2b6f27e7f8792679b30d216a93bbe916.ppt
- Количество слайдов: 31
WAC 2011 Debates 4, 6 December 2011: 10: 45 - 11: 45 Skin test or in-vitro Test for Food Allergy? “In vitro” Motohiro Ebisawa, MD, Ph. D Ig. E

Disclosures of Motohiro Ebisawa, MD, Ph. D 1) Employee of Sagamihara National Hospital 2) Academic activities WAO: Board member at large, AAAAI: International Assembly vice-chair Japanese Society of Allergology: Board member Japanese Society of Pediatric Allergy: Board member 3) Grant support from Japanese government, Ministry of Labor, Health, and Welfare for 12 years as PI 4) No Conflicts of Interests
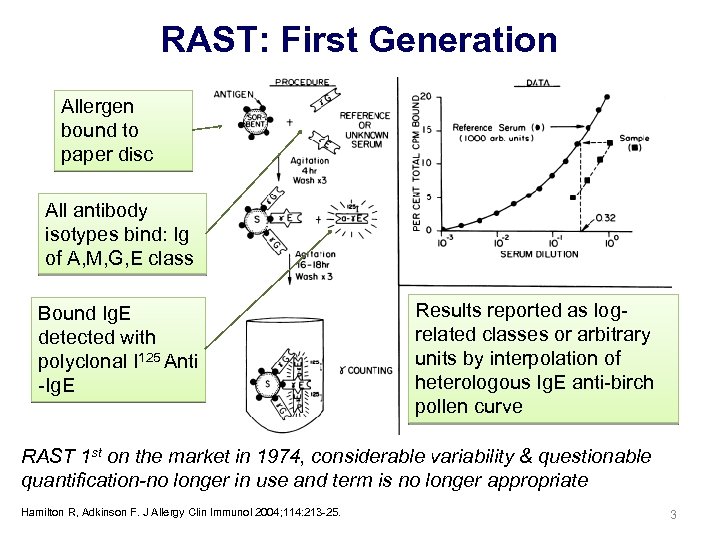
RAST: First Generation Allergen bound to paper disc All antibody isotypes bind: Ig of A, M, G, E class Bound Ig. E detected with polyclonal I 125 Anti -Ig. E Results reported as logrelated classes or arbitrary units by interpolation of heterologous Ig. E anti-birch pollen curve RAST 1 st on the market in 1974, considerable variability & questionable quantification-no longer in use and term is no longer appropriate Hamilton R, Adkinson F. J Allergy Clin Immunol 2004; 114: 213 -25. 3
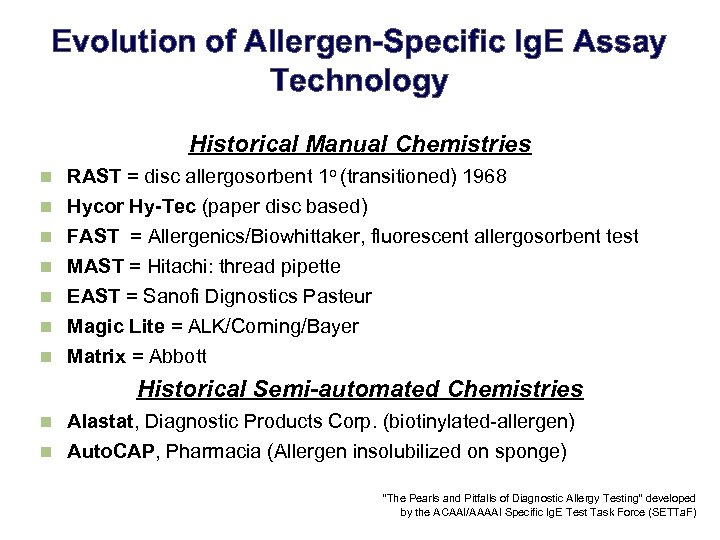
Evolution of Allergen-Specific Ig. E Assay Technology Historical Manual Chemistries n RAST = disc allergosorbent 1 o (transitioned) 1968 n Hycor Hy-Tec (paper disc based) FAST = Allergenics/Biowhittaker, fluorescent allergosorbent test MAST = Hitachi: thread pipette EAST = Sanofi Dignostics Pasteur Magic Lite = ALK/Corning/Bayer Matrix = Abbott n n n Historical