7247aab65aff9c7f82dbd1fb8cd34d83.ppt
- Количество слайдов: 57
 VTE and Cancer The Science and Medicine of Cancer and Thrombosis Management VTE Prophylaxis in the Cancer Patient Scope, Trials, Guidelines and Solutions Samuel Z. Goldhaber, MD Professor of Medicine Harvard Medical School Cardiovascular Division Director, Venous Thromboembolism Research Group Brigham and Women’s Hospital Boston, MA
VTE and Cancer The Science and Medicine of Cancer and Thrombosis Management VTE Prophylaxis in the Cancer Patient Scope, Trials, Guidelines and Solutions Samuel Z. Goldhaber, MD Professor of Medicine Harvard Medical School Cardiovascular Division Director, Venous Thromboembolism Research Group Brigham and Women’s Hospital Boston, MA
 Learning Objectives VTE and Cancer ► Epidemiology/ Scope of the Problem ► Prophylaxis Paradigm Shift ► Surgeon General’s Call To Action ► Medicare’s “Never Events” ► Prophylaxis Modalities ► Electronic, Computerized Alerts ► Human, Physician-to-Physician Alerts ► Guidelines: NCCN, ASCO, ACCP
Learning Objectives VTE and Cancer ► Epidemiology/ Scope of the Problem ► Prophylaxis Paradigm Shift ► Surgeon General’s Call To Action ► Medicare’s “Never Events” ► Prophylaxis Modalities ► Electronic, Computerized Alerts ► Human, Physician-to-Physician Alerts ► Guidelines: NCCN, ASCO, ACCP
 VTE and Cancer Epidemiology: Scope of the Problem
VTE and Cancer Epidemiology: Scope of the Problem
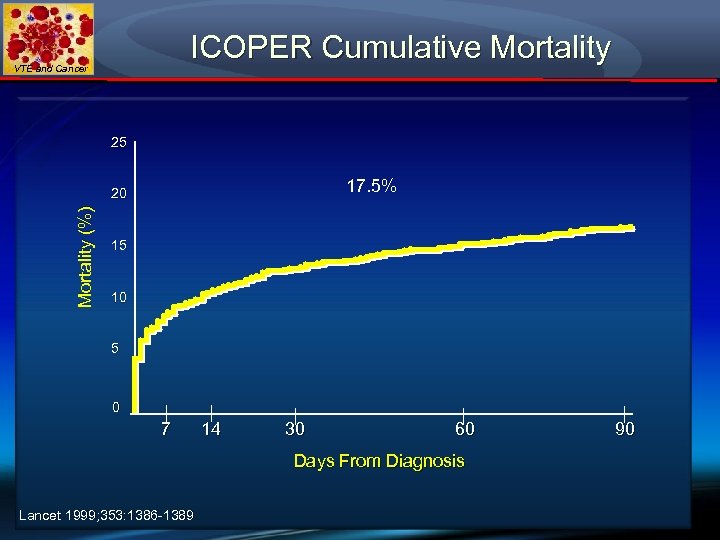 ICOPER Cumulative Mortality VTE and Cancer 25 17. 5% Mortality (%) 20 15 10 5 0 7 14 30 60 Days From Diagnosis Lancet 1999; 353: 1386 -1389 90
ICOPER Cumulative Mortality VTE and Cancer 25 17. 5% Mortality (%) 20 15 10 5 0 7 14 30 60 Days From Diagnosis Lancet 1999; 353: 1386 -1389 90
 VTE and Cancer
VTE and Cancer
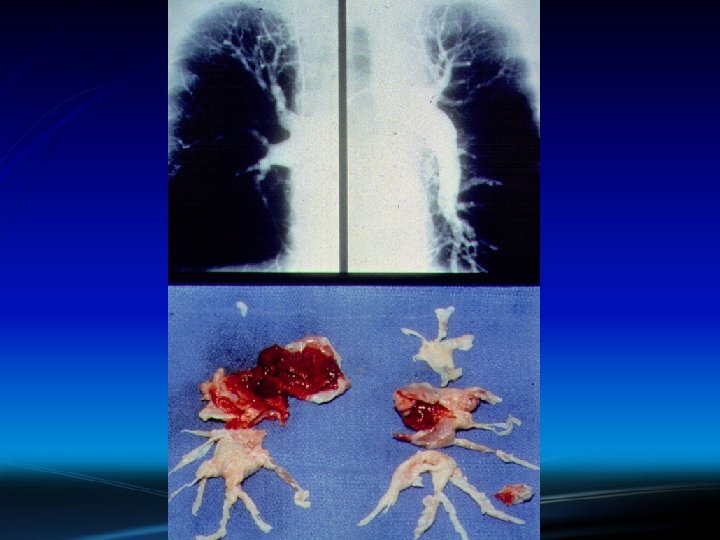
 At-Risk for VTE and Cancer ► The high death rate from PE (exceeding acute MI!) and the high frequency of undiagnosed PE causing “sudden cardiac death” emphasize the need for improved preventive efforts. ► Failure to institute prophylaxis is a much bigger problem with Medical Service patients than Surgical Service patients.
