1a5845deeb4348d6a20066d2c756e369.ppt
- Количество слайдов: 55
 Waldenstrom’s and the Nervous System From Peripheral Nerve to Brain Fred H. Hochberg, MD
Waldenstrom’s and the Nervous System From Peripheral Nerve to Brain Fred H. Hochberg, MD
 Purpose • • Define the effects of WM Ig. M on Nerve Describe the investigations and therapy Describe Bing-Neel syndrome (brain) If Ig. M causes nerve damage does it cause Bing-Neel?
Purpose • • Define the effects of WM Ig. M on Nerve Describe the investigations and therapy Describe Bing-Neel syndrome (brain) If Ig. M causes nerve damage does it cause Bing-Neel?
 WM Causes Neurologic Illness • • • Peripheral neuropathy – POEMS Hyperviscosity > 3 c. P Invasion of CSF with WM cells Transformation into NHL CN, myelopathy. Bing - Neel
WM Causes Neurologic Illness • • • Peripheral neuropathy – POEMS Hyperviscosity > 3 c. P Invasion of CSF with WM cells Transformation into NHL CN, myelopathy. Bing - Neel
 “M spike” Neuropathies • MAG – Myelin Associated Glycoprotein = sensation, gait and tremor • GALOP – Gait, Auto-antibody, Late age, Onset, Polyneuropathy • POEMS – Polyneuropathy, Organomegaly, Edema, M spike, Skin change
“M spike” Neuropathies • MAG – Myelin Associated Glycoprotein = sensation, gait and tremor • GALOP – Gait, Auto-antibody, Late age, Onset, Polyneuropathy • POEMS – Polyneuropathy, Organomegaly, Edema, M spike, Skin change
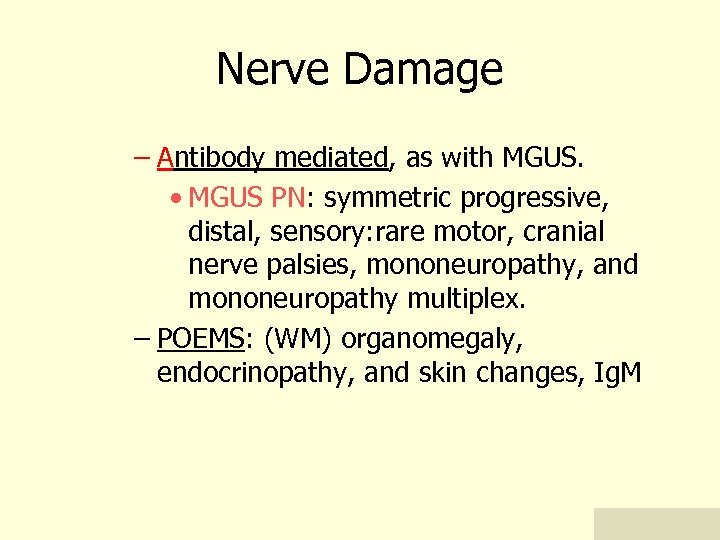 Nerve Damage – Antibody mediated, as with MGUS. • MGUS PN: symmetric progressive, distal, sensory: rare motor, cranial nerve palsies, mononeuropathy, and mononeuropathy multiplex. – POEMS: (WM) organomegaly, endocrinopathy, and skin changes, Ig. M
Nerve Damage – Antibody mediated, as with MGUS. • MGUS PN: symmetric progressive, distal, sensory: rare motor, cranial nerve palsies, mononeuropathy, and mononeuropathy multiplex. – POEMS: (WM) organomegaly, endocrinopathy, and skin changes, Ig. M
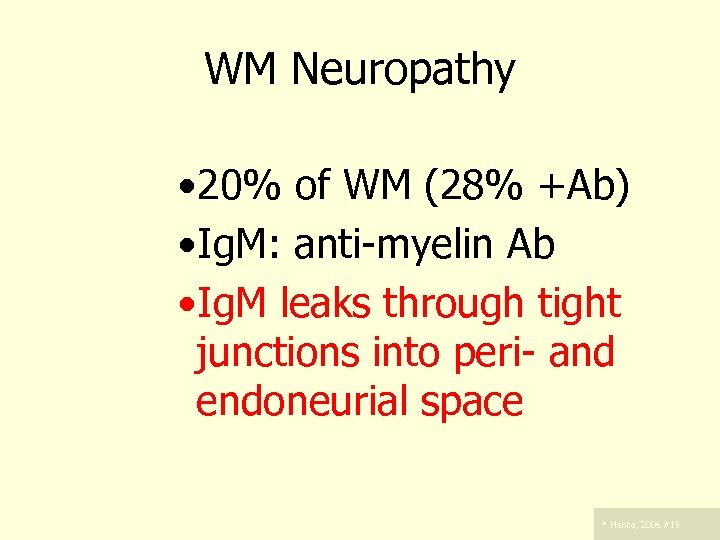 WM Neuropathy – . • 20% of WM (28% +Ab) • Ig. M: anti-myelin Ab • Ig. M leaks through tight junctions into peri- and endoneurial space * Menke, 2006 #15