Semi-automated Chemistries Alastat, Diagnostic Products Corp. (biotinylated-allergen) n Auto. CAP, Pharmacia (Allergen insolubilized on sponge) n “The Pearls and Pitfalls of Diagnostic Allergy Testing” developed by the ACAAI/AAAAI Specific Ig. E Test Task Force (SETTa. F)
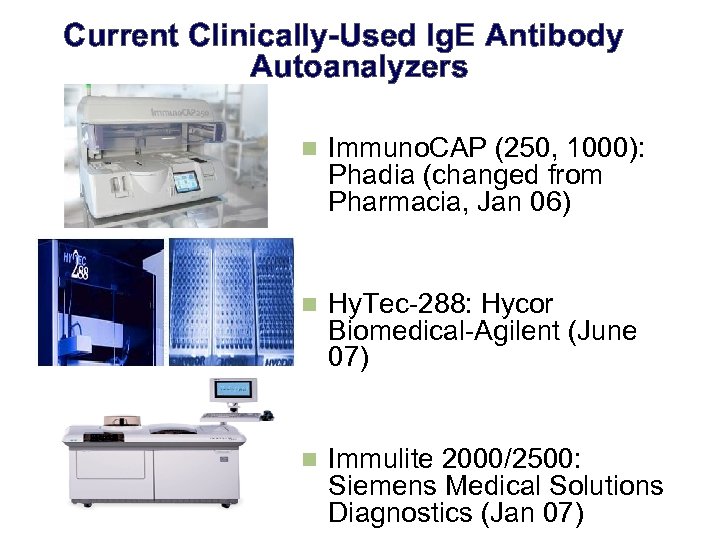
Current Clinically-Used Ig. E Antibody Autoanalyzers n Immuno. CAP (250, 1000): Phadia (changed from Pharmacia, Jan 06) n Hy. Tec-288: Hycor Biomedical-Agilent (June 07) n Immulite 2000/2500: Siemens Medical Solutions Diagnostics (Jan 07)
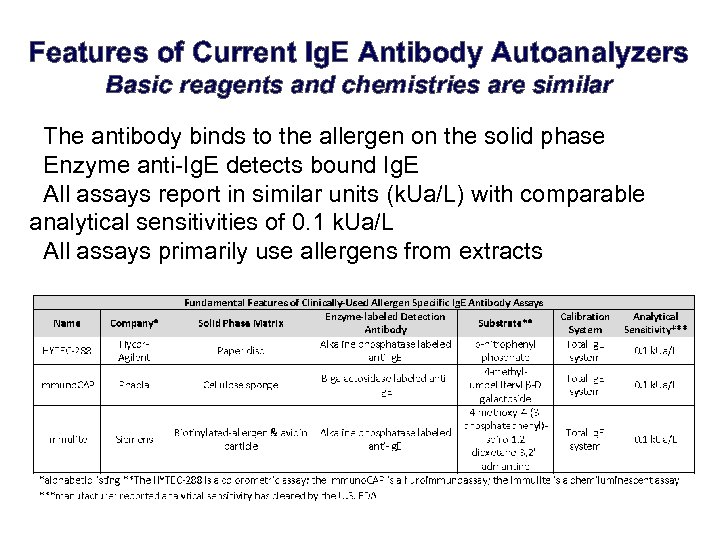
Features of Current Ig. E Antibody Autoanalyzers Basic reagents and chemistries are similar • The antibody binds to the allergen on the solid phase • Enzyme anti-Ig. E detects bound Ig. E • All assays report in similar units (k. Ua/L) with comparable analytical sensitivities of 0. 1 k. Ua/L • All assays primarily use allergens from extracts

Immuno. CAP perceived as gold standard for in vitro Ig. E testing ”The predicitive values associated with clinical evidence for Immuno. CAP cannot be applied to Turbo-MP and Immulite”NIH/NIAID Food allergy guideline. J Allergy Clin Immunol 2010; 126: S 1 -S 58 ”The Pharmacia CAP system is in world wide use and is a de facto standard to which other methods are compared” Dolen WK. Allergy 2003; 58: 717 -723
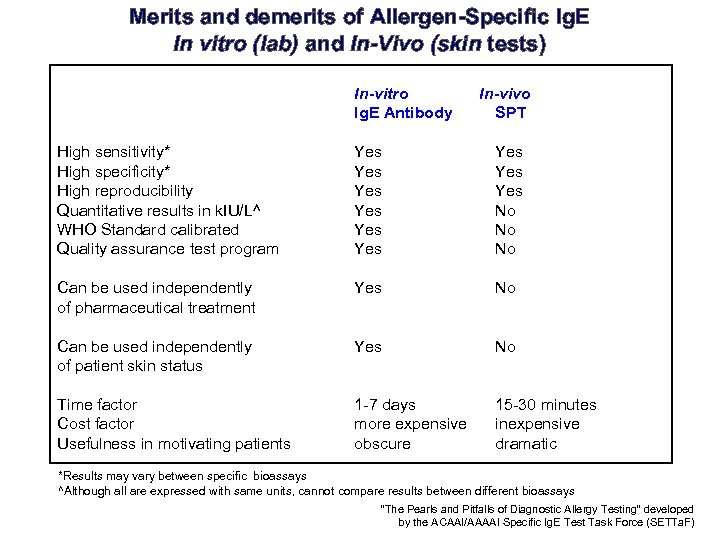