At-Risk for VTE and Cancer ► The high death rate from PE (exceeding acute MI!) and the high frequency of undiagnosed PE causing “sudden cardiac death” emphasize the need for improved preventive efforts. ► Failure to institute prophylaxis is a much bigger problem with Medical Service patients than Surgical Service patients.
 At-Risk for VTE and Cancer ► Two quality improvement initiatives show that among at-risk-for-VTE Medical Service patients, Medical Oncology patients are the least likely group to receive VTE prophylaxis. ► 80% of omitted prophylaxis on Medical Services occurred in Medical Oncology patients.
At-Risk for VTE and Cancer ► Two quality improvement initiatives show that among at-risk-for-VTE Medical Service patients, Medical Oncology patients are the least likely group to receive VTE prophylaxis. ► 80% of omitted prophylaxis on Medical Services occurred in Medical Oncology patients.
 VTE and Cancer Annual At-Risk for VTE: U. S. Hospitals ► 7. 7 million Medical Service inpatients ► 3. 4 million Surgical Service inpatients ► Based upon ACCP guidelines for VTE prophylaxis Anderson FA Jr, et al. Am J Hematol; 2007; 82: 777 -782
VTE and Cancer Annual At-Risk for VTE: U. S. Hospitals ► 7. 7 million Medical Service inpatients ► 3. 4 million Surgical Service inpatients ► Based upon ACCP guidelines for VTE prophylaxis Anderson FA Jr, et al. Am J Hematol; 2007; 82: 777 -782
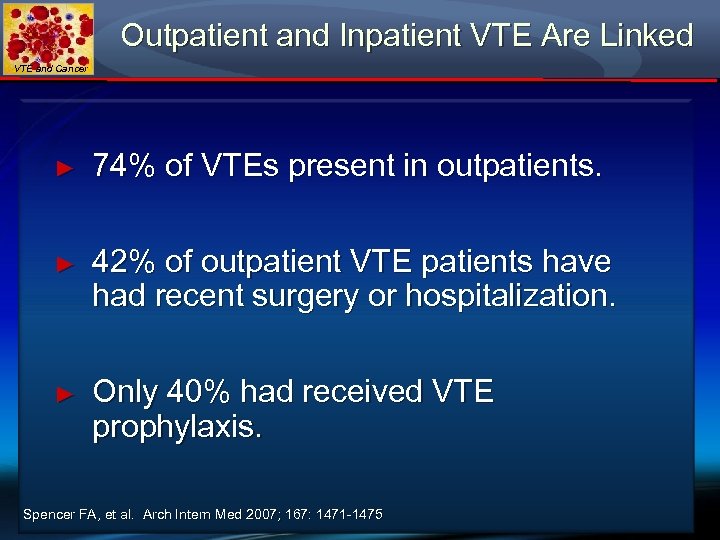 Outpatient and Inpatient VTE Are Linked VTE and Cancer ► 74% of VTEs present in outpatients. ► 42% of outpatient VTE patients have had recent surgery or hospitalization. ► Only 40% had received VTE prophylaxis. Spencer FA, et al. Arch Intern Med 2007; 167: 1471 -1475
Outpatient and Inpatient VTE Are Linked VTE and Cancer ► 74% of VTEs present in outpatients. ► 42% of outpatient VTE patients have had recent surgery or hospitalization. ► Only 40% had received VTE prophylaxis. Spencer FA, et al. Arch Intern Med 2007; 167: 1471 -1475
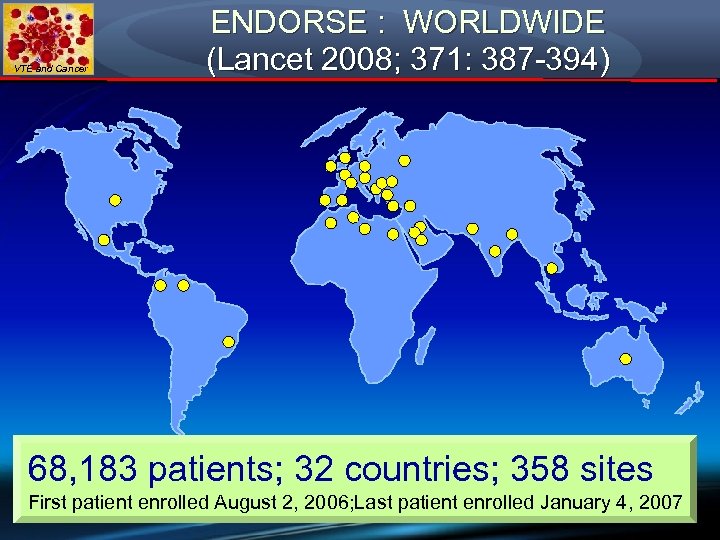 VTE and Cancer ENDORSE : WORLDWIDE (Lancet 2008; 371: 387 -394) 68, 183 patients; 32 countries; 358 sites First patient enrolled August 2, 2006; Last patient enrolled January 4, 2007
VTE and Cancer ENDORSE : WORLDWIDE (Lancet 2008; 371: 387 -394) 68, 183 patients; 32 countries; 358 sites First patient enrolled August 2, 2006; Last patient enrolled January 4, 2007