WM Neuropathy – . • 20% of WM (28% +Ab) • Ig. M: anti-myelin Ab • Ig. M leaks through tight junctions into peri- and endoneurial space * Menke, 2006 #15
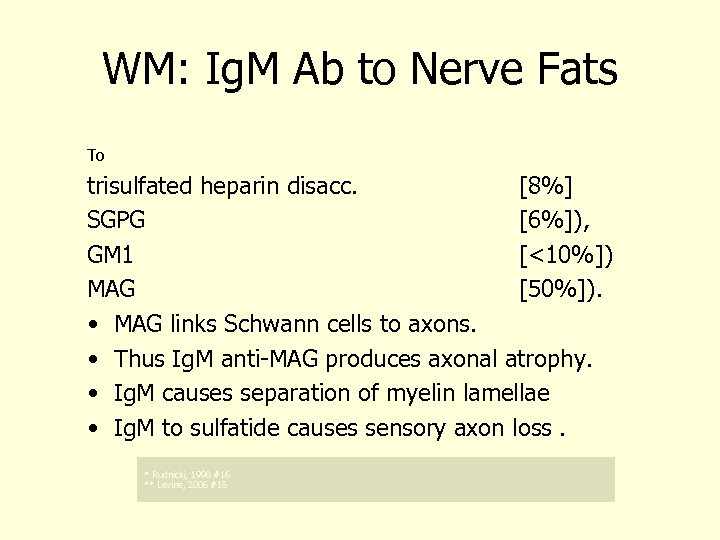 WM: Ig. M Ab to Nerve Fats Motor Neuropathies 1(NICOLE BAUMANN) To trisulfated heparin disacc. [8%] SGPG [6%]), GM 1 [<10%]) MAG [50%]). • MAG links Schwann cells to axons. • Thus Ig. M anti-MAG produces axonal atrophy. • Ig. M causes separation of myelin lamellae • Ig. M to sulfatide causes sensory axon loss. * Rudnicki, 1998 #16 ** Levine, 2006 #18
WM: Ig. M Ab to Nerve Fats Motor Neuropathies 1(NICOLE BAUMANN) To trisulfated heparin disacc. [8%] SGPG [6%]), GM 1 [<10%]) MAG [50%]). • MAG links Schwann cells to axons. • Thus Ig. M anti-MAG produces axonal atrophy. • Ig. M causes separation of myelin lamellae • Ig. M to sulfatide causes sensory axon loss. * Rudnicki, 1998 #16 ** Levine, 2006 #18
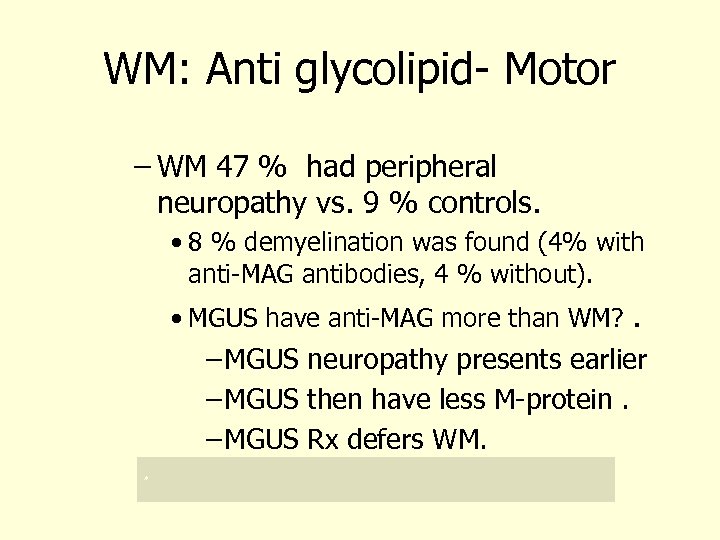 WM: Anti glycolipid- Motor – WM 47 % had peripheral neuropathy vs. 9 % controls. • 8 % demyelination was found (4% with anti-MAG antibodies, 4 % without). • MGUS have anti-MAG more than WM? . – MGUS neuropathy presents earlier – MGUS then have less M-protein. – MGUS Rx defers WM. *
WM: Anti glycolipid- Motor – WM 47 % had peripheral neuropathy vs. 9 % controls. • 8 % demyelination was found (4% with anti-MAG antibodies, 4 % without). • MGUS have anti-MAG more than WM? . – MGUS neuropathy presents earlier – MGUS then have less M-protein. – MGUS Rx defers WM. *
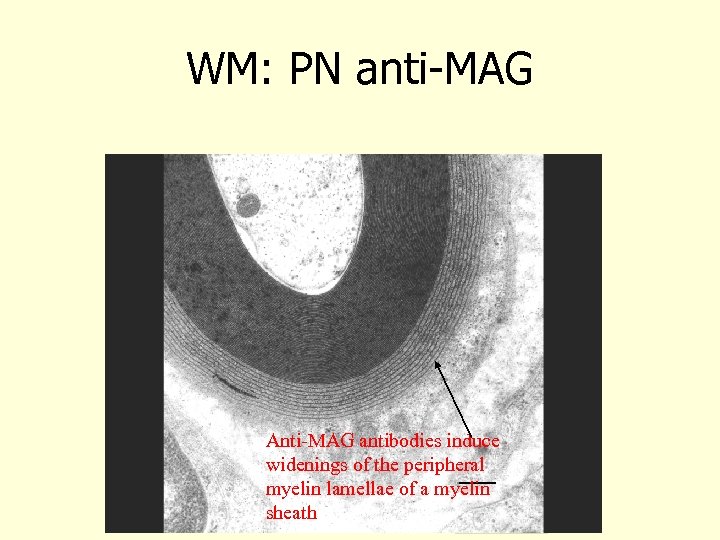 WM: PN anti-MAG Anti-MAG antibodies induce widenings of the peripheral myelin lamellae of a myelin sheath bar = 0. 5 µm
WM: PN anti-MAG Anti-MAG antibodies induce widenings of the peripheral myelin lamellae of a myelin sheath bar = 0. 5 µm
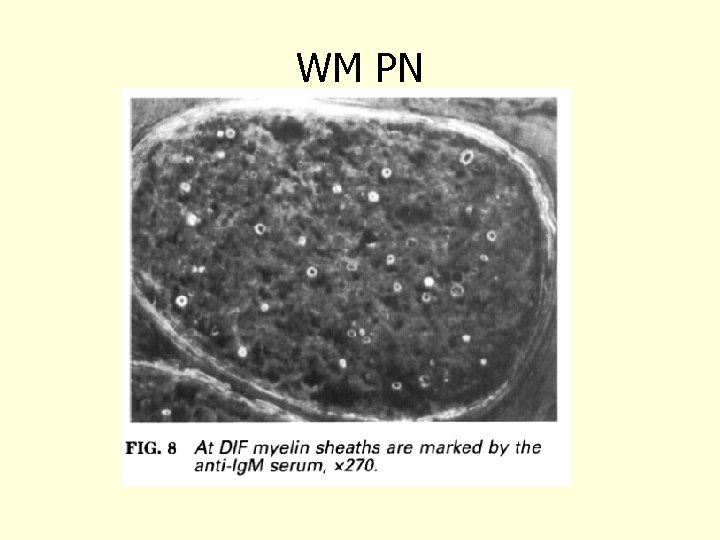 WM PN Vital 1997
WM PN Vital 1997
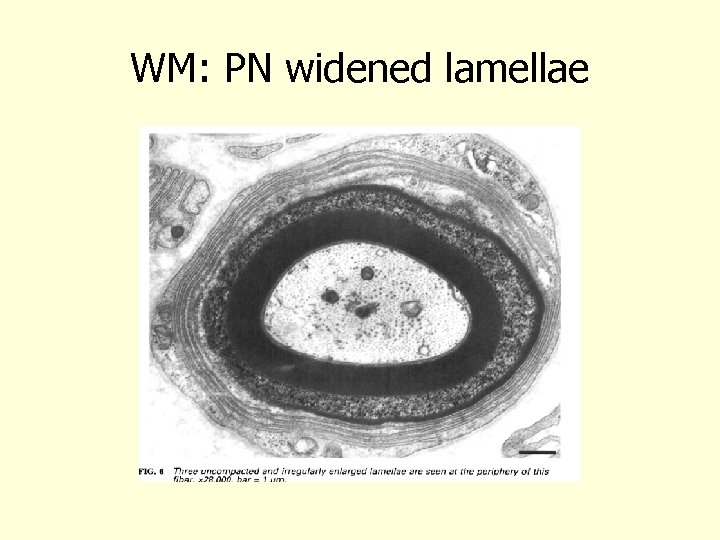 WM: PN widened lamellae Vital 1997
WM: PN widened lamellae Vital 1997
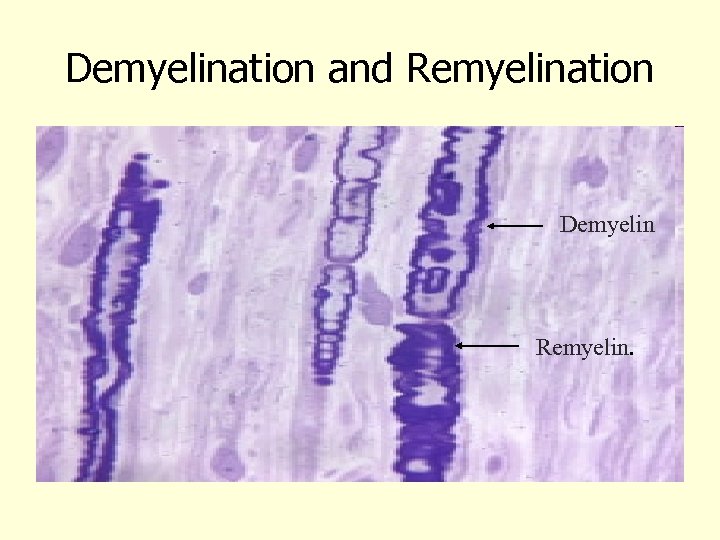 Demyelination and Remyelination Demyelin Remyelin.
Demyelination and Remyelination Demyelin Remyelin.