Merits and demerits of Allergen-Specific Ig. E In vitro (lab) and In-Vivo (skin tests) In-vitro Ig. E Antibody In-vivo SPT High sensitivity* High specificity* High reproducibility Quantitative results in k. IU/L^ WHO Standard calibrated Quality assurance test program Yes Yes Yes No No No Can be used independently of pharmaceutical treatment Yes No Can be used independently of patient skin status Yes No Time factor Cost factor Usefulness in motivating patients 1 -7 days more expensive obscure 15 -30 minutes inexpensive dramatic *Results may vary between specific bioassays ^Although all are expressed with same units, cannot compare results between different bioassays “The Pearls and Pitfalls of Diagnostic Allergy Testing” developed by the ACAAI/AAAAI Specific Ig. E Test Task Force (SETTa. F)
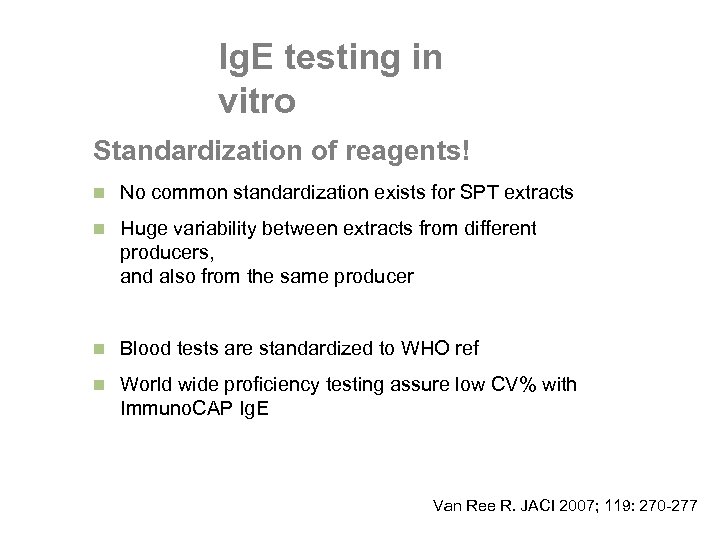
Ig. E testing in vitro Standardization of reagents! n No common standardization exists for SPT extracts n Huge variability between extracts from different producers, and also from the same producer n Blood tests are standardized to WHO ref n World wide proficiency testing assure low CV% with Immuno. CAP Ig. E Van Ree R. JACI 2007; 119: 270 -277
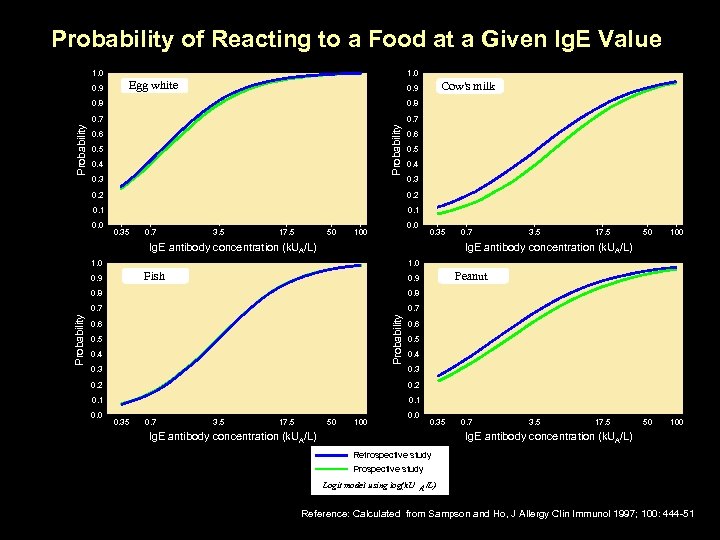
Probability of Reacting to a Food at a Given Ig. E Value 1. 0 0. 9 1. 0 Egg white 0. 8 0. 7 Probability 0. 8 Probability Cow's milk 0. 9 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0. 35 0. 7 3. 5 17. 5 50 0. 0 100 0. 35 Ig. E antibody concentration (k. UA/L) 3. 5 17. 5 50 100 Ig. E antibody concentration (k. UA/L) 1. 0 Fish 0. 9 Peanut 0. 9 0. 8 0. 7 Probability 0. 8 Probability 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0. 35 0. 7 3. 5 17. 5 50 100 0. 