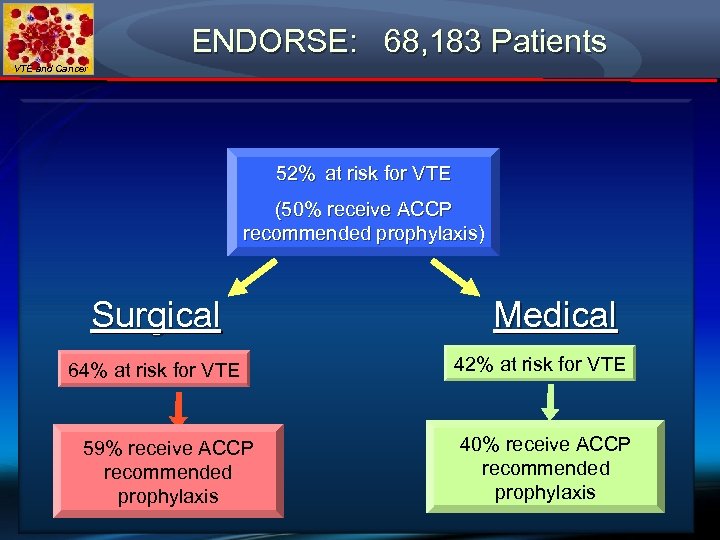 ENDORSE: 68, 183 Patients VTE and Cancer 52% at risk for VTE (50% receive ACCP recommended prophylaxis) Surgical 64% at risk for VTE 59% receive ACCP recommended prophylaxis Medical 42% at risk for VTE 40% receive ACCP recommended prophylaxis
ENDORSE: 68, 183 Patients VTE and Cancer 52% at risk for VTE (50% receive ACCP recommended prophylaxis) Surgical 64% at risk for VTE 59% receive ACCP recommended prophylaxis Medical 42% at risk for VTE 40% receive ACCP recommended prophylaxis
 VTE and Cancer VTE Prophylaxis Paradigm Shift Cancer and Medical Conditions in the Crosshairs
VTE and Cancer VTE Prophylaxis Paradigm Shift Cancer and Medical Conditions in the Crosshairs
 Ten Years Ago… VTE and Cancer ► Most Americans had not heard of DVT (deep vein thrombosis) or PE (pulmonary embolism) ► Virtually no awareness ► Media attention was limited to featuring a few celebrities who were stricken ► No state or congressional resolutions ► No patient advocacy ► No Medicare input
Ten Years Ago… VTE and Cancer ► Most Americans had not heard of DVT (deep vein thrombosis) or PE (pulmonary embolism) ► Virtually no awareness ► Media attention was limited to featuring a few celebrities who were stricken ► No state or congressional resolutions ► No patient advocacy ► No Medicare input
 VTE Awareness in 2009 VTE and Cancer ► Growing interest in VTE’s public health threat ► Known as the most preventable illness in hospitalized patients ► Publicity is increasing among health care professionals and the public ► Patient advocacy is a reality ► Congress and most States have adopted months for “Thrombosis Awareness” ► Medicare has declared certain DVTs or PEs as “Never Events” and will not reimburse
VTE Awareness in 2009 VTE and Cancer ► Growing interest in VTE’s public health threat ► Known as the most preventable illness in hospitalized patients ► Publicity is increasing among health care professionals and the public ► Patient advocacy is a reality ► Congress and most States have adopted months for “Thrombosis Awareness” ► Medicare has declared certain DVTs or PEs as “Never Events” and will not reimburse
 Old Prophylaxis Paradigm VTE and Cancer ► MD individualizes prophylaxis prescription and ultimately has complete “yes” or “no” authority to prescribe or withhold prophylaxis ► Hospital, government auditors, patients, and families do not challenge the MD’s decision to withhold prophylaxis. Instead, they “defer to the physician’s medical judgment”
