73eecdb5dc1e12f04fa2653ae98811d4.ppt
- Количество слайдов: 61
Water Purification System for a Laboratory Facility Millipore Corporation Bioscience Division Christopher Yarima Mike Kelly

Outline n Contaminants in Water n Pure Water Applications and Quality Standards n Water Purification Technologies n Key Water Purification System Design Steps Systems n Questions

Water Chemistry – Contaminants
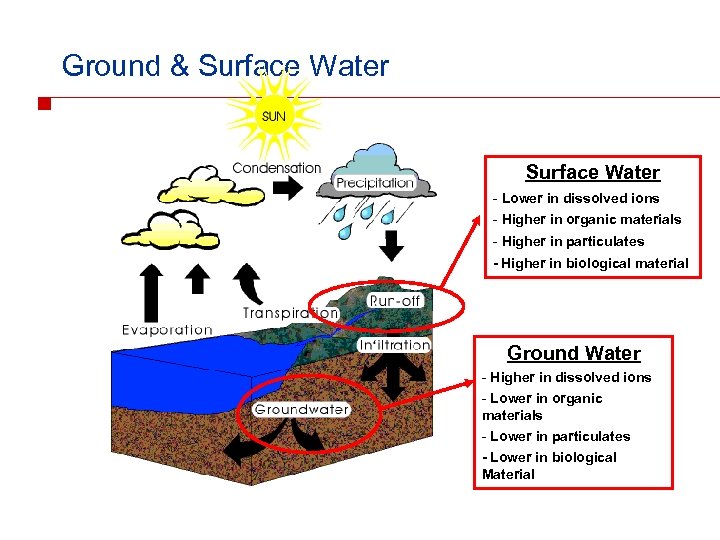
Ground & Surface Water - Lower in dissolved ions - Higher in organic materials - Higher in particulates - Higher in biological material Ground Water - Higher in dissolved ions - Lower in organic materials - Lower in particulates - Lower in biological Material
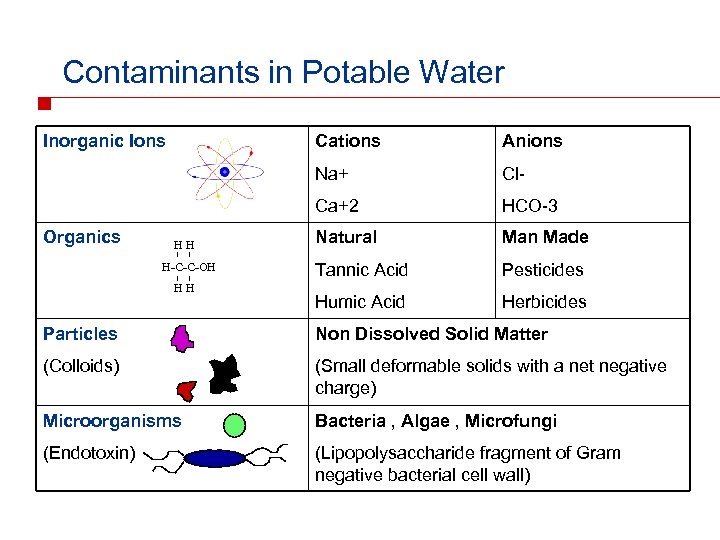
Contaminants in Potable Water Inorganic Ions H-C-C-OH HH Cl- Ca+2 HH Anions Na+ Organics Cations HCO-3 Natural Man Made Tannic Acid Pesticides Humic Acid Herbicides Particles Non Dissolved Solid Matter (Colloids) (Small deformable solids with a net negative charge) Microorganisms Bacteria , Algae , Microfungi (Endotoxin) (Lipopolysaccharide fragment of Gram negative bacterial cell wall)
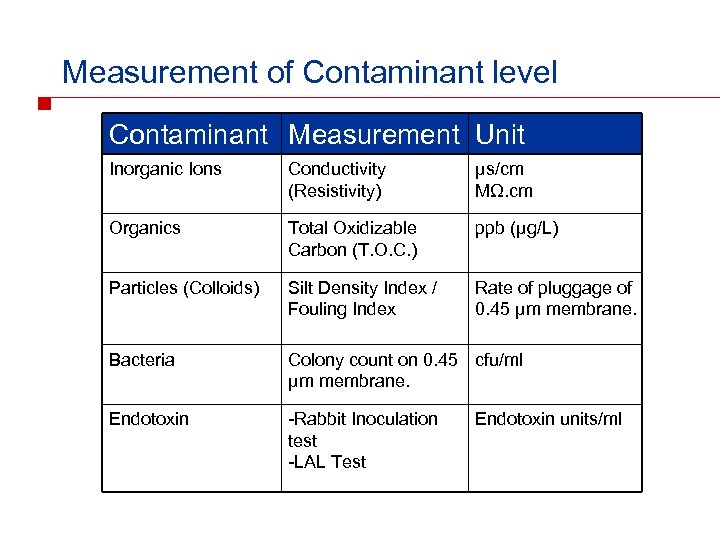
Measurement of Contaminant level Contaminant Measurement Unit Inorganic Ions Conductivity (Resistivity) μs/cm MΩ. cm Organics Total Oxidizable Carbon (T. O. C. ) ppb (μg/L) Particles (Colloids) Silt Density Index / Fouling Index Rate of pluggage of 0. 45 μm membrane. Bacteria Colony count on 0. 45 cfu/ml μm membrane. Endotoxin -Rabbit Inoculation test -LAL Test Endotoxin units/ml
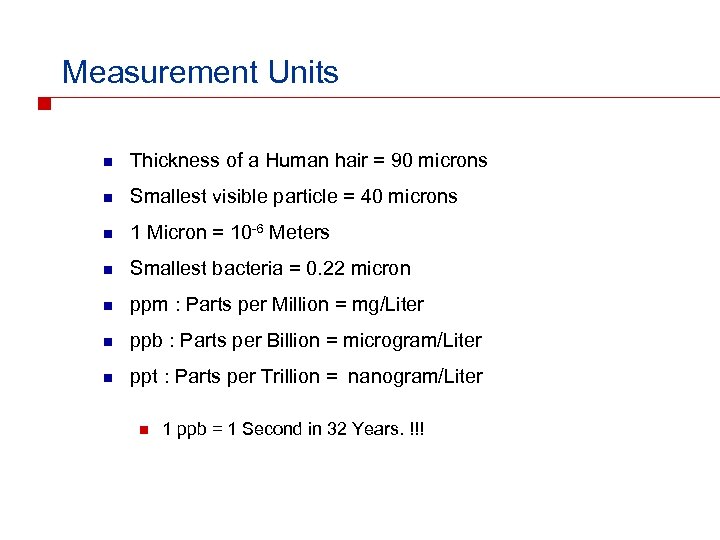
Measurement Units n Thickness of a Human hair = 90 microns n Smallest visible particle = 40 microns n 1 Micron = 10 -6 Meters n Smallest bacteria = 0. 22 micron n ppm : Parts per Million = mg/Liter n ppb : Parts per Billion = microgram/Liter n ppt : Parts per Trillion = nanogram/Liter n 1 ppb = 1 Second in 32 Years. !!!