35 Ig. E antibody concentration (k. UA/L) 0. 7 3. 5 17. 5 Ig. E antibody concentration (k. UA/L) Retrospective study Prospective study Logit model using log(k. U A /L) Reference: Calculated from Sampson and Ho, J Allergy Clin Immunol 1997; 100: 444 -51
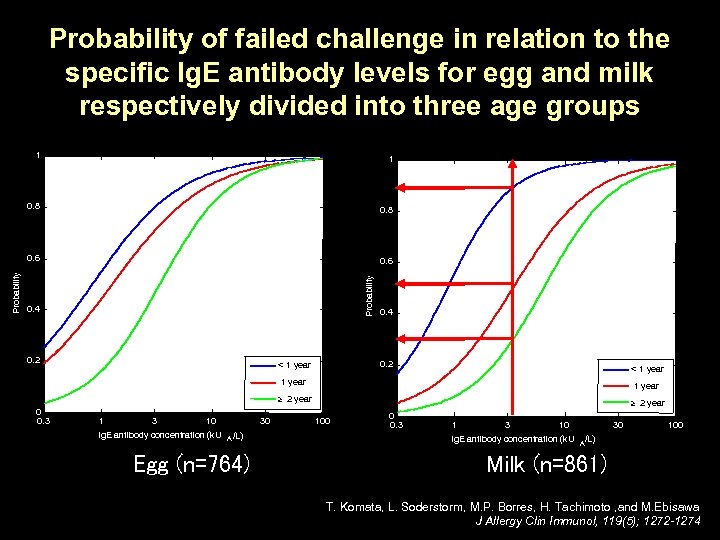
Probability of failed challenge in relation to the specific Ig. E antibody levels for egg and milk respectively divided into three age groups 0. 8 0. 6 Probability 1 0. 8 Probability 1 0. 4 0. 2 < 1 year ³ 2 year 0 0. 3 10 Ig. E antibody concentration (k. U 30 A /L) Egg (n=764) ³ 2 year 100 0 0. 3 10 Ig. E antibody concentration (k. U 30 A 100 /L) Milk (n=861) T. Komata, L. Soderstorm, M. P. Borres, H. Tachimoto , and M. Ebisawa J Allergy Clin Immunol, 119(5); 1272 -1274
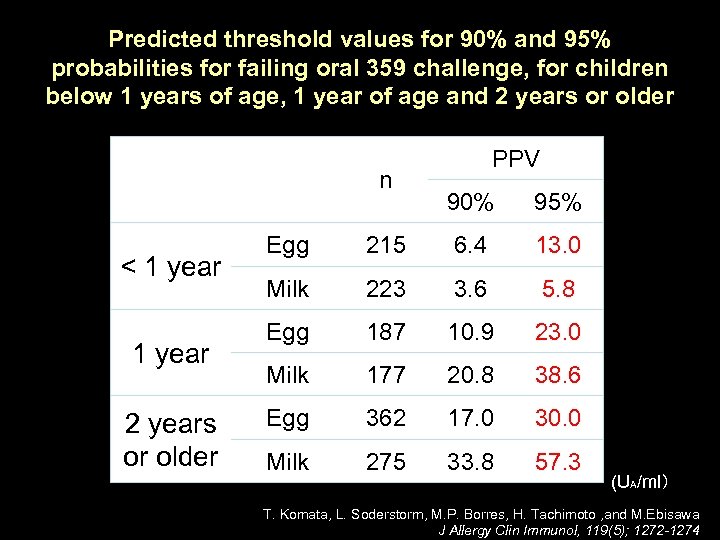
Predicted threshold values for 90% and 95% probabilities for failing oral 359 challenge, for children below 1 years of age, 1 year of age and 2 years or older n < 1 year 2 years or older PPV 90% 95% Egg 215 6. 4 13. 0 Milk 223 3. 6 5. 8 Egg 187 10. 9 23. 0 Milk 177 20. 8 38. 6 Egg 362 17. 0 30. 0 Milk 275 33. 8 57. 3 (UA/ml) T. Komata, L. Soderstorm, M. P. Borres, H. Tachimoto , and M. Ebisawa J Allergy Clin Immunol, 119(5); 1272 -1274
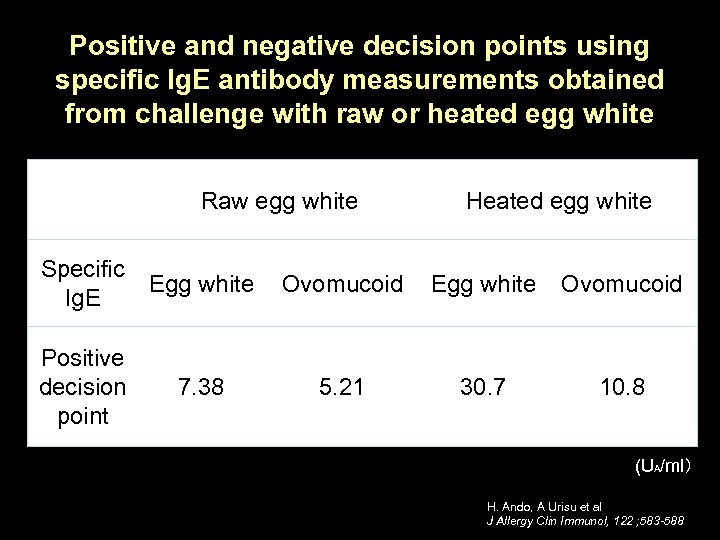
Positive and negative decision points using specific Ig. E antibody measurements obtained from challenge with raw or heated egg white Raw egg white Specific Egg white Ig. E Positive decision point 7. 38 Heated egg white Ovomucoid Egg white Ovomucoid 5. 21 30. 7 10. 8 (UA/ml) H. Ando, A Urisu et al J Allergy Clin Immunol, 122 ; 583 -588