Old Prophylaxis Paradigm VTE and Cancer ► MD individualizes prophylaxis prescription and ultimately has complete “yes” or “no” authority to prescribe or withhold prophylaxis ► Hospital, government auditors, patients, and families do not challenge the MD’s decision to withhold prophylaxis. Instead, they “defer to the physician’s medical judgment”
 New Prophylaxis Paradigm VTE and Cancer ► Hospital monitors VTE prophylaxis prescribing and insists upon guideline-based practice ► Electronic reminders and automated electronic orders ultimately ensure appropriate prophylaxis for at-risk patients ► Hospital’s financial and medicolegal penalty for failure to prophylax may be “passed on” to the responsible attending physician ► Cancer patients represent high-risk, “must prophylax” subgroup
New Prophylaxis Paradigm VTE and Cancer ► Hospital monitors VTE prophylaxis prescribing and insists upon guideline-based practice ► Electronic reminders and automated electronic orders ultimately ensure appropriate prophylaxis for at-risk patients ► Hospital’s financial and medicolegal penalty for failure to prophylax may be “passed on” to the responsible attending physician ► Cancer patients represent high-risk, “must prophylax” subgroup
 VTE and Cancer SURGEON GENERAL: CALL TO ACTION TO PREVENT DVT AND PE September 15, 2008
VTE and Cancer SURGEON GENERAL: CALL TO ACTION TO PREVENT DVT AND PE September 15, 2008
 VTE and Cancer Medicare’s “Never Events”
VTE and Cancer Medicare’s “Never Events”
 Medicare’s “Never Events” VTE and Cancer Medicare’s most recent strategy to reduce medical errors is to withhold payment to hospitals for treatment of serious preventable illnesses or complications termed “never events. ” The initial 3 were: 1) 2) 3) Foreign object retained postop Air embolism removing CVC Blood transfusion incompatibility
Medicare’s “Never Events” VTE and Cancer Medicare’s most recent strategy to reduce medical errors is to withhold payment to hospitals for treatment of serious preventable illnesses or complications termed “never events. ” The initial 3 were: 1) 2) 3) Foreign object retained postop Air embolism removing CVC Blood transfusion incompatibility
 Medicare’s “Never Events” VTE and Cancer ► On October 1, 2008, Medicare added: ► DVT or pulmonary embolism occurring after total knee or hip replacement. ► Medicare will not pay the incremental cost to manage the complication. Nor will the patient be responsible. ► The hospital will bear the additional financial burden.