Water Standards
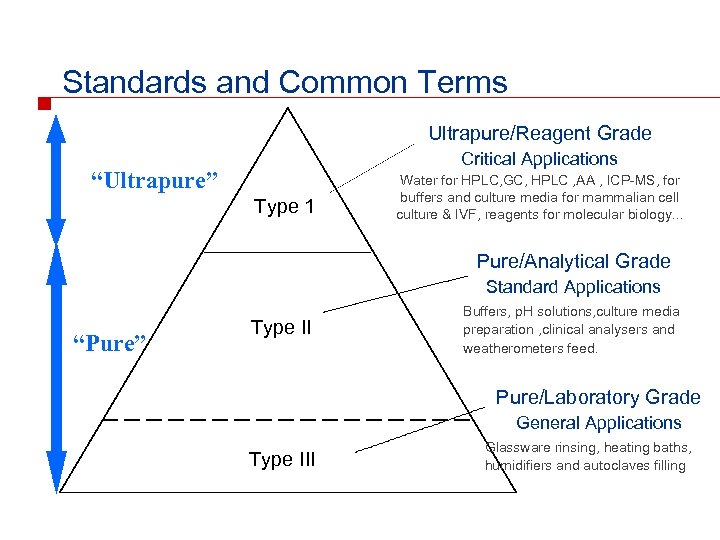
Standards and Common Terms Ultrapure/Reagent Grade Critical Applications “Ultrapure” Type 1 Water for HPLC, GC, HPLC , AA , ICP-MS, for buffers and culture media for mammalian cell culture & IVF, reagents for molecular biology. . . Pure/Analytical Grade Standard Applications “Pure” Type II Buffers, p. H solutions, culture media preparation , clinical analysers and weatherometers feed. Pure/Laboratory Grade General Applications Type III Glassware rinsing, heating baths, humidifiers and autoclaves filling
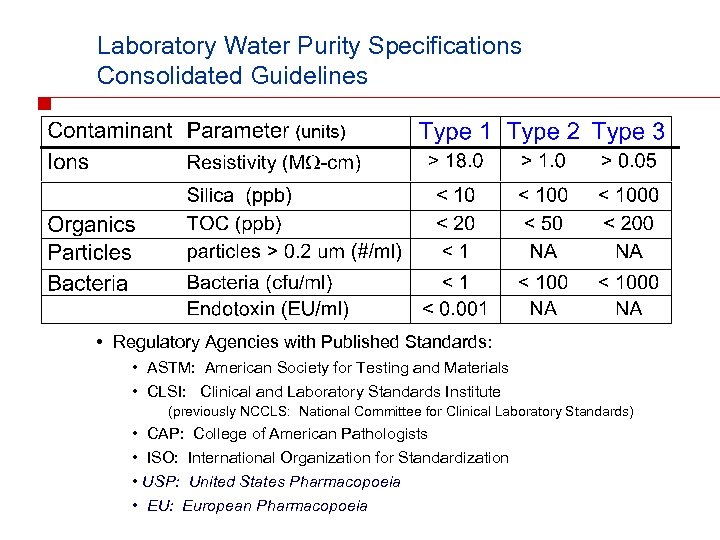
Laboratory Water Purity Specifications Consolidated Guidelines • Regulatory Agencies with Published Standards: • ASTM: American Society for Testing and Materials • CLSI: Clinical and Laboratory Standards Institute (previously NCCLS: National Committee for Clinical Laboratory Standards) • CAP: College of American Pathologists • ISO: International Organization for Standardization • USP: United States Pharmacopoeia • EU: European Pharmacopoeia
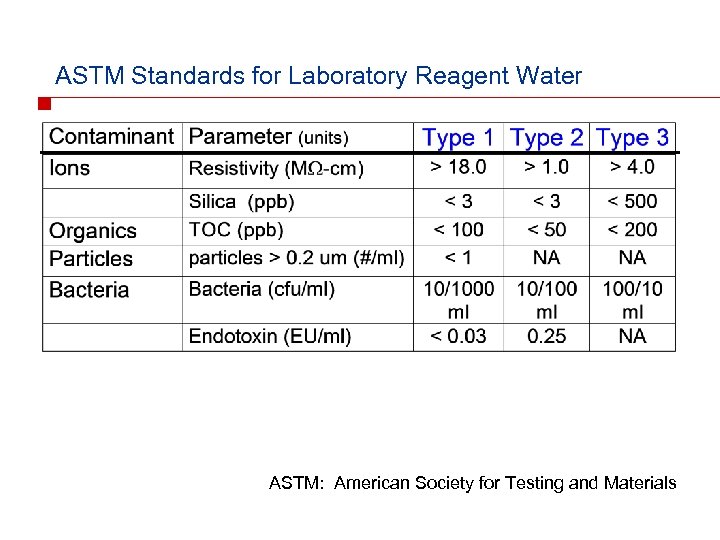
ASTM Standards for Laboratory Reagent Water ASTM: American Society for Testing and Materials
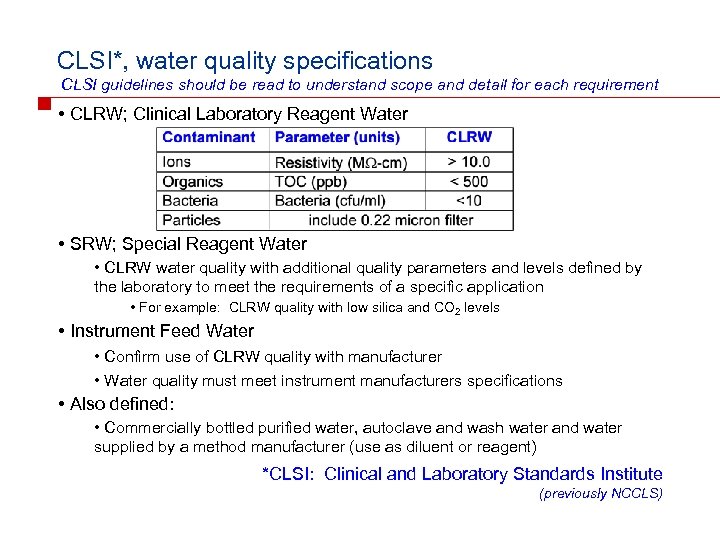
CLSI*, water quality specifications CLSI guidelines should be read to understand scope and detail for each requirement • CLRW; Clinical Laboratory Reagent Water • SRW; Special Reagent Water • CLRW water quality with additional quality parameters and levels defined by the laboratory to meet the requirements of a specific application • For example: CLRW quality with low silica and CO 2 levels • Instrument Feed Water • Confirm use of CLRW quality with manufacturer • Water quality must meet instrument manufacturers specifications • Also defined: • Commercially bottled purified water, autoclave and wash water and water supplied by a method manufacturer (use as diluent or reagent) *CLSI: Clinical and Laboratory Standards Institute (previously NCCLS)
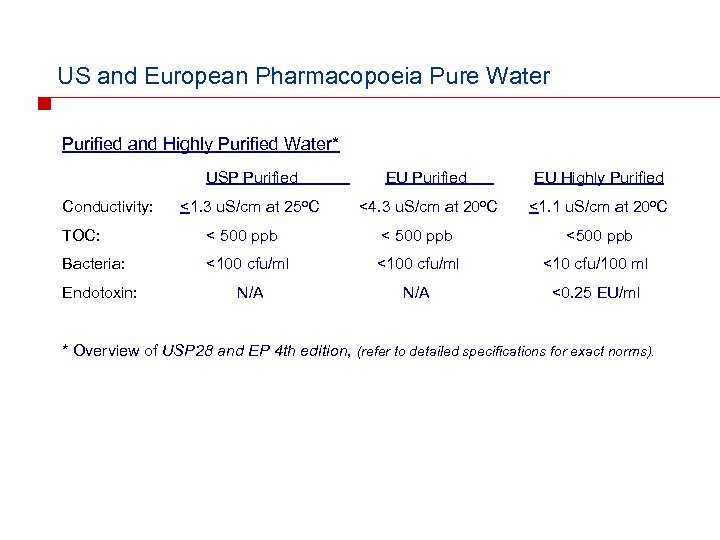
US and European Pharmacopoeia Pure Water Purified and Highly Purified Water* USP Purified Conductivity: EU Purified EU Highly Purified <1. 3 u. S/cm at 25 o. C <4. 3 u. S/cm at 20 o. C <1. 1 u. S/cm at 20 o. C TOC: < 500 ppb <500 ppb Bacteria: <100 cfu/ml <10 cfu/100 ml N/A <0. 25 EU/ml Endotoxin: * Overview of USP 28 and EP 4 th edition, (refer to detailed specifications for exact norms).

