardiovascular events in 15 000 subjects with coronary heart disease or risk equivalents. The Data Safety Monitoring Board (DSMB) recommended the trial be halted due to an "imbalance of mortality and cardiovascular events. " Overall, 82 patients taking the combination of torcetrapib and atorvastatin died, compared with 51 patient deaths in the atorvastatin-alone arm. Clinical Trial Results. org
 Preview of ACC CTR agenda C. Michael Gibson, MS, MD Associate Professor of Medicine Harvard Medical School Chief of Clinical Research Cardiology Division Beth Israel Deaconess Medical Center Boston, MA
Preview of ACC CTR agenda C. Michael Gibson, MS, MD Associate Professor of Medicine Harvard Medical School Chief of Clinical Research Cardiology Division Beth Israel Deaconess Medical Center Boston, MA
 Saturday, March 24, 2007 4: 00 -6: 30 p. m. Michael Gibson, MS, MD, Christopher Cannon, MD & Jeffrey J. Popma, MD Clinical. Trial. Results. org Overview
Saturday, March 24, 2007 4: 00 -6: 30 p. m. Michael Gibson, MS, MD, Christopher Cannon, MD & Jeffrey J. Popma, MD Clinical. Trial. Results. org Overview
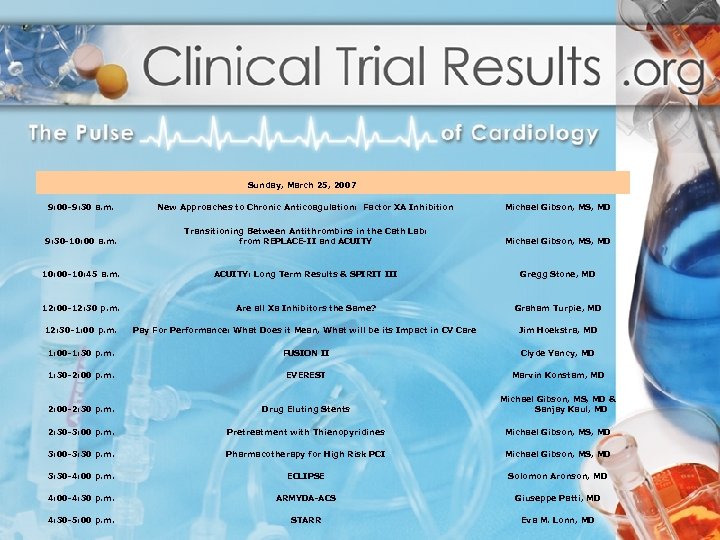 Sunday, March 25, 2007 9: 00 -9: 30 a. m. 9: 30 -10: 00 a. m. 10: 00 -10: 45 a. m. 12: 00 -12: 30 p. m. New Approaches to Chronic Anticoagulation: Factor XA Inhibition Transitioning Between Antithrombins in the Cath Lab: from REPLACE-II and ACUITY: Long Term Results & SPIRIT III Are all Xa Inhibitors the Same? Michael Gibson, MS, MD Gregg Stone, MD Graham Turpie, MD 12: 30 -1: 00 p. m. Pay For Performance: What Does it Mean, What will be its Impact in CV Care Jim Hoekstra, MD 1: 00 -1: 30 p. m. FUSION II Clyde Yancy, MD 1: 30 -2: 00 p. m. EVEREST Marvin Konstam, MD 2: 00 -2: 30 p. m. Drug Eluting Stents Michael Gibson, MS, MD & Sanjay Kaul, MD 2: 30 -3: 00 p. m. Pretreatment with Thienopyridines Michael Gibson, MS, MD 3: 00 -3: 30 p. m. Pharmacotherapy for High Risk PCI Michael Gibson, MS, MD 3: 30 -4: 00 p. m. ECLIPSE Solomon Aronson, MD 4: 00 -4: 30 p. m. ARMYDA-ACS Giuseppe Patti, MD 4: 30 -5: 00 p. m. STARR Eva M. Lonn, MD