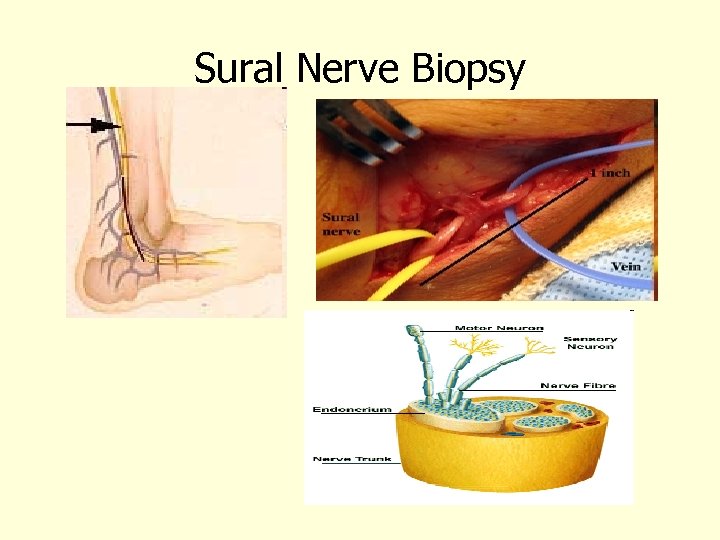 Sural Nerve Biopsy
Sural Nerve Biopsy
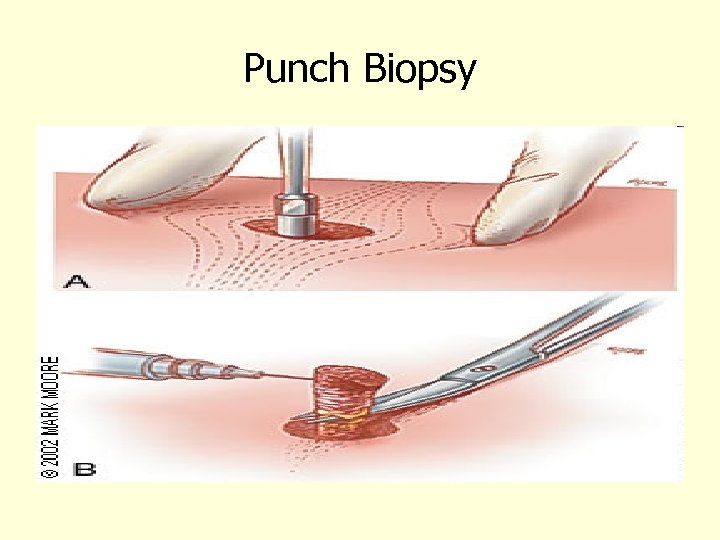 Punch Biopsy
Punch Biopsy
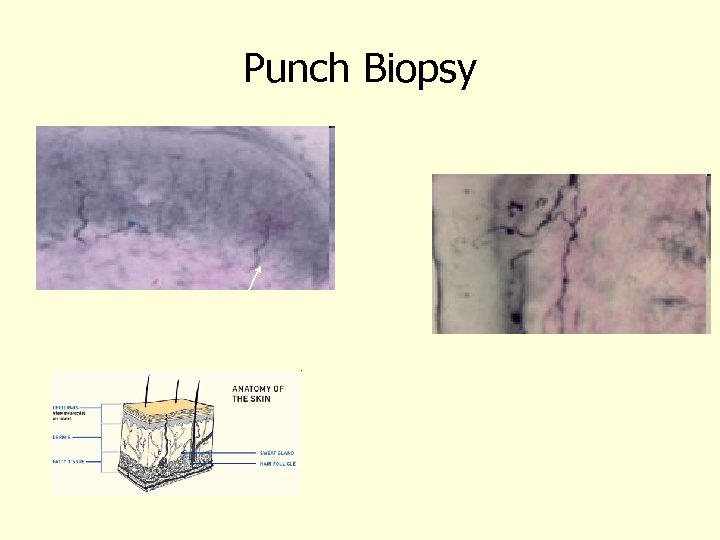 Punch Biopsy
Punch Biopsy
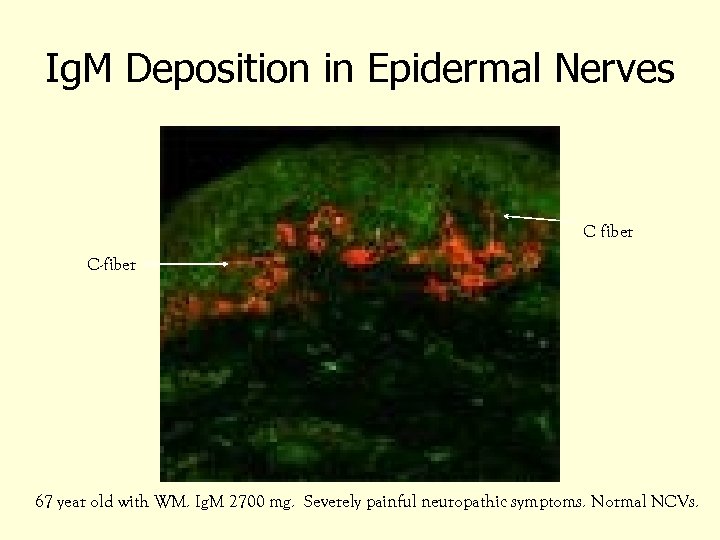 Ig. M Deposition in Epidermal Nerves C fiber C-fiber 67 year old with WM. Ig. M 2700 mg. Severely painful neuropathic symptoms. Normal NCVs.
Ig. M Deposition in Epidermal Nerves C fiber C-fiber 67 year old with WM. Ig. M 2700 mg. Severely painful neuropathic symptoms. Normal NCVs.
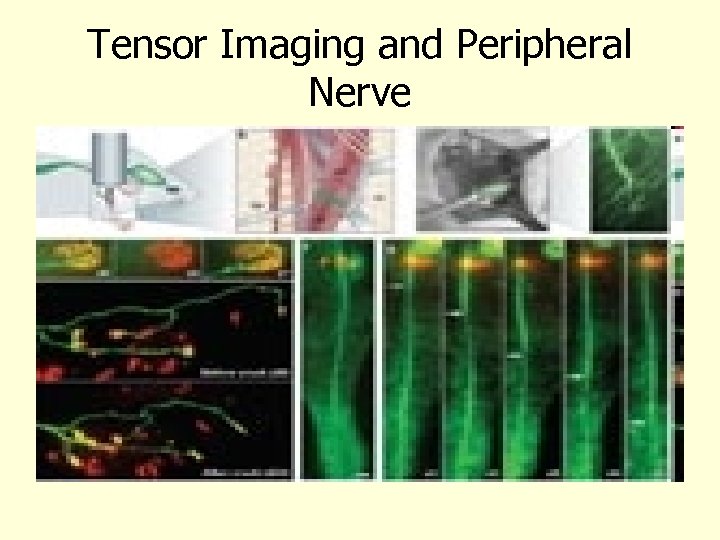 Tensor Imaging and Peripheral Nerve
Tensor Imaging and Peripheral Nerve
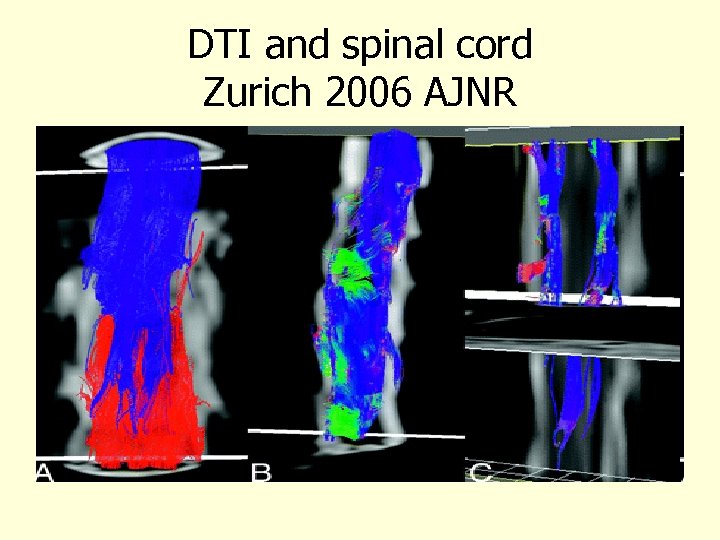 DTI and spinal cord Zurich 2006 AJNR
DTI and spinal cord Zurich 2006 AJNR
 WM: Ig. M PN 3 – sulphatide Ab (5% WM): sensory axon loss; – MAG Ab (4% of WM): sensorimotor axon loss and demyelination. – NO MAG: Demyelination in 4% of WM – Rare PN infiltration by lymphoplasmacytic B cells. – More profound motor involvement is described. – 10% of multifocal motor neuropathy with conduction block have Ig. M monoclonal gammopathy. – Can be severe motor, mononeuropathy multiplex and autonomic (BP, constipation, and impotence). – Nerve compression syndromes (CTS) seen in amyloidosis.
WM: Ig. M PN 3 – sulphatide Ab (5% WM): sensory axon loss; – MAG Ab (4% of WM): sensorimotor axon loss and demyelination. – NO MAG: Demyelination in 4% of WM – Rare PN infiltration by lymphoplasmacytic B cells. – More profound motor involvement is described. – 10% of multifocal motor neuropathy with conduction block have Ig. M monoclonal gammopathy. – Can be severe motor, mononeuropathy multiplex and autonomic (BP, constipation, and impotence). – Nerve compression syndromes (CTS) seen in amyloidosis.
 WM: PN 2 – PN (anti-MAG): • Sensory, sensory ataxia, slowly progressive. • 47% of 119 WM patients (versus 9% of 58 controls) had discomfort and sensory loss in the legs. • 35% lower vibration in WM, • 3. 4 times more likely abnormal pin sensation (Levine, • 5. 5 Neurology, Neurosurgery, and Psychiatry 2006; 77: 224 -228 Pestronk) Journal of times more likely gait disorders.
WM: PN 2 – PN (anti-MAG): • Sensory, sensory ataxia, slowly progressive. • 47% of 119 WM patients (versus 9% of 58 controls) had discomfort and sensory loss in the legs. • 35% lower vibration in WM, • 3. 4 times more likely abnormal pin sensation (Levine, • 5. 5 Neurology, Neurosurgery, and Psychiatry 2006; 77: 224 -228 Pestronk) Journal of times more likely gait disorders.