Purification Technologies Overview of Key Technologies Advantages/Disadvantages Summary

Purification Technologies n Filtration – Depth and Screen Filters n Activated Carbon and chlorine removal n Mineral scale control – Softening and Sequestering n Distillation n Reverse Osmosis n Deionization n Electrodeionization n Ultraviolet light
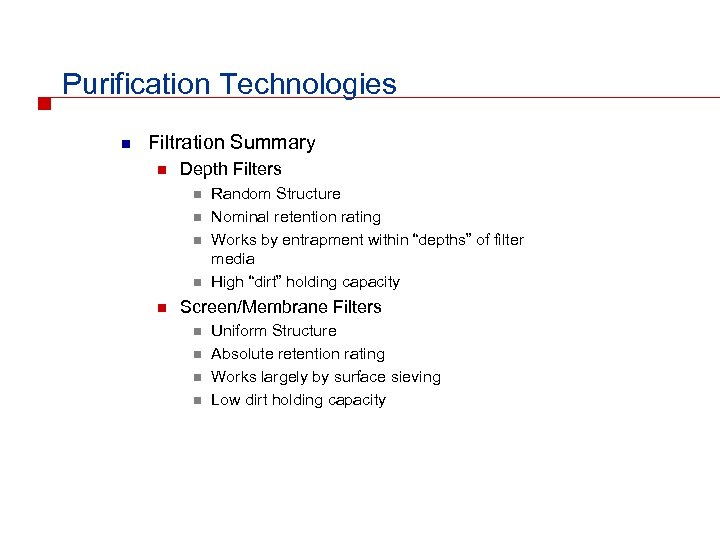
Purification Technologies n Filtration Summary n Depth Filters n n n Random Structure Nominal retention rating Works by entrapment within “depths” of filter media High “dirt” holding capacity Screen/Membrane Filters n n Uniform Structure Absolute retention rating Works largely by surface sieving Low dirt holding capacity
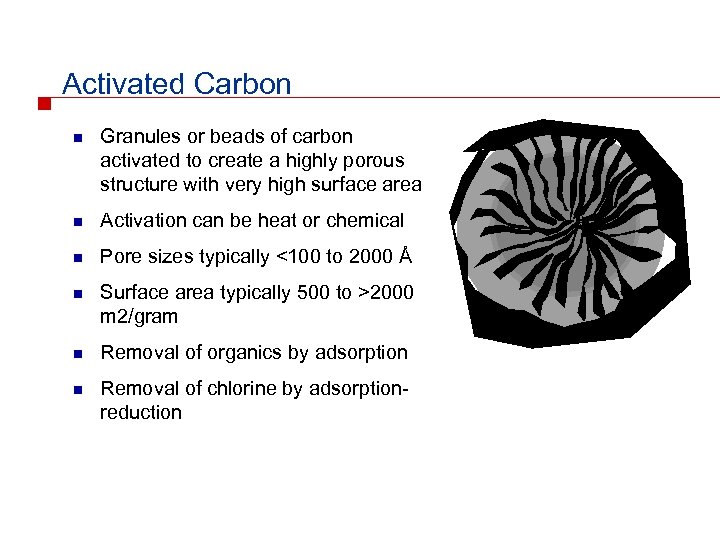
Activated Carbon n Granules or beads of carbon activated to create a highly porous structure with very high surface area n Activation can be heat or chemical n Pore sizes typically <100 to 2000 Å n Surface area typically 500 to >2000 m 2/gram n Removal of organics by adsorption n Removal of chlorine by adsorptionreduction
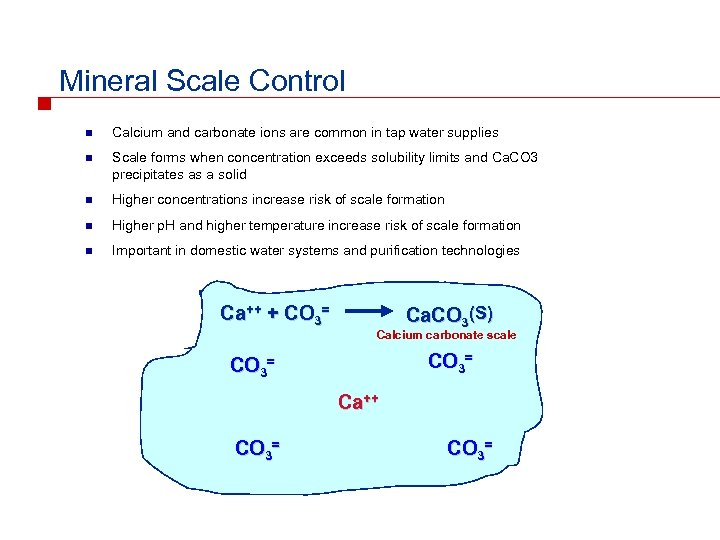
Mineral Scale Control n Calcium and carbonate ions are common in tap water supplies n Scale forms when concentration exceeds solubility limits and Ca. CO 3 precipitates as a solid n Higher concentrations increase risk of scale formation n Higher p. H and higher temperature increase risk of scale formation n Important in domestic water systems and purification technologies Ca++ + CO 3= Ca. CO 3(S) Calcium carbonate scale CO 3= Ca++ CO 3=
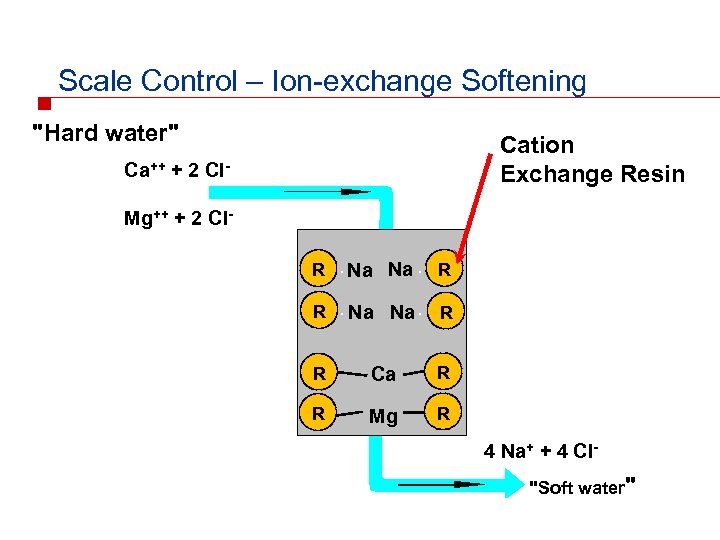
Scale Control – Ion-exchange Softening "Hard water" Cation Exchange Resin Ca++ + 2 Cl. Mg++ + 2 Cl. R Na Na R R Ca R R Mg R 4 Na+ + 4 Cl"Soft water"
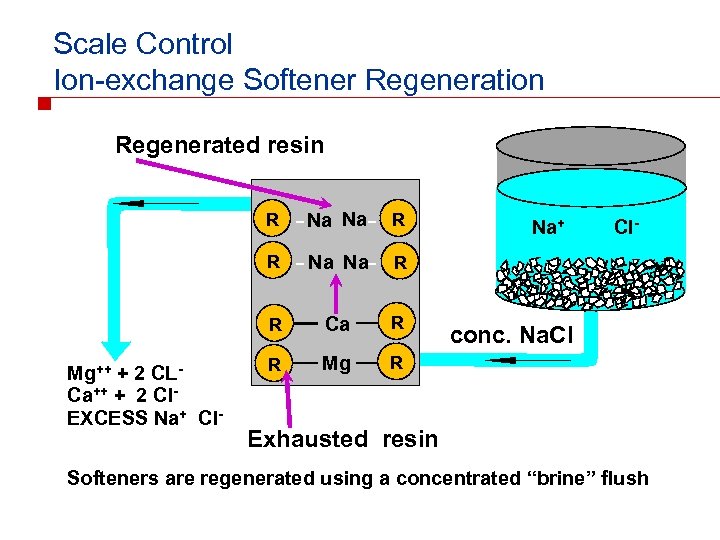
Scale Control Ion-exchange Softener Regeneration Regenerated resin R R R Na Na R R Mg++ + 2 CLCa++ + 2 Cl. EXCESS Na+ Cl- Na Na Ca R R Mg R Na+ Cl- conc. Na. Cl Exhausted resin Softeners are regenerated using a concentrated “brine” flush
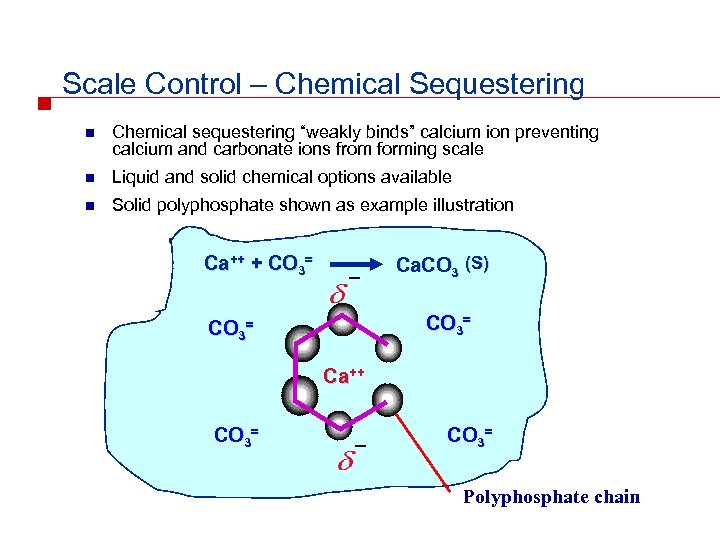
Scale Control – Chemical Sequestering n Chemical sequestering “weakly binds” calcium ion preventing calcium and carbonate ions from forming scale n Liquid and solid chemical options available n Solid polyphosphate shown as example illustration Ca++ + CO 3= _ Ca. CO 3 (S) CO 3= Ca++ CO 3= _ CO 3= Polyphosphate chain
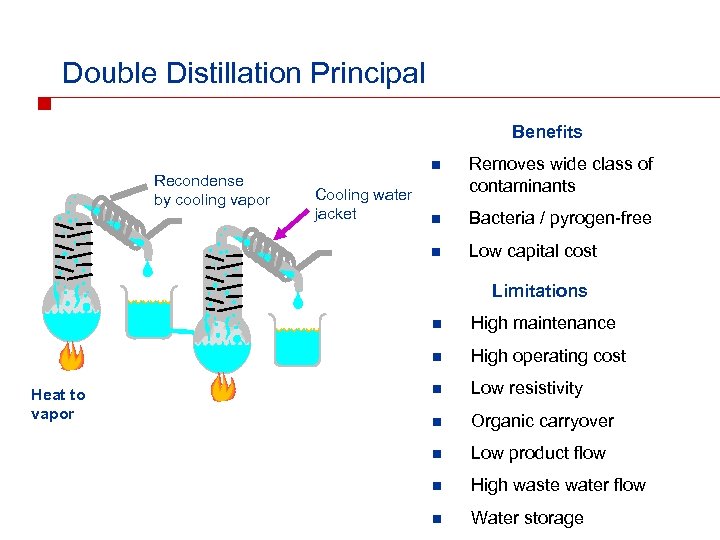
Double Distillation Principal Benefits Cooling water jacket Removes wide class of contaminants n Bacteria / pyrogen-free n Recondense by cooling vapor n Low capital cost Limitations n n Heat to vapor High maintenance High operating cost n Low resistivity n Organic carryover n Low product flow n High waste water flow n Water storage
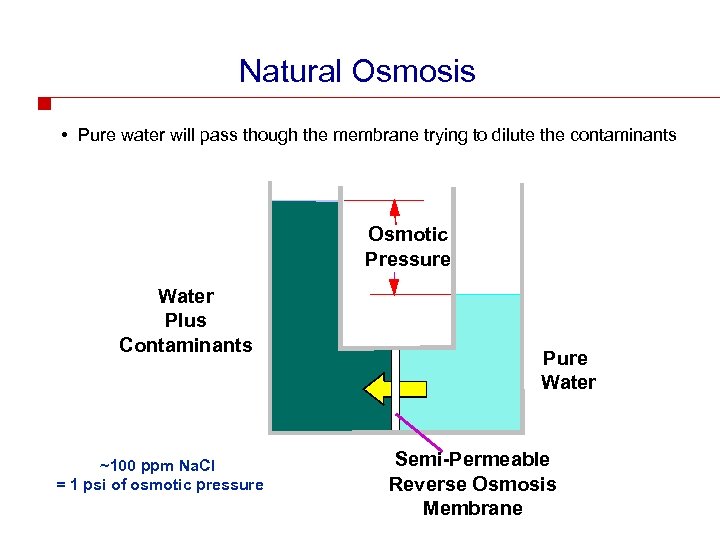