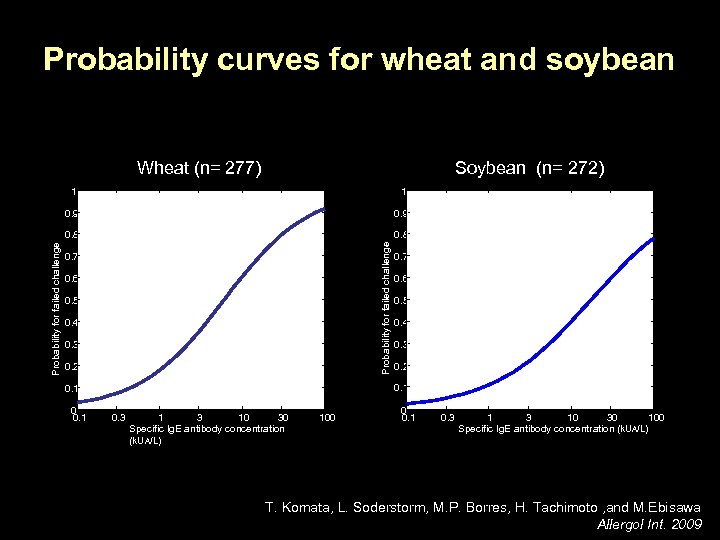
Probability curves for wheat and soybean Wheat (n= 277) Soybean (n= 272) 0. 9 0. 8 Probability for failed challenge 1 0. 9 Probability for failed challenge 1 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 7 0. 3 10 30 Specific Ig. E antibody concentration (k. UA/L) 100 0 0. 1 0. 3 10 30 100 Specific Ig. E antibody concentration (k. UA/L) T. Komata, L. Soderstorm, M. P. Borres, H. Tachimoto , and M. Ebisawa Allergol Int. 2009
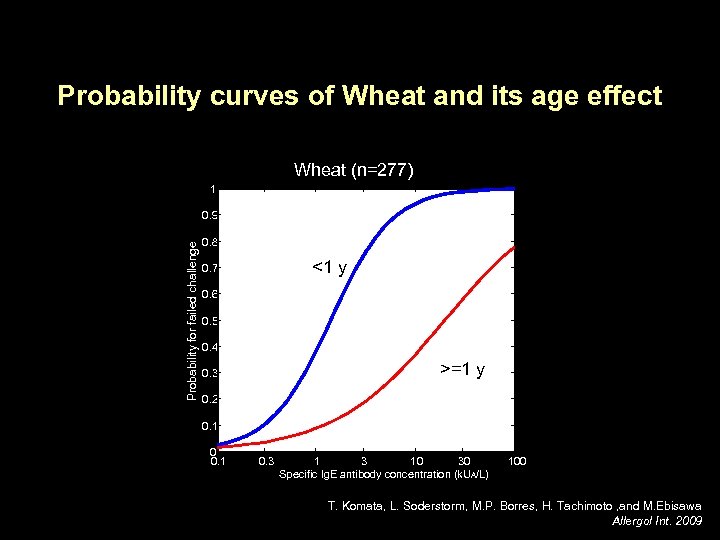
Probability curves of Wheat and its age effect Wheat (n=277) 1 Probability for failed challenge 0. 9 0. 8 <1 y 0. 7 0. 6 0. 5 0. 4 >=1 y 0. 3 0. 2 0. 1 0. 3 10 30 Specific Ig. E antibody concentration (k. UA/L) 100 T. Komata, L. Soderstorm, M. P. Borres, H. Tachimoto , and M. Ebisawa Allergol Int. 2009
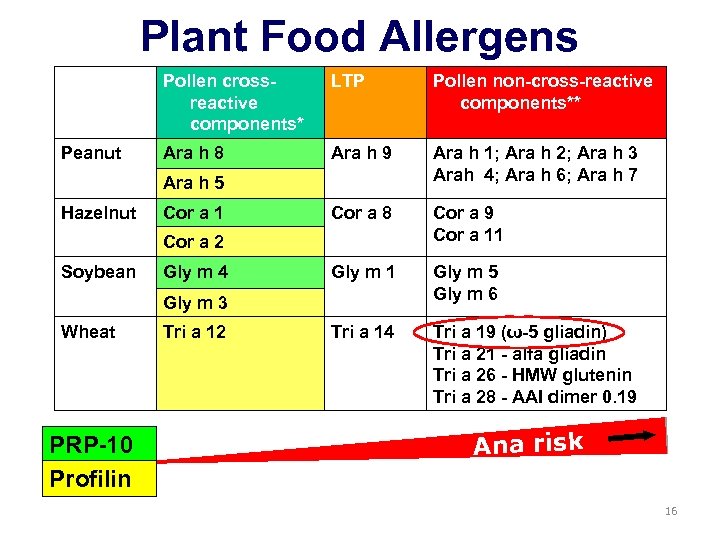
Plant Food Allergens Pollen crossreactive components* Peanut LTP Pollen non-cross-reactive components** Ara h 8 Ara h 9 Ara h 1; Ara h 2; Ara h 3 Arah 4; Ara h 6; Ara h 7 Cor a 8 Cor a 9 Cor a 11 Gly m 5 Gly m 6 Tri a 14 Tri a 19 (ω-5 gliadin) Tri a 21 - alfa gliadin Tri a 26 - HMW glutenin Tri a 28 - AAI dimer 0. 19 Ara h 5 Hazelnut Cor a 1 Cor a 2 Soybean Gly m 4 Gly m 3 Wheat PRP-10 Profilin Tri a 12 Ana risk 16
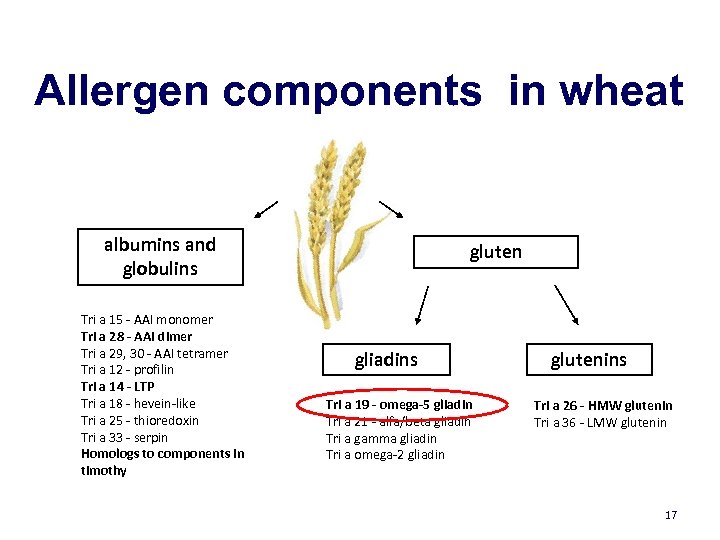