Medicare’s “Never Events” VTE and Cancer ► On October 1, 2008, Medicare added: ► DVT or pulmonary embolism occurring after total knee or hip replacement. ► Medicare will not pay the incremental cost to manage the complication. Nor will the patient be responsible. ► The hospital will bear the additional financial burden.
 VTE and Cancer Prophylaxis Modalities
VTE and Cancer Prophylaxis Modalities
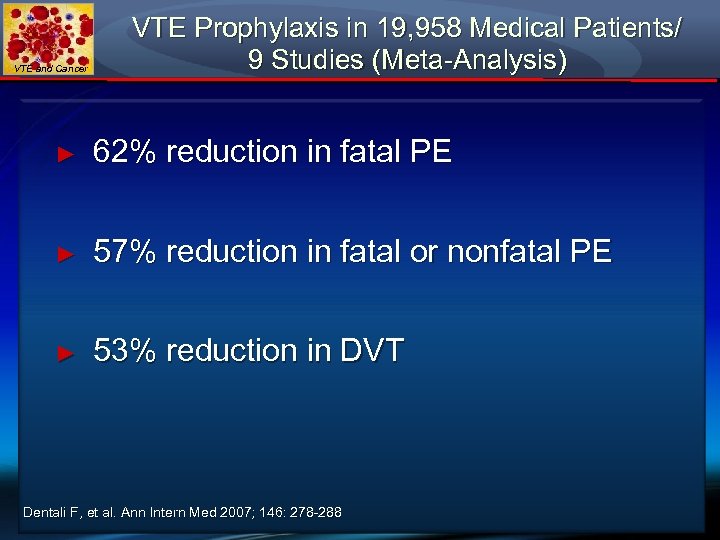 VTE and Cancer VTE Prophylaxis in 19, 958 Medical Patients/ 9 Studies (Meta-Analysis) ► 62% reduction in fatal PE ► 57% reduction in fatal or nonfatal PE ► 53% reduction in DVT Dentali F, et al. Ann Intern Med 2007; 146: 278 -288
VTE and Cancer VTE Prophylaxis in 19, 958 Medical Patients/ 9 Studies (Meta-Analysis) ► 62% reduction in fatal PE ► 57% reduction in fatal or nonfatal PE ► 53% reduction in DVT Dentali F, et al. Ann Intern Med 2007; 146: 278 -288
 VTE and Cancer VTE Prophylaxis in Medical Patients is Cost-Effective ► $1, 264 per patient for LMWH ► $2, 245 for No Prophylaxis Deitelzweig et al. Thromb Haemostas 2008; 100: 810 -820
VTE and Cancer VTE Prophylaxis in Medical Patients is Cost-Effective ► $1, 264 per patient for LMWH ► $2, 245 for No Prophylaxis Deitelzweig et al. Thromb Haemostas 2008; 100: 810 -820
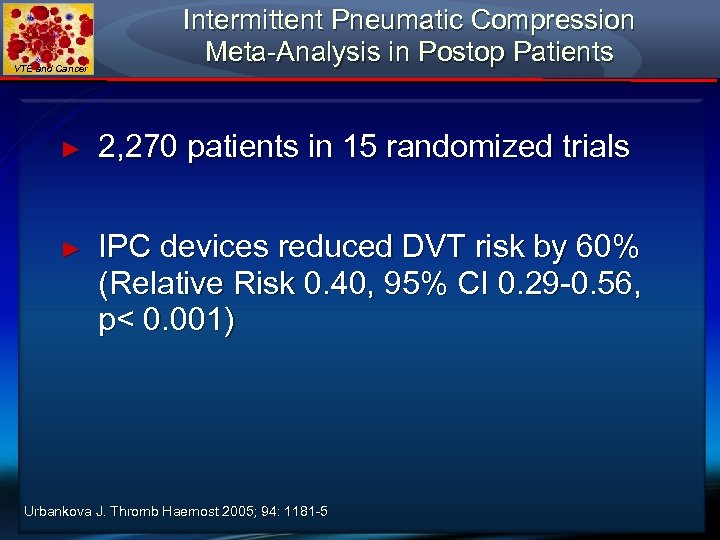 VTE and Cancer Intermittent Pneumatic Compression Meta-Analysis in Postop Patients ► 2, 270 patients in 15 randomized trials ► IPC devices reduced DVT risk by 60% (Relative Risk 0. 40, 95% CI 0. 29 -0. 56, p< 0. 001) Urbankova J. Thromb Haemost 2005; 94: 1181 -5