Natural Osmosis • Pure water will pass though the membrane trying to dilute the contaminants Osmotic Pressure Water Plus Contaminants ~100 ppm Na. Cl = 1 psi of osmotic pressure Pure Water Semi-Permeable Reverse Osmosis Membrane
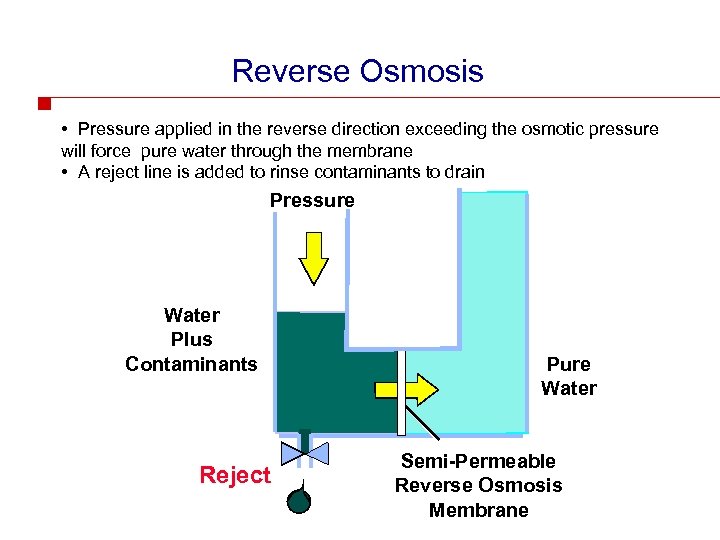
Reverse Osmosis • Pressure applied in the reverse direction exceeding the osmotic pressure will force pure water through the membrane • A reject line is added to rinse contaminants to drain Pressure Water Plus Contaminants Reject Pure Water Semi-Permeable Reverse Osmosis Membrane
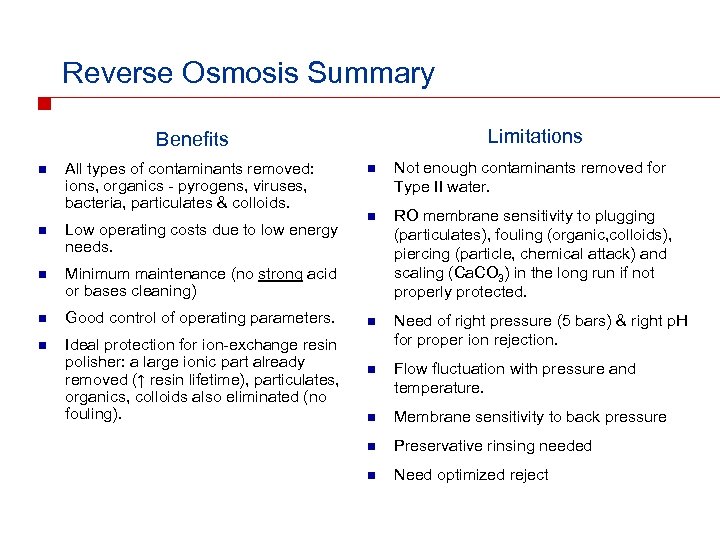
Reverse Osmosis Summary Limitations Benefits n All types of contaminants removed: ions, organics - pyrogens, viruses, bacteria, particulates & colloids. n Low operating costs due to low energy needs. n Good control of operating parameters. n Ideal protection for ion-exchange resin polisher: a large ionic part already removed (↑ resin lifetime), particulates, organics, colloids also eliminated (no fouling). Not enough contaminants removed for Type II water. n RO membrane sensitivity to plugging (particulates), fouling (organic, colloids), piercing (particle, chemical attack) and scaling (Ca. CO 3) in the long run if not properly protected. n Need of right pressure (5 bars) & right p. H for proper ion rejection. n Flow fluctuation with pressure and temperature. n Membrane sensitivity to back pressure n Preservative rinsing needed n Need optimized reject Minimum maintenance (no strong acid or bases cleaning) n n
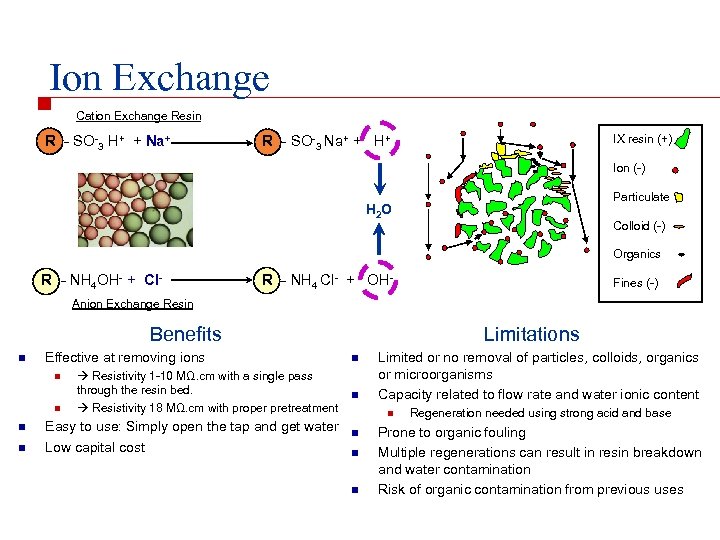
Ion Exchange Cation Exchange Resin R - SO-3 H+ + Na+ IX resin (+) R - SO-3 Na+ + H+ Ion (-) Particulate H 2 O Colloid (-) Organics R - NH 4 OH- + Cl- R - NH 4 Cl- + OH- Fines (-) Anion Exchange Resin Benefits n Effective at removing ions n Easy to use: Simply open the tap and get water Low capital cost n n Resistivity 1 -10 MΩ. cm with a single pass through the resin bed. Resistivity 18 MΩ. cm with proper pretreatment n n Limitations Limited or no removal of particles, colloids, organics or microorganisms Capacity related to flow rate and water ionic content n n n Regeneration needed using strong acid and base Prone to organic fouling Multiple regenerations can result in resin breakdown and water contamination Risk of organic contamination from previous uses
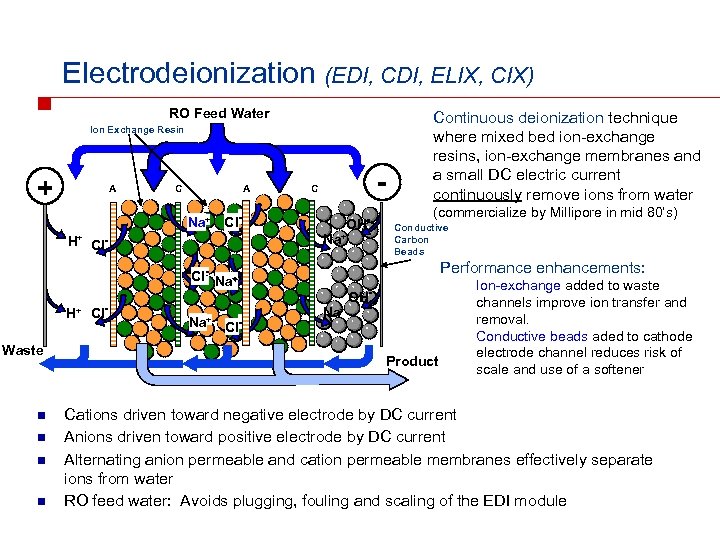
Electrodeionization (EDI, CDI, ELIX, CIX) RO Feed Water Ion Exchange Resin + A C A Na+ - C Cl. Na+ H+ Cl- Waste n n Na+ Cl- (commercialize by Millipore in mid 80’s) Conductive Carbon Beads Performance enhancements: Cl- Na+ H+ Cl- OH- Continuous deionization technique where mixed bed ion-exchange resins, ion-exchange membranes and a small DC electric current continuously remove ions from water OHNa+ Product Ion-exchange added to waste channels improve ion transfer and removal. Conductive beads aded to cathode electrode channel reduces risk of scale and use of a softener Cations driven toward negative electrode by DC current Anions driven toward positive electrode by DC current Alternating anion permeable and cation permeable membranes effectively separate ions from water RO feed water: Avoids plugging, fouling and scaling of the EDI module