Allergen components in wheat albumins and globulins Tri a 15 - AAI monomer Tri a 28 - AAI dimer Tri a 29, 30 - AAI tetramer Tri a 12 - profilin Tri a 14 - LTP Tri a 18 - hevein-like Tri a 25 - thioredoxin Tri a 33 - serpin Homologs to components in timothy gluten gliadins Tri a 19 - omega-5 gliadin Tri a 21 - alfa/beta gliadin Tri a gamma gliadin Tri a omega-2 gliadin glutenins Tri a 26 - HMW glutenin Tri a 36 - LMW glutenin 17

Subjects and Methods • 343 patients with suspected wheat allergy from 3 different hospitals in Japan. • Age range: 6 months - 20. 4 years. Median age: 2. 3 years old. Oral wheat challenge were performed for 339 children. Four children were included with a recent convincing case history of anaphylaxis in relation to wheat. Ebisawa M, Söderström L, Ito K, Shibata R, Sato S, Tanaka A, Borres M and Morita E , EAACI ’ 09
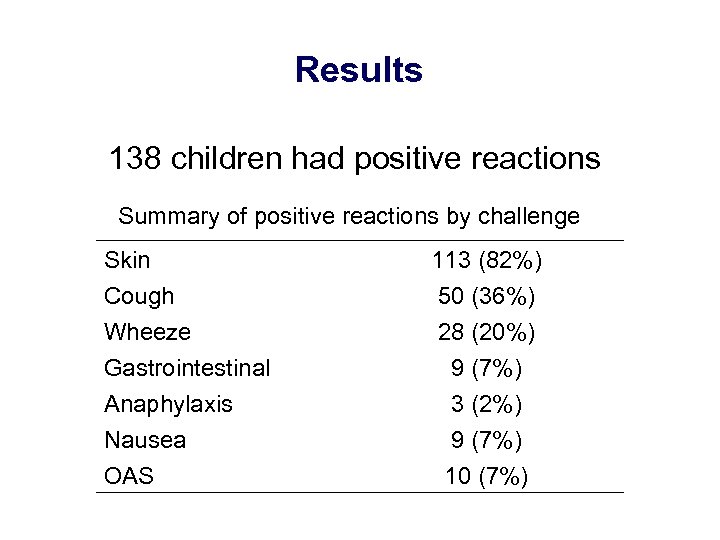
Results 138 children had positive reactions Summary of positive reactions by challenge Skin Cough Wheeze Gastrointestinal Anaphylaxis Nausea OAS 113 (82%) 50 (36%) 28 (20%) 9 (7%) 3 (2%) 9 (7%) 10 (7%)
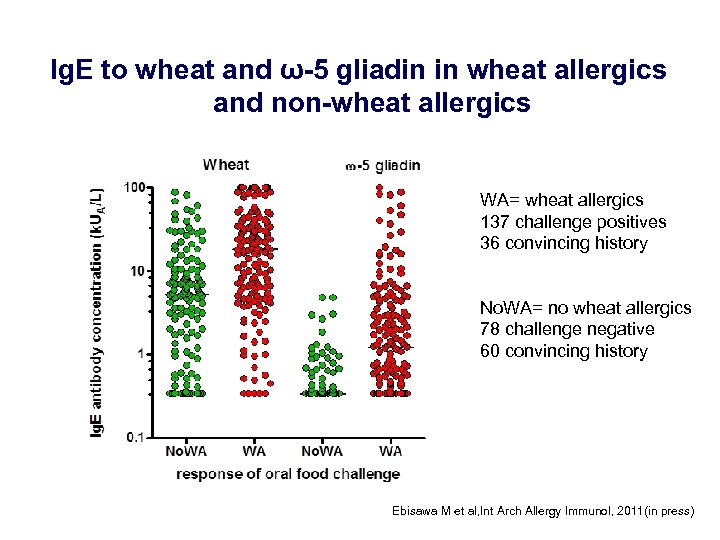
Ig. E to wheat and ω-5 gliadin in wheat allergics and non-wheat allergics WA= wheat allergics 137 challenge positives 36 convincing history No. WA= no wheat allergics 78 challenge negative 60 convincing history Ebisawa M et al, Int Arch Allergy Immunol, 2011(in press)
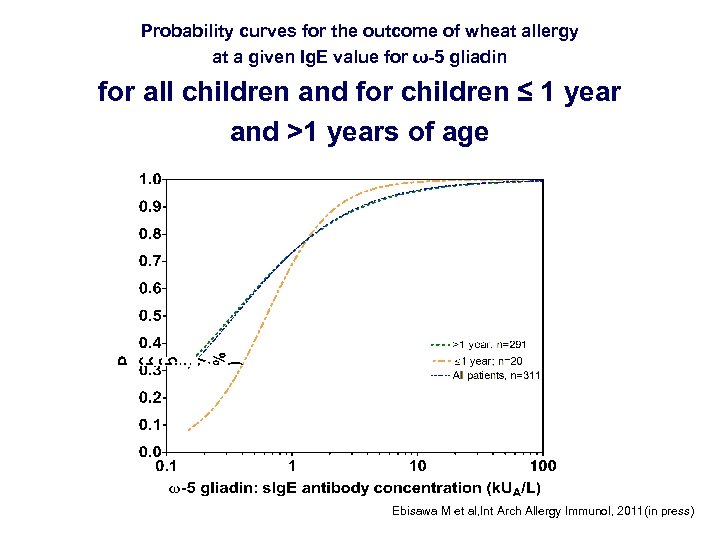
Probability curves for the outcome of wheat allergy at a given Ig. E value for ω-5 gliadin for all children and for children ≤ 1 year and >1 years of age Ebisawa M et al, Int Arch Allergy Immunol, 2011(in press)
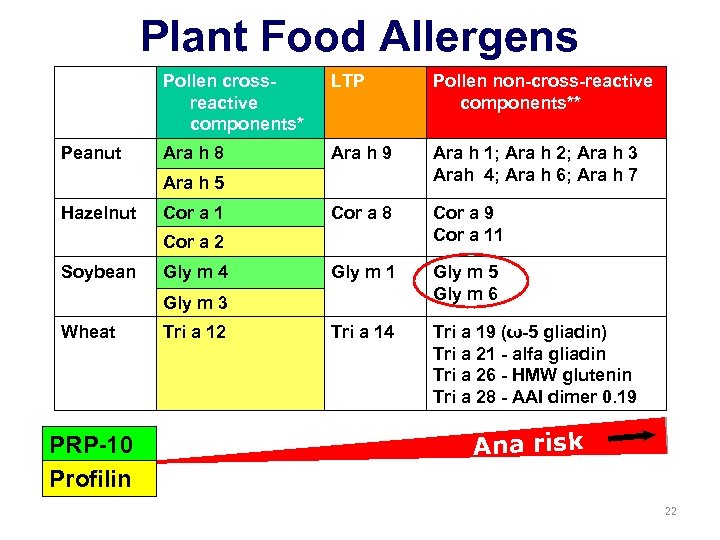