 POEMS • Vision: pressure 29% to 55% blurring, bleeding retina, inflammation • Skin thickening/shiney, blood flow • Swelling-Belly, Fingers or whole body • Hormonal-Diabetes (25%), Thyroid low (25%), breast tissue (60%), menses (60%), impotence (70%); Erectile • Large Spleen, Lymph nodes • Kidney failure • Thrombotic events
POEMS • Vision: pressure 29% to 55% blurring, bleeding retina, inflammation • Skin thickening/shiney, blood flow • Swelling-Belly, Fingers or whole body • Hormonal-Diabetes (25%), Thyroid low (25%), breast tissue (60%), menses (60%), impotence (70%); Erectile • Large Spleen, Lymph nodes • Kidney failure • Thrombotic events
 Bortezomib-Velcade Neuropathy • SUMMIT trial: 80% P. N. prior to therapy – 34% peripheral neuropathy with therapy, – 22% grade 1 or 2. • APEX study: – 36% neuropathy, – 7% grade 3 or 4 toxicity. – >50% better within 3 months of therapy • The dose is reduced if sensory neuropathy
Bortezomib-Velcade Neuropathy • SUMMIT trial: 80% P. N. prior to therapy – 34% peripheral neuropathy with therapy, – 22% grade 1 or 2. • APEX study: – 36% neuropathy, – 7% grade 3 or 4 toxicity. – >50% better within 3 months of therapy • The dose is reduced if sensory neuropathy
 Sensory Testing at Home How to spend the evening • Sensation: 10 -g fishing line touches first toe. Repeat four times (R and L): guess when it was not perceived. • Vibration testing (On/Off): 128 -Hz tuning fork to first toe. Do twice with and without dampening. • Vibration: report the end of vibration • Superficial pain: Neurotip (Owen Mumford, Oxford, U. K. ) to determine Perkins Toronto 2001 - DM number of times the pain was not perceived.
Sensory Testing at Home How to spend the evening • Sensation: 10 -g fishing line touches first toe. Repeat four times (R and L): guess when it was not perceived. • Vibration testing (On/Off): 128 -Hz tuning fork to first toe. Do twice with and without dampening. • Vibration: report the end of vibration • Superficial pain: Neurotip (Owen Mumford, Oxford, U. K. ) to determine Perkins Toronto 2001 - DM number of times the pain was not perceived.
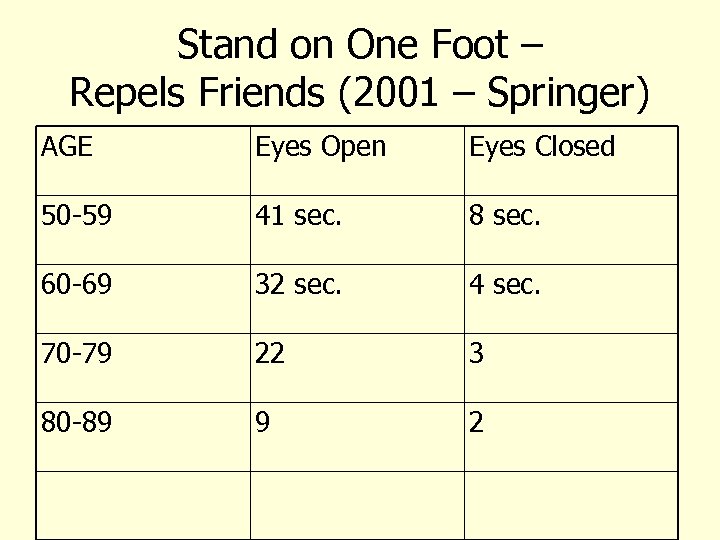 Stand on One Foot – Repels Friends (2001 – Springer) AGE Eyes Open Eyes Closed 50 -59 41 sec. 8 sec. 60 -69 32 sec. 4 sec. 70 -79 22 3 80 -89 9 2
Stand on One Foot – Repels Friends (2001 – Springer) AGE Eyes Open Eyes Closed 50 -59 41 sec. 8 sec. 60 -69 32 sec. 4 sec. 70 -79 22 3 80 -89 9 2
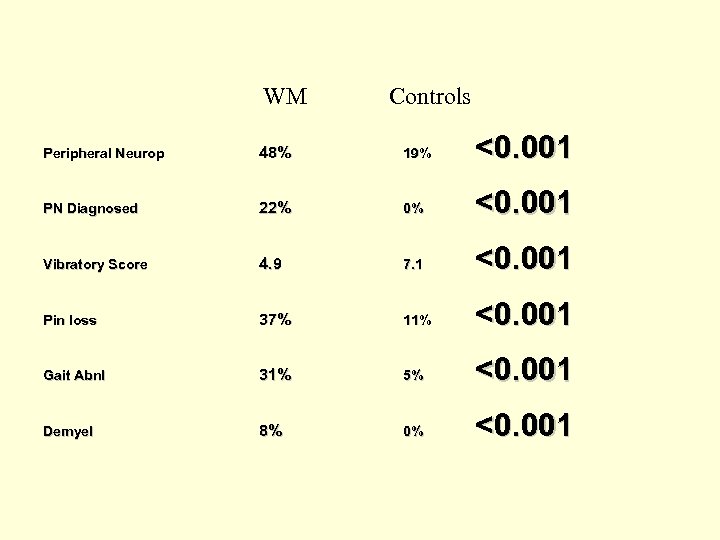 WM Controls Peripheral Neurop 48% 19% <0. 001 PN Diagnosed 22% 0% <0. 001 Vibratory Score 4. 9 7. 1 <0. 001 Pin loss 37% 11% <0. 001 Gait Abnl 31% 5% <0. 001 Demyel 8% 0% <0. 001
WM Controls Peripheral Neurop 48% 19% <0. 001 PN Diagnosed 22% 0% <0. 001 Vibratory Score 4. 9 7. 1 <0. 001 Pin loss 37% 11% <0. 001 Gait Abnl 31% 5% <0. 001 Demyel 8% 0% <0. 001
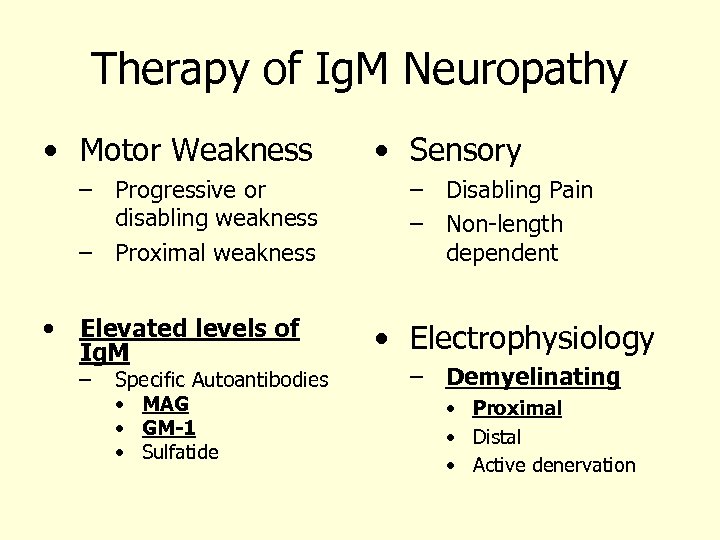 Therapy of Ig. M Neuropathy • Motor Weakness – Progressive or disabling weakness – Proximal weakness • Elevated levels of Ig. M – Specific Autoantibodies • MAG • GM-1 • Sulfatide • Sensory – Disabling Pain – Non-length dependent • Electrophysiology – Demyelinating • Proximal • Distal • Active denervation
Therapy of Ig. M Neuropathy • Motor Weakness – Progressive or disabling weakness – Proximal weakness • Elevated levels of Ig. M – Specific Autoantibodies • MAG • GM-1 • Sulfatide • Sensory – Disabling Pain – Non-length dependent • Electrophysiology – Demyelinating • Proximal • Distal • Active denervation
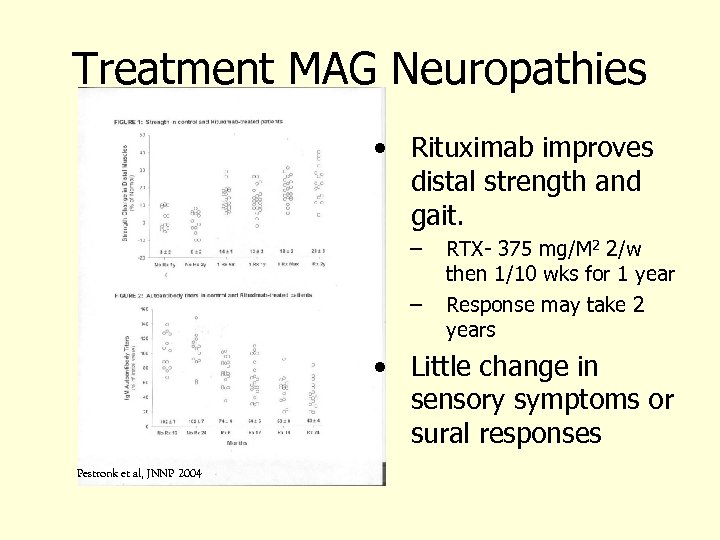 Treatment MAG Neuropathies • Rituximab improves distal strength and gait. – – RTX- 375 mg/M 2 2/w then 1/10 wks for 1 year Response may take 2 years • Little change in sensory symptoms or sural responses Pestronk et al, JNNP 2004
Treatment MAG Neuropathies • Rituximab improves distal strength and gait. – – RTX- 375 mg/M 2 2/w then 1/10 wks for 1 year Response may take 2 years • Little change in sensory symptoms or sural responses Pestronk et al, JNNP 2004
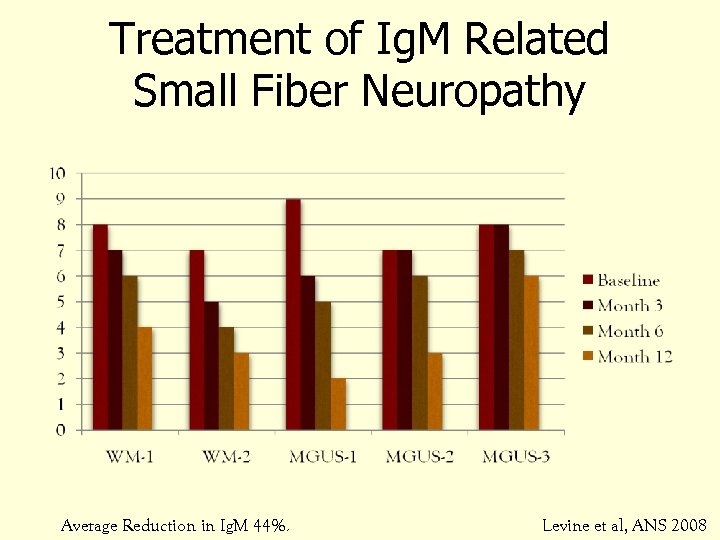 Treatment of Ig. M Related Small Fiber Neuropathy Average Reduction in Ig. M 44%. Levine et al, ANS 2008
Treatment of Ig. M Related Small Fiber Neuropathy Average Reduction in Ig. M 44%. Levine et al, ANS 2008
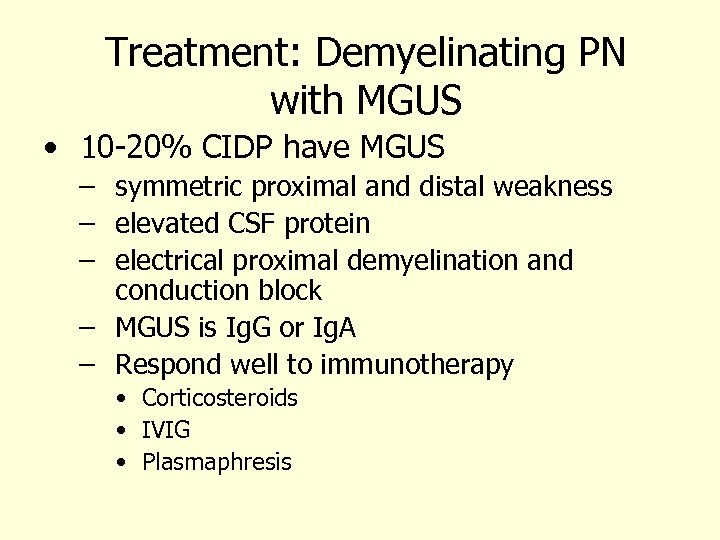 Treatment: Demyelinating PN with MGUS • 10 -20% CIDP have MGUS – symmetric proximal and distal weakness – elevated CSF protein – electrical proximal demyelination and conduction block – MGUS is Ig. G or Ig. A – Respond well to immunotherapy • Corticosteroids • IVIG • Plasmaphresis