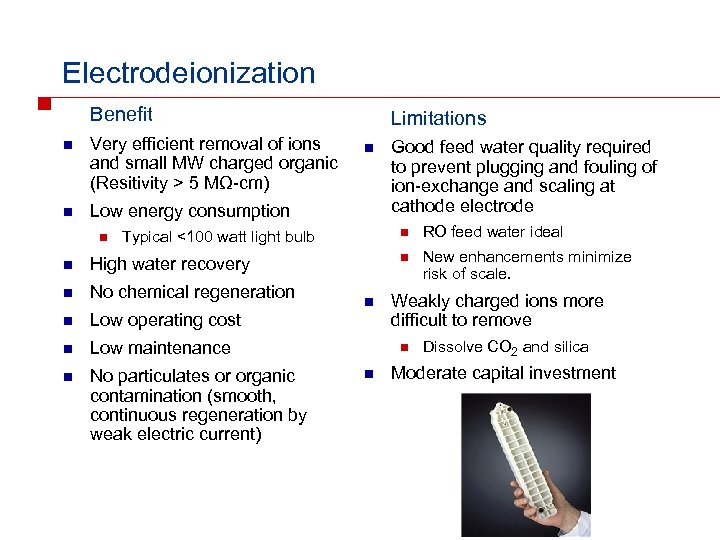
Electrodeionization Benefit n Very efficient removal of ions and small MW charged organic (Resitivity > 5 MΩ-cm) n Limitations Low energy consumption n High water recovery n No chemical regeneration n Low operating cost n Low maintenance n No particulates or organic contamination (smooth, continuous regeneration by weak electric current) n RO feed water ideal n Typical <100 watt light bulb n Good feed water quality required to prevent plugging and fouling of ion-exchange and scaling at cathode electrode New enhancements minimize risk of scale. Weakly charged ions more difficult to remove n n Dissolve CO 2 and silica Moderate capital investment
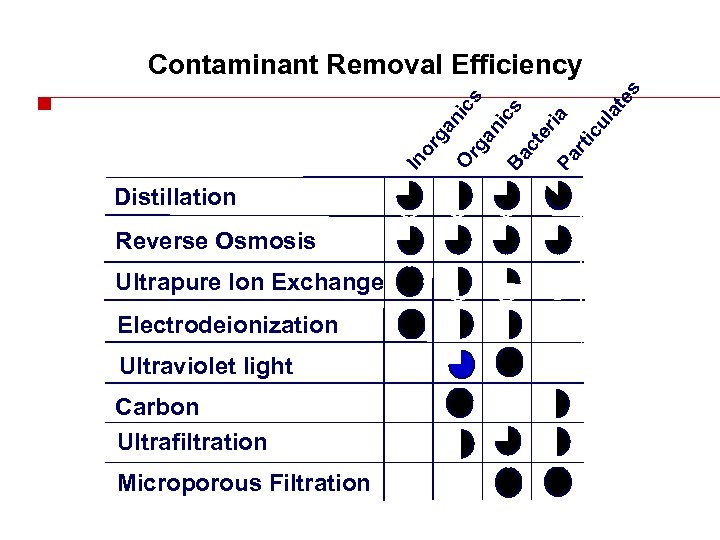
In or ga ni O cs rg an ic Ba s ct er Pa ia rti cu la te s Contaminant Removal Efficiency Distillation Reverse Osmosis Ultrapure Ion Exchange Electrodeionization Ultraviolet light Carbon Ultrafiltration Microporous Filtration 2311 BD 10
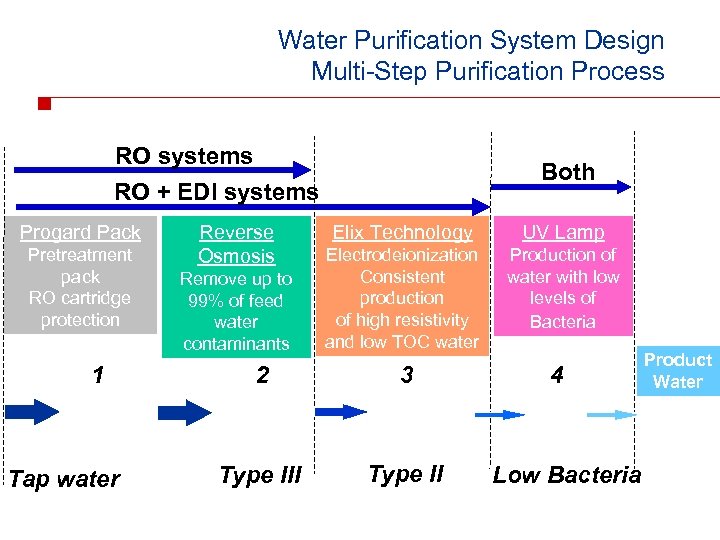
Water Purification System Design Multi-Step Purification Process RO systems Both RO + EDI systems Progard Pack Pretreatment pack RO cartridge protection 1 Tap water Reverse Osmosis Remove up to 99% of feed water contaminants Elix Technology UV Lamp Electrodeionization Consistent production of high resistivity and low TOC water Production of water with low levels of Bacteria 2 3 Type III Type II 4 Low Bacteria Product Water

Water Purification System Overview of Design Considerations
Major phases in a project n Definition of the needs n Design of a total solution n Budget estimation n Tender (Bid) process n Delivery of the units, accessories and consumables n Installation n Users training/Commissioning n Additional phases n n Preventive maintenance Full support for validation
Major phases in a project n Definition of the needs n Design of a total solution n Budget estimation n Tender (Bid) process n Delivery of the units, accessories and consumables n Installation n Users training/Commissioning n Additional phases n n Preventive maintenance Full support for validation
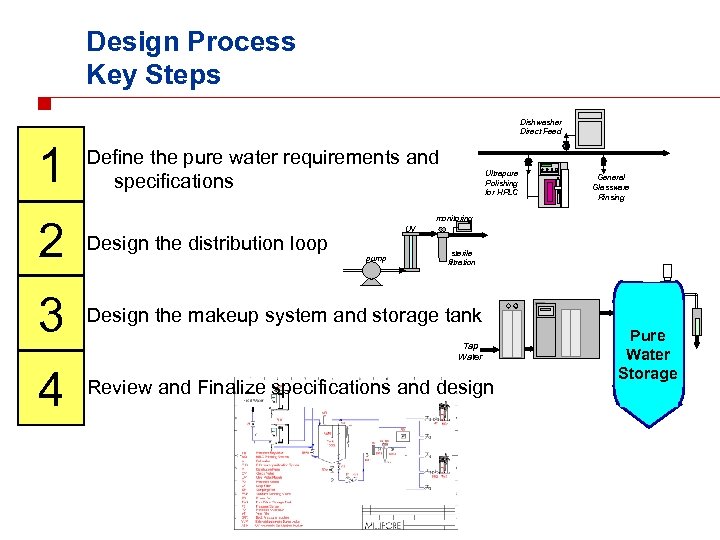
Design Process Key Steps Dishwasher Direct Feed 1 Define the pure water requirements and specifications Ultrapure Polishing for HPLC 2 Design the distribution loop 3 Design the makeup system and storage tank 4 Review and Finalize specifications and design General Glassware Rinsing monitoring UV pump sterile filtration Tap Water Pure Water Storage
1 Design Process: Step 1 n Defining the pure water requirements and specifications n n Dishwasher Direct Feed What purity level? How much water? n When is it needed? n Where is it needed? Ultrapure Polishing for HPLC General Glassware Rinsing