Plant Food Allergens Pollen crossreactive components* Peanut LTP Pollen non-cross-reactive components** Ara h 8 Ara h 9 Ara h 1; Ara h 2; Ara h 3 Arah 4; Ara h 6; Ara h 7 Cor a 8 Cor a 9 Cor a 11 Gly m 5 Gly m 6 Tri a 14 Tri a 19 (ω-5 gliadin) Tri a 21 - alfa gliadin Tri a 26 - HMW glutenin Tri a 28 - AAI dimer 0. 19 Ara h 5 Hazelnut Cor a 1 Cor a 2 Soybean Gly m 4 Gly m 3 Wheat PRP-10 Profilin Tri a 12 Ana risk 22
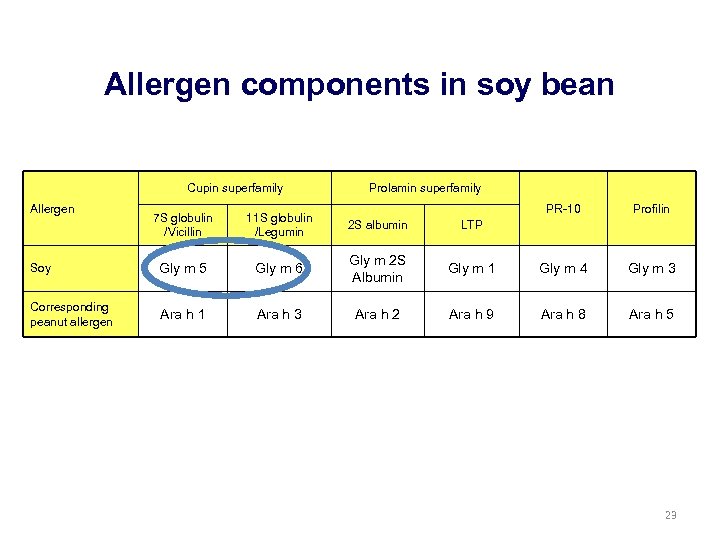
Allergen components in soy bean Cupin superfamily Allergen Prolamin superfamily PR-10 Profilin Gly m 1 Gly m 4 Gly m 3 Ara h 9 Ara h 8 Ara h 5 7 S globulin /Vicillin 11 S globulin /Legumin 2 S albumin LTP Soy Gly m 5 Gly m 6 Gly m 2 S Albumin Corresponding peanut allergen Ara h 1 Ara h 3 Ara h 2 23
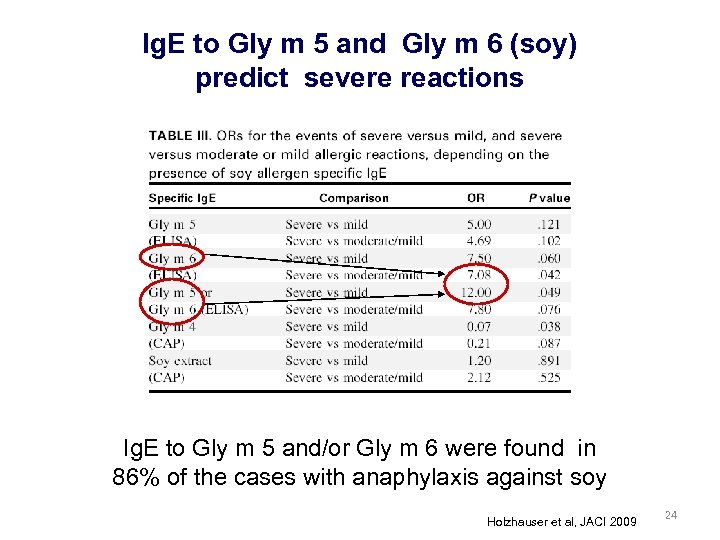
Ig. E to Gly m 5 and Gly m 6 (soy) predict severe reactions Ig. E to Gly m 5 and/or Gly m 6 were found in 86% of the cases with anaphylaxis against soy Holzhauser et al, JACI 2009 24
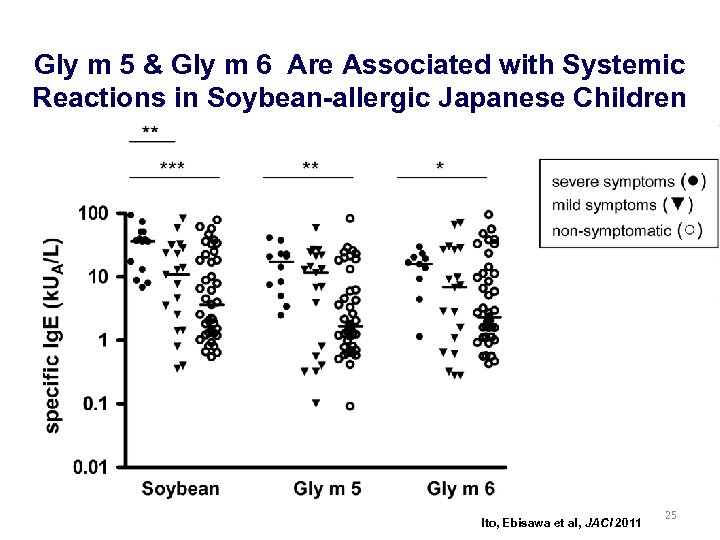
Gly m 5 & Gly m 6 Are Associated with Systemic Reactions in Soybean-allergic Japanese Children Ito, Ebisawa et al, JACI 2011 25
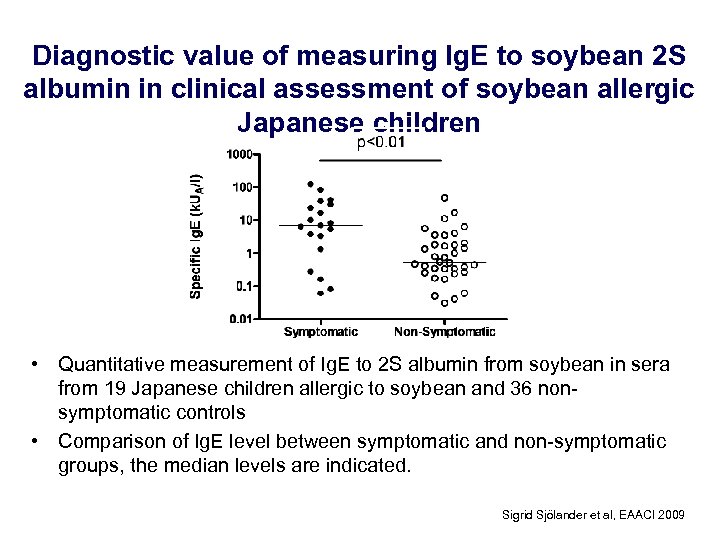
Diagnostic value of measuring Ig. E to soybean 2 S albumin in clinical assessment of soybean allergic Japanese children • Quantitative measurement of Ig. E to 2 S albumin from soybean in sera from 19 Japanese children allergic to soybean and 36 nonsymptomatic controls • Comparison of Ig. E level between symptomatic and non-symptomatic groups, the median levels are indicated. Sigrid Sjölander et al, EAACI 2009