Treatment: Demyelinating PN with MGUS • 10 -20% CIDP have MGUS – symmetric proximal and distal weakness – elevated CSF protein – electrical proximal demyelination and conduction block – MGUS is Ig. G or Ig. A – Respond well to immunotherapy • Corticosteroids • IVIG • Plasmaphresis
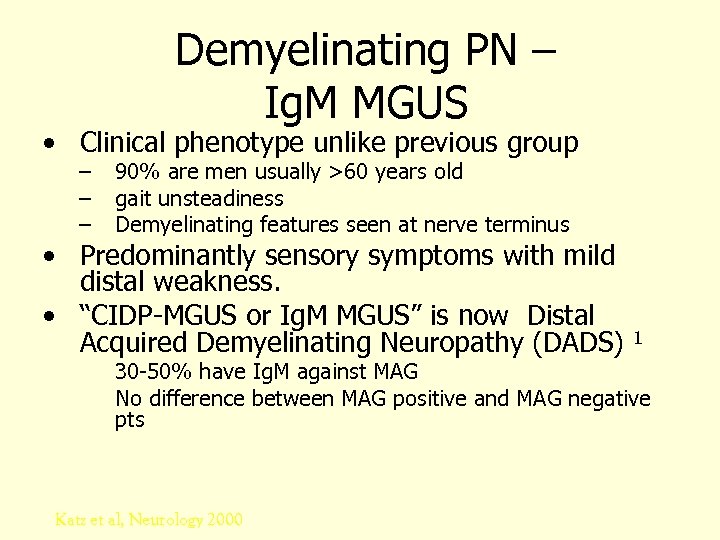 Demyelinating PN – Ig. M MGUS • Clinical phenotype unlike previous group – – – 90% are men usually >60 years old gait unsteadiness Demyelinating features seen at nerve terminus • Predominantly sensory symptoms with mild distal weakness. • “CIDP-MGUS or Ig. M MGUS” is now Distal Acquired Demyelinating Neuropathy (DADS) 1 30 -50% have Ig. M against MAG No difference between MAG positive and MAG negative pts Katz et al, Neurology 2000
Demyelinating PN – Ig. M MGUS • Clinical phenotype unlike previous group – – – 90% are men usually >60 years old gait unsteadiness Demyelinating features seen at nerve terminus • Predominantly sensory symptoms with mild distal weakness. • “CIDP-MGUS or Ig. M MGUS” is now Distal Acquired Demyelinating Neuropathy (DADS) 1 30 -50% have Ig. M against MAG No difference between MAG positive and MAG negative pts Katz et al, Neurology 2000
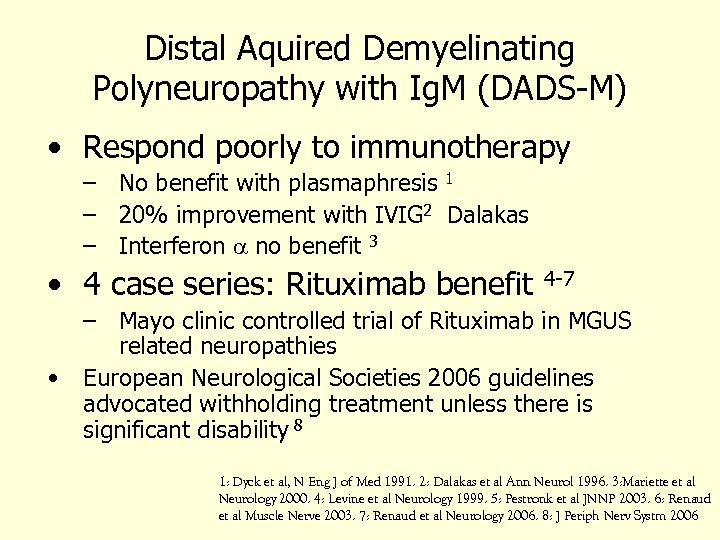 Distal Aquired Demyelinating Polyneuropathy with Ig. M (DADS-M) • Respond poorly to immunotherapy – No benefit with plasmaphresis 1 – 20% improvement with IVIG 2 Dalakas – Interferon a no benefit 3 • 4 case series: Rituximab benefit • 4 -7 – Mayo clinic controlled trial of Rituximab in MGUS related neuropathies European Neurological Societies 2006 guidelines advocated withholding treatment unless there is significant disability 8 1: Dyck et al, N Eng J of Med 1991. 2: Dalakas et al Ann Neurol 1996. 3: Mariette et al Neurology 2000. 4: Levine et al Neurology 1999. 5: Pestronk et al JNNP 2003. 6: Renaud et al Muscle Nerve 2003. 7: Renaud et al Neurology 2006. 8: J Periph Nerv Systm 2006
Distal Aquired Demyelinating Polyneuropathy with Ig. M (DADS-M) • Respond poorly to immunotherapy – No benefit with plasmaphresis 1 – 20% improvement with IVIG 2 Dalakas – Interferon a no benefit 3 • 4 case series: Rituximab benefit • 4 -7 – Mayo clinic controlled trial of Rituximab in MGUS related neuropathies European Neurological Societies 2006 guidelines advocated withholding treatment unless there is significant disability 8 1: Dyck et al, N Eng J of Med 1991. 2: Dalakas et al Ann Neurol 1996. 3: Mariette et al Neurology 2000. 4: Levine et al Neurology 1999. 5: Pestronk et al JNNP 2003. 6: Renaud et al Muscle Nerve 2003. 7: Renaud et al Neurology 2006. 8: J Periph Nerv Systm 2006
 Axonal PN with Ig. M Disorders • Sulfatide – 30% may have motor weakness and gait disorder. – 3% of CIDP pts – 4% of WM pts – No controlled studies of therapy. • Potential benefit from cyclophosphamide or Rituximab. • Ab negative – Unclear relationship between Ig. M and PN – often pain and sensory symptoms without motor. – Search for other causes of PN or mimics of PN – Treatment:
Axonal PN with Ig. M Disorders • Sulfatide – 30% may have motor weakness and gait disorder. – 3% of CIDP pts – 4% of WM pts – No controlled studies of therapy. • Potential benefit from cyclophosphamide or Rituximab. • Ab negative – Unclear relationship between Ig. M and PN – often pain and sensory symptoms without motor. – Search for other causes of PN or mimics of PN – Treatment:
 Potential Aides in Neuropathy • Biotin, choline, inositol, thiamine • B 12 • Alpha-lipoic acid useful reduction in pain and numbness in diabetes – Approved in Germany. – antioxidant • Gamma linolenic acid in oils (borage, grape seed, black currant) – double-blind: gradual reversal of nerve damage
Potential Aides in Neuropathy • Biotin, choline, inositol, thiamine • B 12 • Alpha-lipoic acid useful reduction in pain and numbness in diabetes – Approved in Germany. – antioxidant • Gamma linolenic acid in oils (borage, grape seed, black currant) – double-blind: gradual reversal of nerve damage
 Neurontin • Diabetic neuropathy – significant reduction in pain – 26 percent were pain-free vs. 15 percent with placebo • Post-herpetic neuralgia (zoster) (double blind) – significant reduction in average daily pain – Neurontin (16%) pain-free vs. placebo (8. 8%) • Neurontin improved sleep and life quality. Fatigue, dizziness and tremor
Neurontin • Diabetic neuropathy – significant reduction in pain – 26 percent were pain-free vs. 15 percent with placebo • Post-herpetic neuralgia (zoster) (double blind) – significant reduction in average daily pain – Neurontin (16%) pain-free vs. placebo (8. 8%) • Neurontin improved sleep and life quality. Fatigue, dizziness and tremor
 Skin stem cells reform nerves. Midha, Calgary
Skin stem cells reform nerves. Midha, Calgary