Sunday, March 25, 2007 9: 00 -9: 30 a. m. 9: 30 -10: 00 a. m. 10: 00 -10: 45 a. m. 12: 00 -12: 30 p. m. New Approaches to Chronic Anticoagulation: Factor XA Inhibition Transitioning Between Antithrombins in the Cath Lab: from REPLACE-II and ACUITY: Long Term Results & SPIRIT III Are all Xa Inhibitors the Same? Michael Gibson, MS, MD Gregg Stone, MD Graham Turpie, MD 12: 30 -1: 00 p. m. Pay For Performance: What Does it Mean, What will be its Impact in CV Care Jim Hoekstra, MD 1: 00 -1: 30 p. m. FUSION II Clyde Yancy, MD 1: 30 -2: 00 p. m. EVEREST Marvin Konstam, MD 2: 00 -2: 30 p. m. Drug Eluting Stents Michael Gibson, MS, MD & Sanjay Kaul, MD 2: 30 -3: 00 p. m. Pretreatment with Thienopyridines Michael Gibson, MS, MD 3: 00 -3: 30 p. m. Pharmacotherapy for High Risk PCI Michael Gibson, MS, MD 3: 30 -4: 00 p. m. ECLIPSE Solomon Aronson, MD 4: 00 -4: 30 p. m. ARMYDA-ACS Giuseppe Patti, MD 4: 30 -5: 00 p. m. STARR Eva M. Lonn, MD
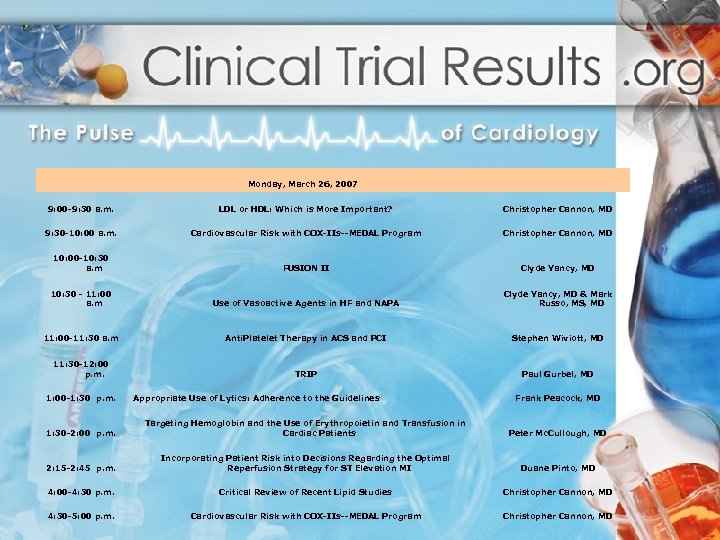 Monday, March 26, 2007 9: 00 -9: 30 a. m. LDL or HDL: Which is More Important? Christopher Cannon, MD 9: 30 -10: 00 a. m. Cardiovascular Risk with COX-IIs--MEDAL Program Christopher Cannon, MD 10: 00 -10: 30 a. m FUSION II Clyde Yancy, MD 10: 30 - 11: 00 a. m Use of Vasoactive Agents in HF and NAPA Clyde Yancy, MD & Mark Russo, MS, MD 11: 00 -11: 30 a. m Anti. Platelet Therapy in ACS and PCI Stephen Wiviott, MD TRIP Paul Gurbel, MD 11: 30 -12: 00 p. m. 1: 00 -1: 30 p. m. Appropriate Use of Lytics: Adherence to the Guidelines Frank Peacock, MD 1: 30 -2: 00 p. m. Targeting Hemoglobin and the Use of Erythropoietin and Transfusion in Cardiac Patients Peter Mc. Cullough, MD 2: 15 -2: 45 p. m. Incorporating Patient Risk into Decisions Regarding the Optimal Reperfusion Strategy for ST Elevation MI Duane Pinto, MD 4: 00 -4: 30 p. m. Critical Review of Recent Lipid Studies Christopher Cannon, MD 4: 30 -5: 00 p. m. Cardiovascular Risk with COX-IIs--MEDAL Program Christopher Cannon, MD