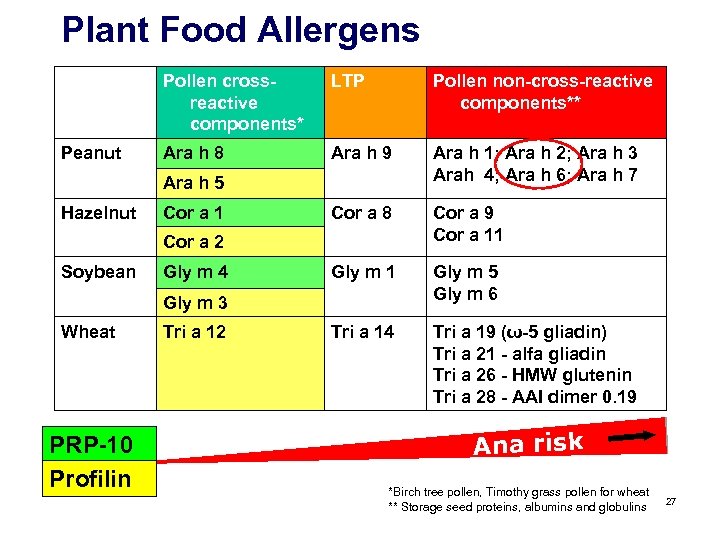
Plant Food Allergens Pollen crossreactive components* Peanut LTP Pollen non-cross-reactive components** Ara h 8 Ara h 9 Ara h 1; Ara h 2; Ara h 3 Arah 4; Ara h 6; Ara h 7 Cor a 8 Cor a 9 Cor a 11 Gly m 5 Gly m 6 Tri a 14 Tri a 19 (ω-5 gliadin) Tri a 21 - alfa gliadin Tri a 26 - HMW glutenin Tri a 28 - AAI dimer 0. 19 Ara h 5 Hazelnut Cor a 1 Cor a 2 Soybean Gly m 4 Gly m 3 Wheat PRP-10 Profilin Tri a 12 Ana risk *Birch tree pollen, Timothy grass pollen for wheat ** Storage seed proteins, albumins and globulins 27
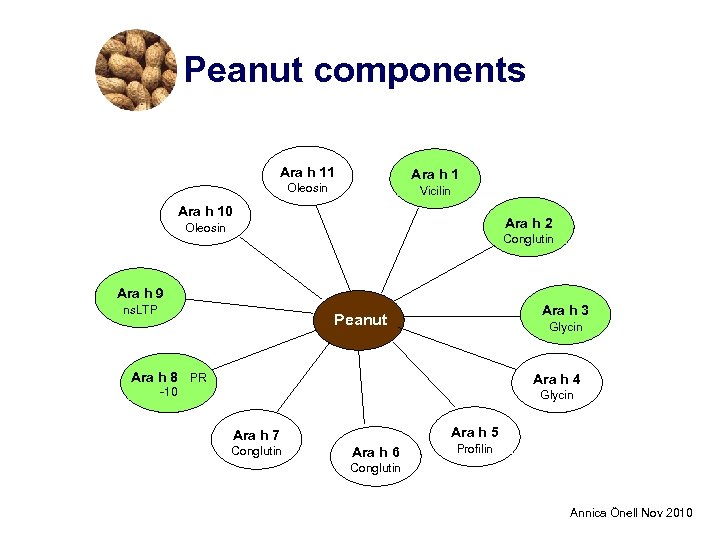
Peanut components Ara h 11 Ara h 1 Oleosin Vicilin Ara h 10 Ara h 2 Oleosin Conglutin Ara h 9 ns. LTP Ara h 3 Peanut Glycin Ara h 8 PR Ara h 4 -10 Glycin Ara h 5 Ara h 7 Conglutin Ara h 6 Profilin Conglutin Annica Önell Nov 2010
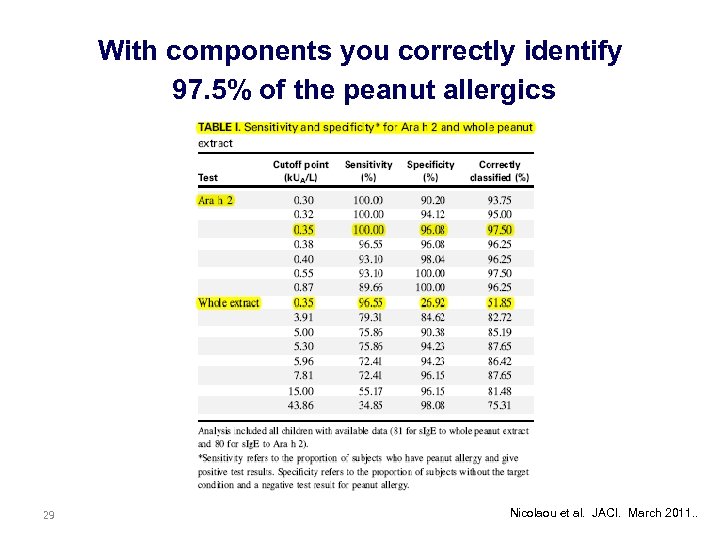
With components you correctly identify 97. 5% of the peanut allergics 29 Nicolaou et al. JACI. March 2011. .
Allergy testing in the 21 st century For primary care by eye or instrument For specialists

Quantitative s. Ig. E reduces the risk and need for oral food challenge n Sampson et al reduced the need with 40% n Österballe et. Allergy Clin Immunol 1997; 100: 444 -51 Sampson and Ho. J al reduced the need Österballe M. with 60%J Allergy Clin Immunol 2003; 112: 196 -201 “Shifted to Immuno CAP from SPT, more and more” by Dr. Hugh Sampson APAPARI 2011, in Fukuoka (Japan)
2b6f27e7f8792679b30d216a93bbe916.ppt