 Rate of regeneration • Myelin repair begins within 24 hours • Axon repair starts at same time • Axon sprouts diminish further away from the cell body • Rate of growth is. 5 to 9 mm/day • Regeneration can occur even a year after damage
Rate of regeneration • Myelin repair begins within 24 hours • Axon repair starts at same time • Axon sprouts diminish further away from the cell body • Rate of growth is. 5 to 9 mm/day • Regeneration can occur even a year after damage
 ALADIN I, ALADIN III, SYDNEY, NATHAN II Ziegler 1258 ALA or placebo patients • alpha-lipoic acid intravenously 3 weeks • Total Symptom Score (TSS) in the feet. – TSS (baseline to the end of i. v. ) ALA> Placebo by 24% – daily changes in TSS: ALA daily improved pain, burning, numb – responder rates (> or =50% improved TSS): 53%: 37%, – Neuropathy Impairment Score (NIS) LExt, ALA>Placebo by 16% • pin-prick and touch-pressure sensation • adverse events did not differ between the groups.
ALADIN I, ALADIN III, SYDNEY, NATHAN II Ziegler 1258 ALA or placebo patients • alpha-lipoic acid intravenously 3 weeks • Total Symptom Score (TSS) in the feet. – TSS (baseline to the end of i. v. ) ALA> Placebo by 24% – daily changes in TSS: ALA daily improved pain, burning, numb – responder rates (> or =50% improved TSS): 53%: 37%, – Neuropathy Impairment Score (NIS) LExt, ALA>Placebo by 16% • pin-prick and touch-pressure sensation • adverse events did not differ between the groups.
 GLA - Glasgow • Twenty-two: distal diabetic PN • double-blind, placebo study – 360 mg oral gamma-linolenic acid. • 6 months: . – improved neuropathy symptom score – median nerve motor conductions – compound muscle action potential amplitude – median and sural sensory nerve action potentials
GLA - Glasgow • Twenty-two: distal diabetic PN • double-blind, placebo study – 360 mg oral gamma-linolenic acid. • 6 months: . – improved neuropathy symptom score – median nerve motor conductions – compound muscle action potential amplitude – median and sural sensory nerve action potentials
 What is ‘Bing-Neel’ ? • Jens Bing and Axel Neel (1936*) • nerve root + cord dysfunction with WMassociated hyperglobulinemia • Two cases combined lymphoplasmacytic infiltrates (meninges, roots and cord) with antibody-mediated damage; one the latter • ‘universal toxic effect…and not to a local effect’. Marrow not studied. • No myeloma…clinical, x-ray, autopsy” * Bing, J; Neel, A. Two Cases of Hyperglobulinaemia with Affection of the Central Nervous System on a Toxi. Infectious Basis (Myelitis, polyradiculitis, Spinal Fluid Chages) Acta Medica Scandinavica 88: fascicle V-V 1, 492 -506, 1936
What is ‘Bing-Neel’ ? • Jens Bing and Axel Neel (1936*) • nerve root + cord dysfunction with WMassociated hyperglobulinemia • Two cases combined lymphoplasmacytic infiltrates (meninges, roots and cord) with antibody-mediated damage; one the latter • ‘universal toxic effect…and not to a local effect’. Marrow not studied. • No myeloma…clinical, x-ray, autopsy” * Bing, J; Neel, A. Two Cases of Hyperglobulinaemia with Affection of the Central Nervous System on a Toxi. Infectious Basis (Myelitis, polyradiculitis, Spinal Fluid Chages) Acta Medica Scandinavica 88: fascicle V-V 1, 492 -506, 1936
 Case 2 con. – ‘Toxic Infective’ ‘Polyradiculitis and ‘myeloencephalopathia’ with degenerative changes in the spinal cord, anterior/posterior roots – Anterior horn cells-ganglion cells: fatty changes One single medullary vessel had round cell infiltrate – No mention was made of lymphoplasmacytic infiltrate nor of bone marrow findings.
Case 2 con. – ‘Toxic Infective’ ‘Polyradiculitis and ‘myeloencephalopathia’ with degenerative changes in the spinal cord, anterior/posterior roots – Anterior horn cells-ganglion cells: fatty changes One single medullary vessel had round cell infiltrate – No mention was made of lymphoplasmacytic infiltrate nor of bone marrow findings.
 Presentation • Presented with generalized fatigue and hiccoughs in February 2003. Symptoms likely began in September 2002 and became manifest following a vacation to Northern Germany. • “Every five or six seconds the hiccough came and much stronger and louder in the night perhaps from being in the horizontal position. ” Spared were lips and tongue and speech during the spells. These hiccoughs could not be stopped.
Presentation • Presented with generalized fatigue and hiccoughs in February 2003. Symptoms likely began in September 2002 and became manifest following a vacation to Northern Germany. • “Every five or six seconds the hiccough came and much stronger and louder in the night perhaps from being in the horizontal position. ” Spared were lips and tongue and speech during the spells. These hiccoughs could not be stopped.