Monday, March 26, 2007 9: 00 -9: 30 a. m. LDL or HDL: Which is More Important? Christopher Cannon, MD 9: 30 -10: 00 a. m. Cardiovascular Risk with COX-IIs--MEDAL Program Christopher Cannon, MD 10: 00 -10: 30 a. m FUSION II Clyde Yancy, MD 10: 30 - 11: 00 a. m Use of Vasoactive Agents in HF and NAPA Clyde Yancy, MD & Mark Russo, MS, MD 11: 00 -11: 30 a. m Anti. Platelet Therapy in ACS and PCI Stephen Wiviott, MD TRIP Paul Gurbel, MD 11: 30 -12: 00 p. m. 1: 00 -1: 30 p. m. Appropriate Use of Lytics: Adherence to the Guidelines Frank Peacock, MD 1: 30 -2: 00 p. m. Targeting Hemoglobin and the Use of Erythropoietin and Transfusion in Cardiac Patients Peter Mc. Cullough, MD 2: 15 -2: 45 p. m. Incorporating Patient Risk into Decisions Regarding the Optimal Reperfusion Strategy for ST Elevation MI Duane Pinto, MD 4: 00 -4: 30 p. m. Critical Review of Recent Lipid Studies Christopher Cannon, MD 4: 30 -5: 00 p. m. Cardiovascular Risk with COX-IIs--MEDAL Program Christopher Cannon, MD
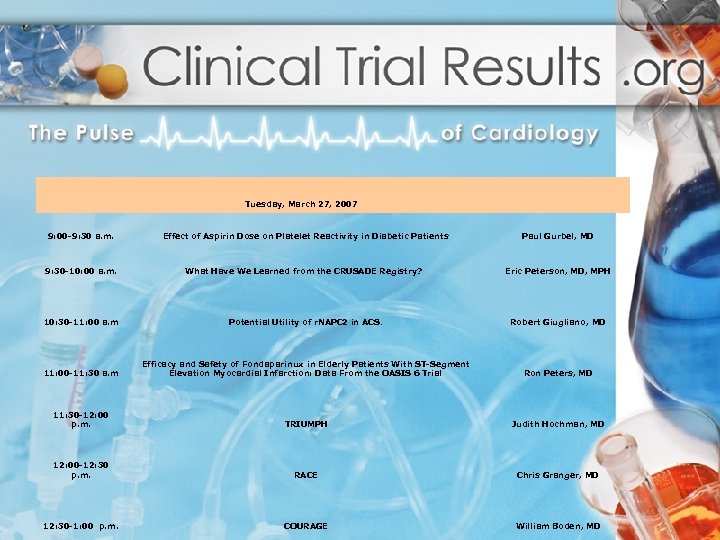 9: 00 -9: 30 a. m. 9: 30 -10: 00 a. m. Tuesday, March 27, 2007 Effect of Aspirin Dose on Platelet Reactivity in Diabetic Patients What Have We Learned from the CRUSADE Registry? Paul Gurbel, MD Eric Peterson, MD, MPH 10: 30 -11: 00 a. m Potential Utility of r. NAPC 2 in ACS. Robert Giugliano, MD 11: 00 -11: 30 a. m Efficacy and Safety of Fondaparinux in Elderly Patients With ST-Segment Elevation Myocardial Infarction: Data From the OASIS 6 Trial Ron Peters, MD 11: 30 -12: 00 p. m. TRIUMPH Judith Hochman, MD 12: 00 -12: 30 p. m. RACE Chris Granger, MD COURAGE William Boden, MD 12: 30 -1: 00 p. m.
9: 00 -9: 30 a. m. 9: 30 -10: 00 a. m. Tuesday, March 27, 2007 Effect of Aspirin Dose on Platelet Reactivity in Diabetic Patients What Have We Learned from the CRUSADE Registry? Paul Gurbel, MD Eric Peterson, MD, MPH 10: 30 -11: 00 a. m Potential Utility of r. NAPC 2 in ACS. Robert Giugliano, MD 11: 00 -11: 30 a. m Efficacy and Safety of Fondaparinux in Elderly Patients With ST-Segment Elevation Myocardial Infarction: Data From the OASIS 6 Trial Ron Peters, MD 11: 30 -12: 00 p. m. TRIUMPH Judith Hochman, MD 12: 00 -12: 30 p. m. RACE Chris Granger, MD COURAGE William Boden, MD 12: 30 -1: 00 p. m.
 Question & Answer
Question & Answer
 Thank You! Please make sure to hand in your evaluation and pick up a Clinical. Trial. Results. org flash drive
Thank You! Please make sure to hand in your evaluation and pick up a Clinical. Trial. Results. org flash drive