Defining the pure water requirements and specifications n What purity level? n What labs and locations need purified water? n What kind of work will be carried out in each lab, at each location? n n Are there instruments that will need pure water? n n n Glassware washers, steam sterilizers, autoclaves…. . ? Are there any “maximum” purity level requirements? What water quality is needed for each location? n n General rinsing/washing to sensitive trace analysis, …? Ionic, Organic, and Microbiological Quality? Are there alert and action levels? Are there standard specifications to follow? How much water? When? Where? Dishwasher Direct Feed Ultrapure Polishing for HPLC General Glassware Rinsing 1
1 Definition of the needs Questions to select the right configuration and design n n What purity level? How much water? When? Where? n How much water is needed each day? n n n How is the demand distributed during the day? n n In each lab, at each location, . . ? By the individual users, instruments, ultrapure polishing systems? Steady demand over the course of a day? Peak demands at certain times of the day? How many floors need water? Where is each location? n Are there remote locations that need water? n What are the distances between each location? Dishwasher Direct Feed Ultrapure Polishing for HPLC General Glassware Rinsing
Defining the pure water requirements and specifications n What purity level? How much water? When? Where? n Additional questions: n n Does the equipment need to be validated? n n n n At all locations? Who will do the maintenance? Is a service/maintenance contact required? Are the water quality requirements similar between locations? How many researchers/scientists will work in each lab? Dishwasher Direct Feed Where can the equipment be located (space)? Where can piping be run? Ultrapure Are there plans for future expansion? Polishing for HPLC General Glassware Rinsing 1
Step 2: Designing the Distribution Loop n Define the distribution piping n n n Design Layout Materials, welding method, valve type, pipe diameter Design Considerations n Define Loop Purification and Monitoring Equipment n Determine distribution pump performance n Flow rate and pressure 2
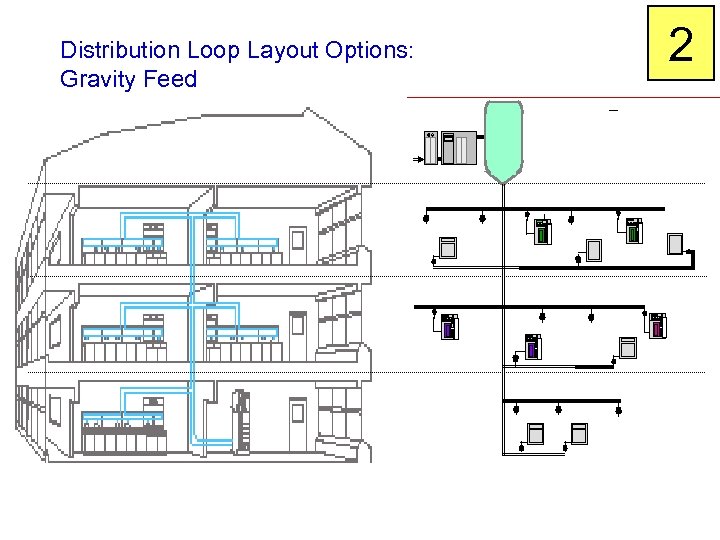
Distribution Loop Layout Options: Gravity Feed 2
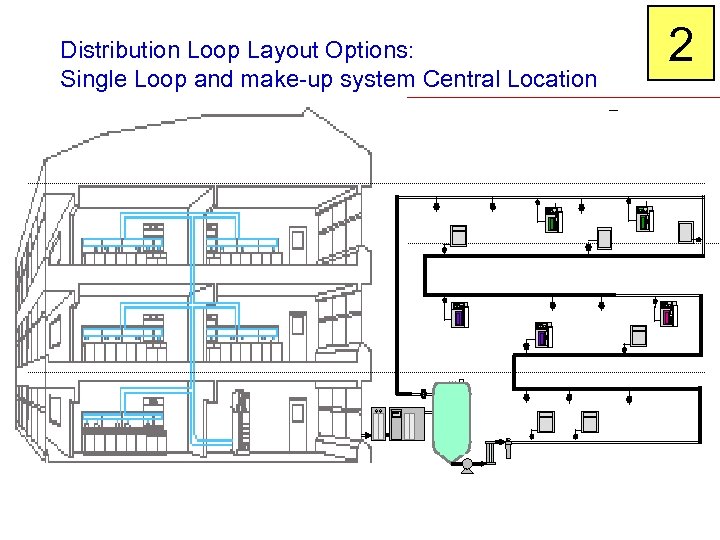
Distribution Loop Layout Options: Single Loop and make-up system Central Location 2
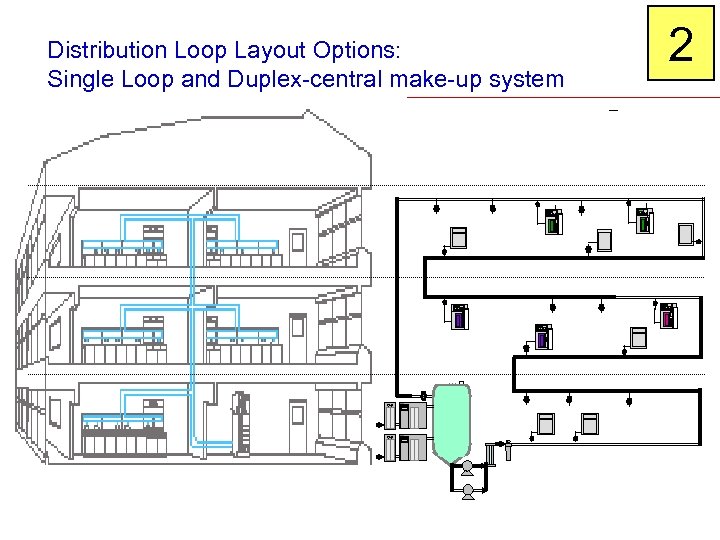
Distribution Loop Layout Options: Single Loop and Duplex-central make-up system 2
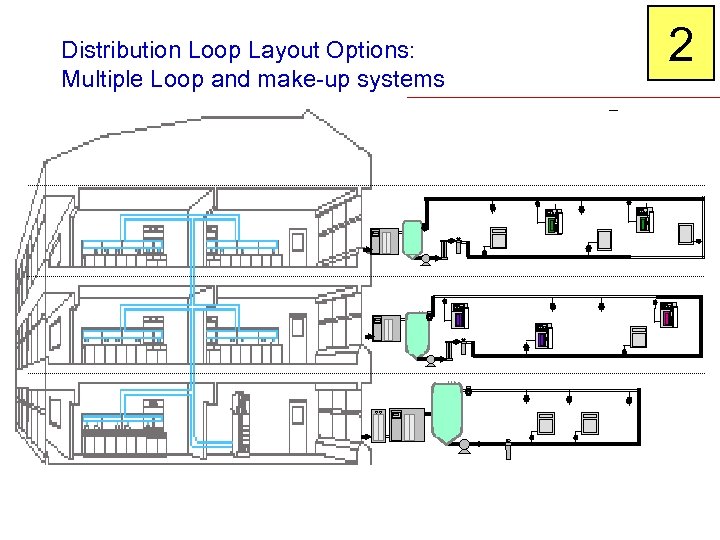
Distribution Loop Layout Options: Multiple Loop and make-up systems 2
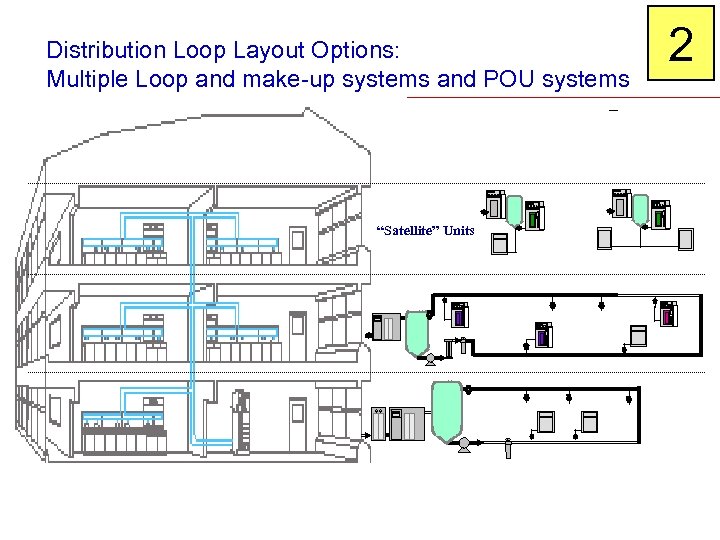
Distribution Loop Layout Options: Multiple Loop and make-up systems and POU systems “Satellite” Units 2