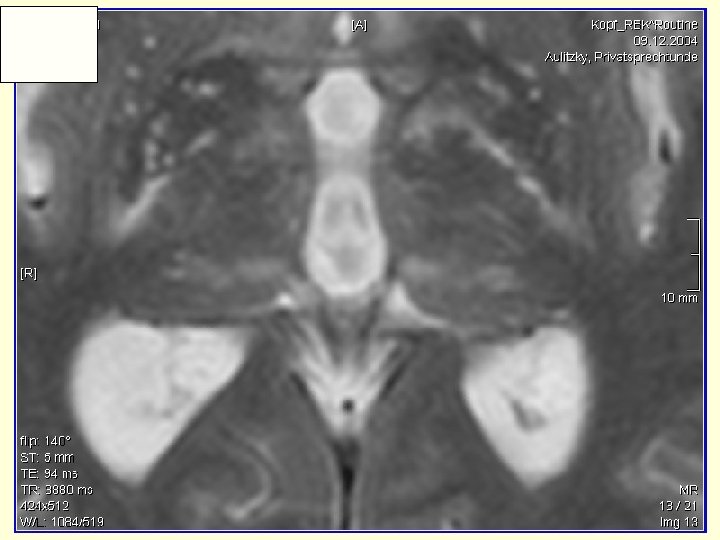
 Presentation • Headaches • Psychiatric changes • Visual hallucinations • Seizures – hiccoughs and LOC • Gait changes • Right hand alterations
Presentation • Headaches • Psychiatric changes • Visual hallucinations • Seizures – hiccoughs and LOC • Gait changes • Right hand alterations
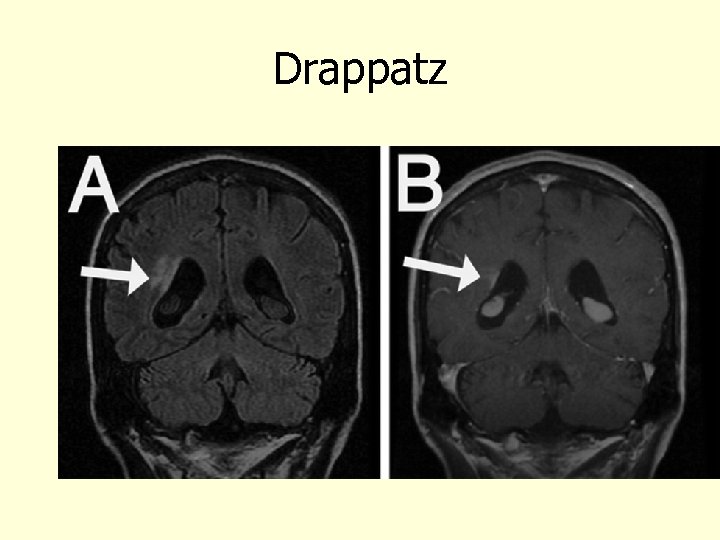 Drappatz
Drappatz
 Classical Bing-Neel Syndrome Overview Three cases: Bing and Neel (1936) and Bing, Fog et al. (1937) Waldenstrom’s Macroglobulinemia (WM) with central nervous system (CNS) infiltration Clinical: encephalopathy, strokes, seizures, hemorrhages, Bell’s Palsy, neuropathy, ocular involvement, numbness, weakness, headaches
Classical Bing-Neel Syndrome Overview Three cases: Bing and Neel (1936) and Bing, Fog et al. (1937) Waldenstrom’s Macroglobulinemia (WM) with central nervous system (CNS) infiltration Clinical: encephalopathy, strokes, seizures, hemorrhages, Bell’s Palsy, neuropathy, ocular involvement, numbness, weakness, headaches
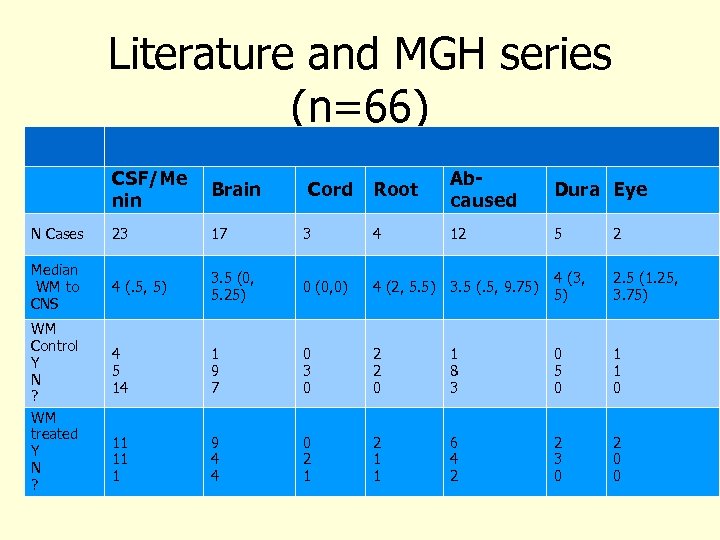 N Cases Median WM to CNS WM Control Y N ? WM treated Y N ? Literature and MGH series (n=66) Manifestation Root Bing-Neel of Ab. CSF/Me Brain Cord Dura Eye nin caused Syndrome 23 17 3 4 4 (. 5, 5) 3. 5 (0, 5. 25) 0 (0, 0) 4 5 14 1 9 7 11 11 1 9 4 4 12 5 2 4 (2, 5. 5) 3. 5 (. 5, 9. 75) 4 (3, 5) 2. 5 (1. 25, 3. 75) 0 3 0 2 2 0 1 8 3 0 5 0 1 1 0 0 2 1 1 6 4 2 2 3 0 2 0 0
N Cases Median WM to CNS WM Control Y N ? WM treated Y N ? Literature and MGH series (n=66) Manifestation Root Bing-Neel of Ab. CSF/Me Brain Cord Dura Eye nin caused Syndrome 23 17 3 4 4 (. 5, 5) 3. 5 (0, 5. 25) 0 (0, 0) 4 5 14 1 9 7 11 11 1 9 4 4 12 5 2 4 (2, 5. 5) 3. 5 (. 5, 9. 75) 4 (3, 5) 2. 5 (1. 25, 3. 75) 0 3 0 2 2 0 1 8 3 0 5 0 1 1 0 0 2 1 1 6 4 2 2 3 0 2 0 0
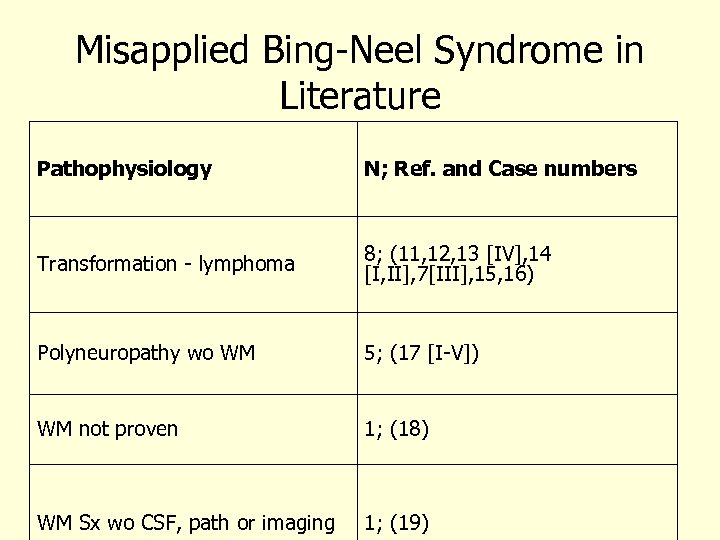 Misapplied Bing-Neel Syndrome in Literature Pathophysiology N; Ref. and Case numbers Transformation - lymphoma 8; (11, 12, 13 [IV], 14 [I, II], 7[III], 15, 16) Polyneuropathy wo WM 5; (17 [I-V]) WM not proven 1; (18) WM Sx wo CSF, path or imaging 1; (19)
Misapplied Bing-Neel Syndrome in Literature Pathophysiology N; Ref. and Case numbers Transformation - lymphoma 8; (11, 12, 13 [IV], 14 [I, II], 7[III], 15, 16) Polyneuropathy wo WM 5; (17 [I-V]) WM not proven 1; (18) WM Sx wo CSF, path or imaging 1; (19)
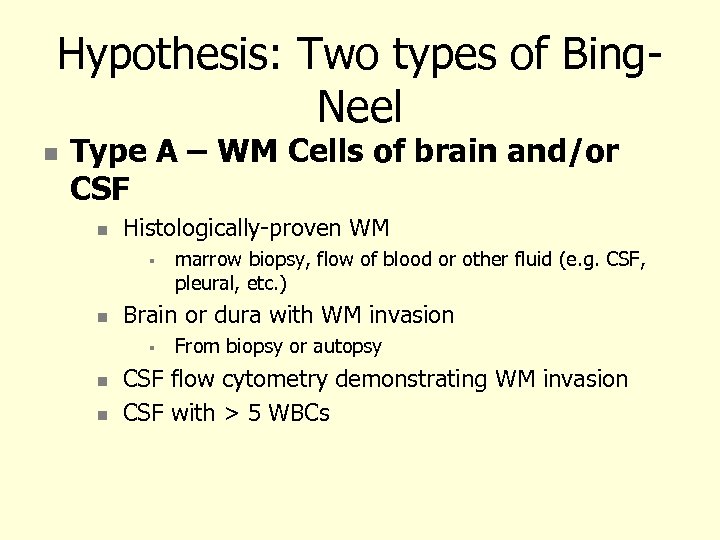 Hypothesis: Two types of Bing. Neel Type A – WM Cells of brain and/or CSF Histologically-proven WM Brain or dura with WM invasion marrow biopsy, flow of blood or other fluid (e. g. CSF, pleural, etc. ) From biopsy or autopsy CSF flow cytometry demonstrating WM invasion CSF with > 5 WBCs
Hypothesis: Two types of Bing. Neel Type A – WM Cells of brain and/or CSF Histologically-proven WM Brain or dura with WM invasion marrow biopsy, flow of blood or other fluid (e. g. CSF, pleural, etc. ) From biopsy or autopsy CSF flow cytometry demonstrating WM invasion CSF with > 5 WBCs
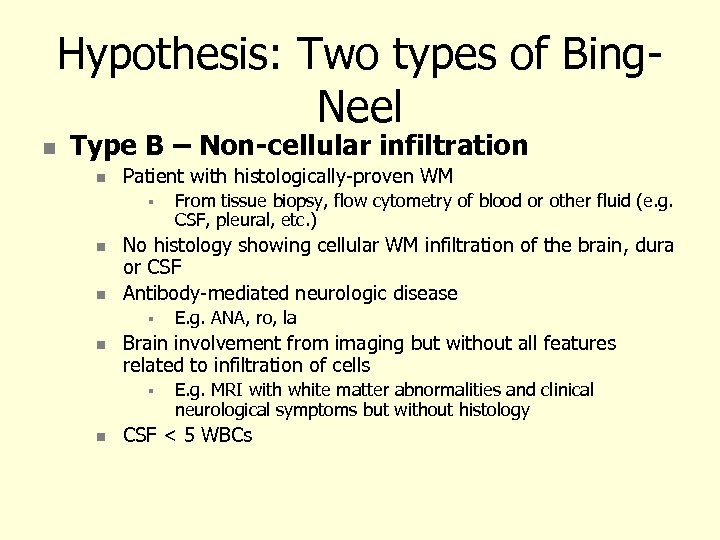 Hypothesis: Two types of Bing. Neel Type B – Non-cellular infiltration Patient with histologically-proven WM No histology showing cellular WM infiltration of the brain, dura or CSF Antibody-mediated neurologic disease E. g. ANA, ro, la Brain involvement from imaging but without all features related to infiltration of cells From tissue biopsy, flow cytometry of blood or other fluid (e. g. CSF, pleural, etc. ) E. g. MRI with white matter abnormalities and clinical neurological symptoms but without histology CSF < 5 WBCs