Design Considerations; Avoid Dead legs 2 n “ 6 D rule” CFR 212 regulations of 1976 n Good Engineering practice requires minimizing the length of dead legs and there are many good instrument and valve designs available to do so. “ 6 D rule” Ø 0. 59” Ø Maximum dead leg = 6 times the pipe diameter Ø 0. 59” X 6 = 3. 5” Maximum dead length of 3. 5 inches Maximum length 6 X pipe diameter (our example max is 3. 5 inches)
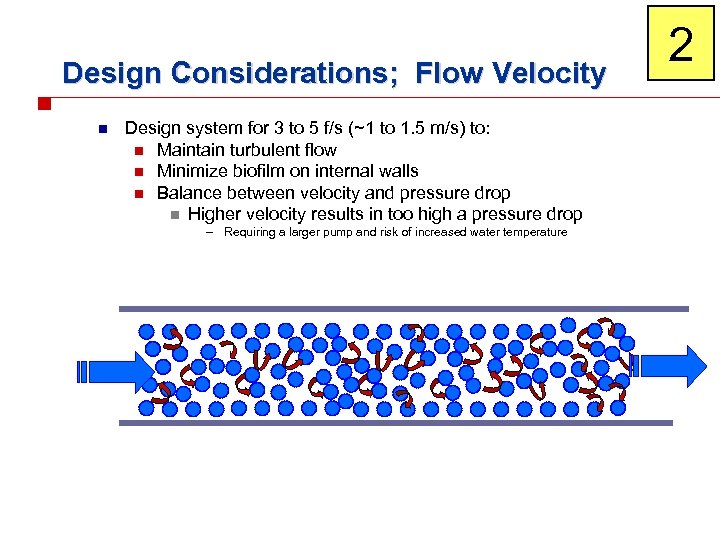
Design Considerations; Flow Velocity n Design system for 3 to 5 f/s (~1 to 1. 5 m/s) to: n Maintain turbulent flow n Minimize biofilm on internal walls n Balance between velocity and pressure drop n Higher velocity results in too high a pressure drop – Requiring a larger pump and risk of increased water temperature 2
Define Loop Purification and Monitoring Equipment n Loop purification equipment to maintain water – UV lamp » Bacteria control » TOC Reduction – Filtration » Membranes for Bacteria and particle control » Ultra-filtration for Pyrogen removal – Deionization – Ion removal n Loop Water Purity Monitoring – – – Resistivity TOC Bacteria Temperature Sanitant Monitors (Ozone) 2 quality
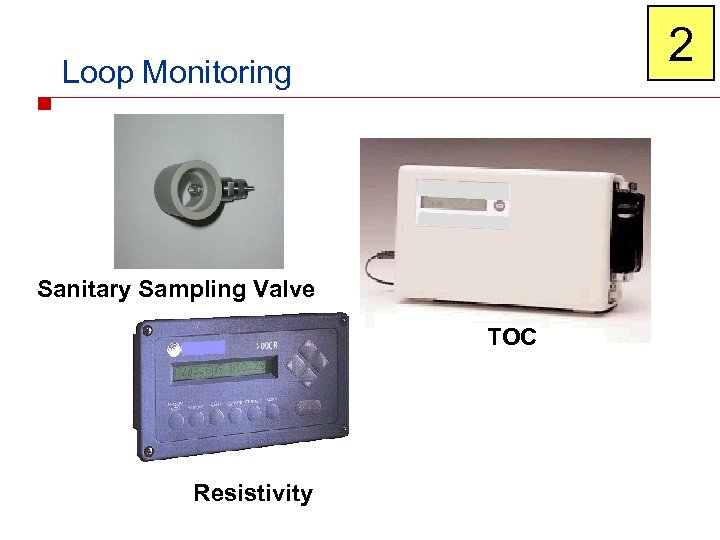
2 Loop Monitoring Sanitary Sampling Valve TOC Resistivity
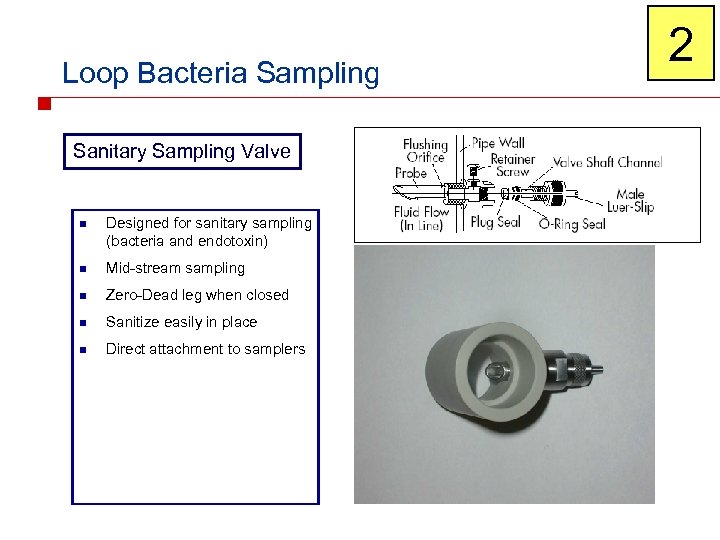
Loop Bacteria Sampling Sanitary Sampling Valve n Designed for sanitary sampling (bacteria and endotoxin) n Mid-stream sampling n Zero-Dead leg when closed n Sanitize easily in place n Direct attachment to samplers 2
Determine the Distribution Pump Requirements n 2 Pump selection is based on flow rate and pressure requirements n n Flow rate required defined in step 1 Pressure requirement Total Pressure requirement can be estimated by adding: piping pressure loss + loop equipment pressure loss + pressure due to elevation changes + pressure required at furthest point of use (25 psi typical) n n n Select a pump that delivers the required flow rate and pressure Reduce pressure loss by increasing pipe diameter, (keeping balance with flow required and target velocity) For added reliability a duplex pumping system can be used
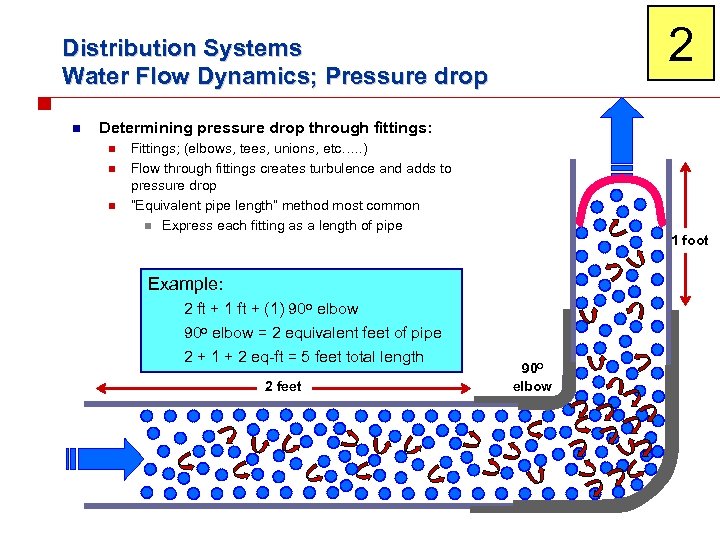
2 Distribution Systems Water Flow Dynamics; Pressure drop n Determining pressure drop through fittings: n n n Fittings; (elbows, tees, unions, etc…. . ) Flow through fittings creates turbulence and adds to pressure drop “Equivalent pipe length” method most common n Express each fitting as a length of pipe 1 foot Example: 2 ft + 1 ft + (1) 90 o elbow = 2 equivalent feet of pipe 2 + 1 + 2 eq-ft = 5 feet total length 2 feet 90 o elbow
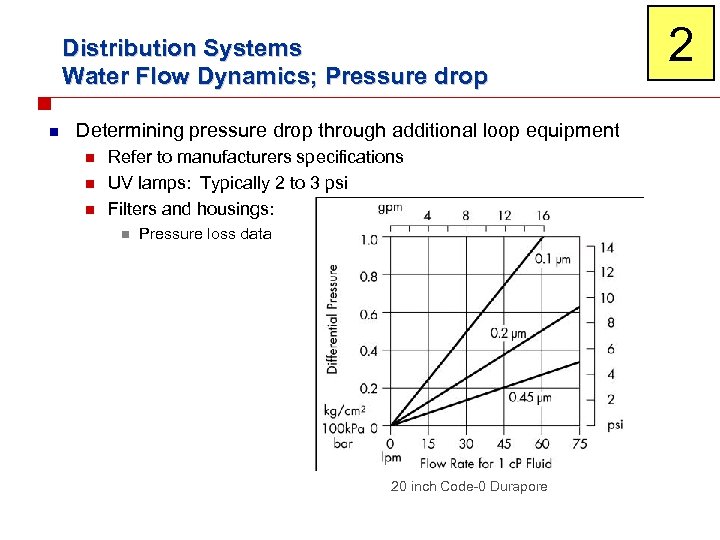
Distribution Systems Water Flow Dynamics; Pressure drop n Determining pressure drop through additional loop equipment n n n Refer to manufacturers specifications UV lamps: Typically 2 to 3 psi Filters and housings: n Pressure loss data 20 inch Code-0 Durapore 2
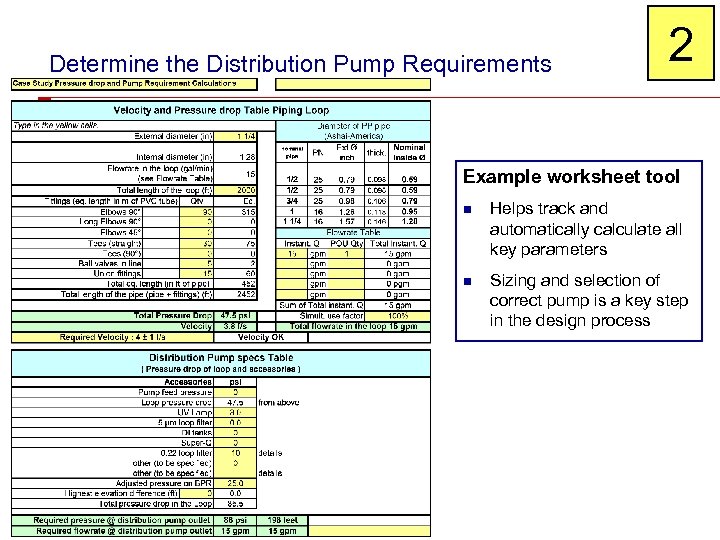