Hypothesis: Two types of Bing. Neel Type B – Non-cellular infiltration Patient with histologically-proven WM No histology showing cellular WM infiltration of the brain, dura or CSF Antibody-mediated neurologic disease E. g. ANA, ro, la Brain involvement from imaging but without all features related to infiltration of cells From tissue biopsy, flow cytometry of blood or other fluid (e. g. CSF, pleural, etc. ) E. g. MRI with white matter abnormalities and clinical neurological symptoms but without histology CSF < 5 WBCs
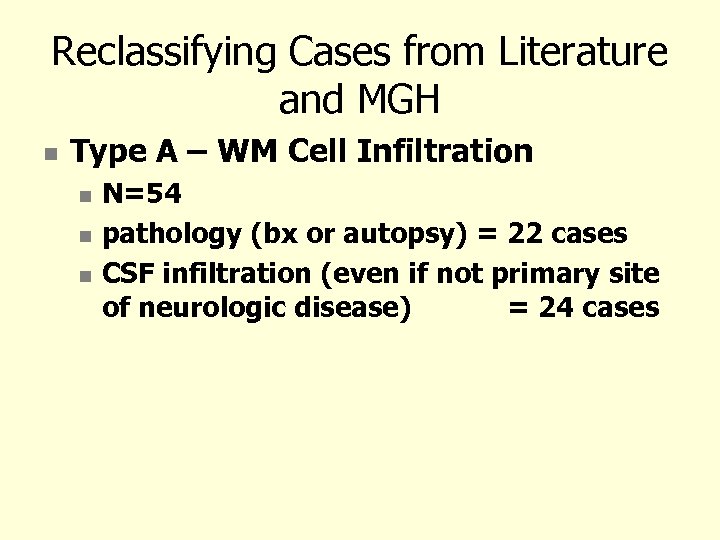 Reclassifying Cases from Literature and MGH Type A – WM Cell Infiltration N=54 pathology (bx or autopsy) = 22 cases CSF infiltration (even if not primary site of neurologic disease) = 24 cases
Reclassifying Cases from Literature and MGH Type A – WM Cell Infiltration N=54 pathology (bx or autopsy) = 22 cases CSF infiltration (even if not primary site of neurologic disease) = 24 cases
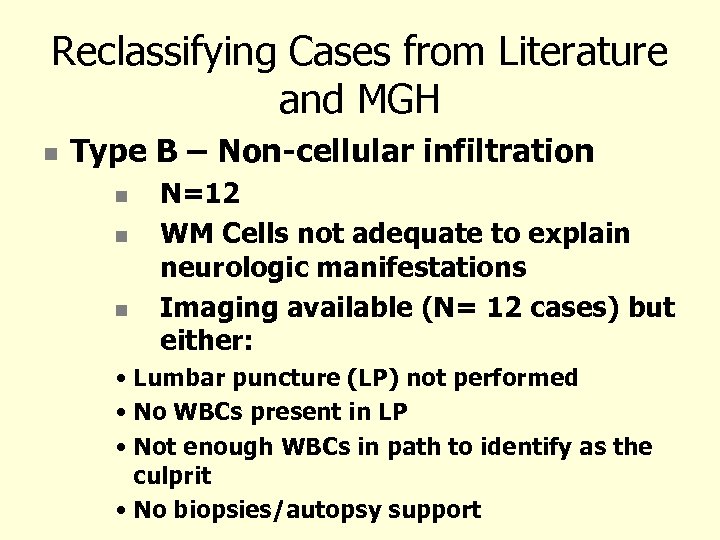 Reclassifying Cases from Literature and MGH Type B – Non-cellular infiltration N=12 WM Cells not adequate to explain neurologic manifestations Imaging available (N= 12 cases) but either: • Lumbar puncture (LP) not performed • No WBCs present in LP • Not enough WBCs in path to identify as the culprit • No biopsies/autopsy support
Reclassifying Cases from Literature and MGH Type B – Non-cellular infiltration N=12 WM Cells not adequate to explain neurologic manifestations Imaging available (N= 12 cases) but either: • Lumbar puncture (LP) not performed • No WBCs present in LP • Not enough WBCs in path to identify as the culprit • No biopsies/autopsy support
 What Information a Prospective BN Study Could Offer Risk factors (? prior WM therapies). No data for 9/66 Prior WM controlled in 24/66 Images not performed for 20/66 patients No CSF data on 4/10 patients at MGH
What Information a Prospective BN Study Could Offer Risk factors (? prior WM therapies). No data for 9/66 Prior WM controlled in 24/66 Images not performed for 20/66 patients No CSF data on 4/10 patients at MGH
 Why a Prospective Study is Useful May identify prognostic factors for CNS invasion B-N as a model for the study of leukocyte trafficking across blood-brain (BBB), CSF-choroid plexus CSF-blood barriers Is B-N an Antibody-mediated CNS disease? Create a working definition of Bing-Neel
Why a Prospective Study is Useful May identify prognostic factors for CNS invasion B-N as a model for the study of leukocyte trafficking across blood-brain (BBB), CSF-choroid plexus CSF-blood barriers Is B-N an Antibody-mediated CNS disease? Create a working definition of Bing-Neel
 Summary • WM is associated with Ab mediated damage to peripheral nerve myelin and central myelin • The central effects (Bing-Neel) likely are more common but the antibodies have not been explored. • Modern therapy will explore Ab control for both diseases
Summary • WM is associated with Ab mediated damage to peripheral nerve myelin and central myelin • The central effects (Bing-Neel) likely are more common but the antibodies have not been explored. • Modern therapy will explore Ab control for both diseases