Determine the Distribution Pump Requirements 2 Example worksheet tool n Helps track and automatically calculate all key parameters n Sizing and selection of correct pump is a key step in the design process
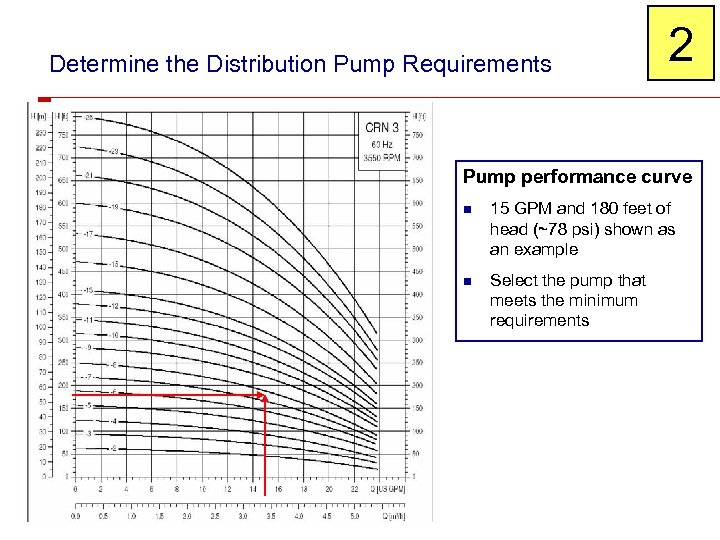
Determine the Distribution Pump Requirements 2 Pump performance curve n 15 GPM and 180 feet of head (~78 psi) shown as an example n Select the pump that meets the minimum requirements

Step 3 - Design the Makeup Purification System and Storage Tank n Select the make-up purification system to match the water quality required n Size the makeup purification system to match the quantity required per day n Size the storage tank to meet peak demands during the day n Determine the pretreatment needed 3

Makeup System Sizing and Quality n Match to the quality requirement (defined in step 1) n RO/EDI or RO/DI system for Type 2 pure water applications n RO system for Type 3 more general applications n Size the makeup system to match the quantity required per day (defined in step 1) n Plans for future expansion? n Are Duplex systems needed? – Back-up for maintenance-down time. – Option to add for future expansion 3

Sizing Makeup System and Tank Sizing the makeup system is done in conjunction with the storage tank Sizing Examples: n Company A needs water to clean vessels in the first two hours of the day shift. They need a total of 1200 Gallons in two hours. n n 1500 Gallon Tank with 100 gph make-up rate Company B needs pure water to feed automated Filling machine. They need 200 gallons per hour for an 8 hour shift. n 200 Gallon Tank with 200 gph make-up rate 3

Determine the pretreatment needed for the makeup water system n Determine feed flow rate base on the make-up system water recovery rate n n Complete feed water analysis n n Feed Flow Rate = RO Product / RO recovery rate conductivity, chlorine, fouling index, p. H, hardness, alkalinity……. . Select pretreatment options based on feed water analysis and manufacturers recommendations n n Multimedia Sand – Particulate contamination Carbon Filters – Chlorine and some organic removal Softeners – Hard water (Mg++ or Ca++ contamination) Cartridge Filters – Particulate and carbon options 3

Design Process Step 4 n Step 4 - Finalize Design n n n Prepare Process Flow Diagram (PFD), supporting documents and specifications Design Controls and Monitoring Review Validation requirements Review who will maintain the equipment n Consider service/maintenance plans Review requirements, specifications, design, equipment and PFD with customer/client Update and Finalize design as needed 4

Outline n Contaminants in Water n Pure Water Applications and Quality Standards n Water Purification Technologies n Key Water Purification System Design Steps Systems n Questions ? ? ?

Water Purification System for a Laboratory Facility Thank You!! Millipore Corporation Bioscience Division Christopher Yarima Mike Kelly
73eecdb5dc1e12f04fa2653ae98811d4.ppt