VTE and Cancer Intermittent Pneumatic Compression Meta-Analysis in Postop Patients ► 2, 270 patients in 15 randomized trials ► IPC devices reduced DVT risk by 60% (Relative Risk 0. 40, 95% CI 0. 29 -0. 56, p< 0. 001) Urbankova J. Thromb Haemost 2005; 94: 1181 -5
 Reversible Risk Factors VTE and Cancer 1. Nutrition: eat fruits, veggies, fish; less red meat (Circulation 2007; 115: 188 -195) 2. Quit cigarettes 3. Lose weight/ exercise 4. Prevent DM/ metabolic syndrome 5. Control hypertension 6. Lower cholesterol 7. Avoid air pollution Arch Intern Med 2008; 168: 920 -927)
Reversible Risk Factors VTE and Cancer 1. Nutrition: eat fruits, veggies, fish; less red meat (Circulation 2007; 115: 188 -195) 2. Quit cigarettes 3. Lose weight/ exercise 4. Prevent DM/ metabolic syndrome 5. Control hypertension 6. Lower cholesterol 7. Avoid air pollution Arch Intern Med 2008; 168: 920 -927)
 VTE and Cancer Statins Prevent PE and DVT!
VTE and Cancer Statins Prevent PE and DVT!
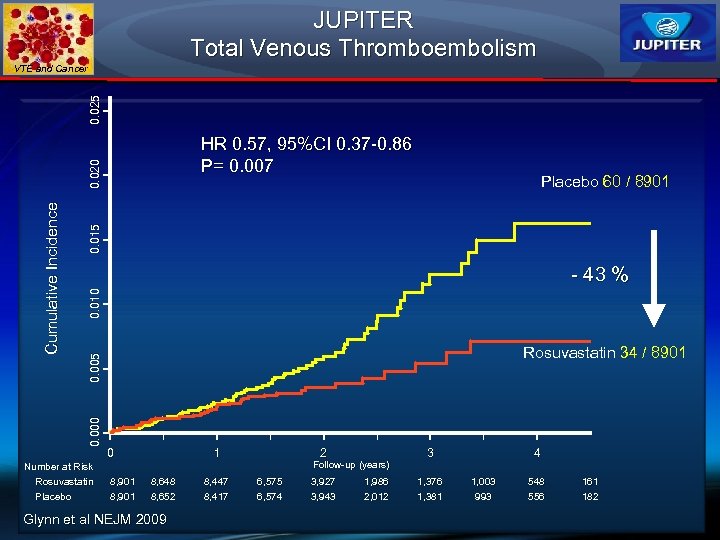 JUPITER Total Venous Thromboembolism 0. 025 VTE and Cancer 0. 015 Placebo 60 / 8901 0. 010 - 43 % Rosuvastatin 34 / 8901 0. 005 0. 000 Cumulative Incidence 0. 020 HR 0. 57, 95%CI 0. 37 -0. 86 P= 0. 007 0 1 2 Follow-up (years) Number at Risk Rosuvastatin Placebo 8, 901 8, 648 8, 652 Glynn et al NEJM 2009 8, 447 8, 417 6, 575 6, 574 3, 927 3, 943 1, 986 2, 012 3 1, 376 1, 381 4 1, 003 993 548 556 161 182
JUPITER Total Venous Thromboembolism 0. 025 VTE and Cancer 0. 015 Placebo 60 / 8901 0. 010 - 43 % Rosuvastatin 34 / 8901 0. 005 0. 000 Cumulative Incidence 0. 020 HR 0. 57, 95%CI 0. 37 -0. 86 P= 0. 007 0 1 2 Follow-up (years) Number at Risk Rosuvastatin Placebo 8, 901 8, 648 8, 652 Glynn et al NEJM 2009 8, 447 8, 417 6, 575 6, 574 3, 927 3, 943 1, 986 2, 012 3 1, 376 1, 381 4 1, 003 993 548 556 161 182
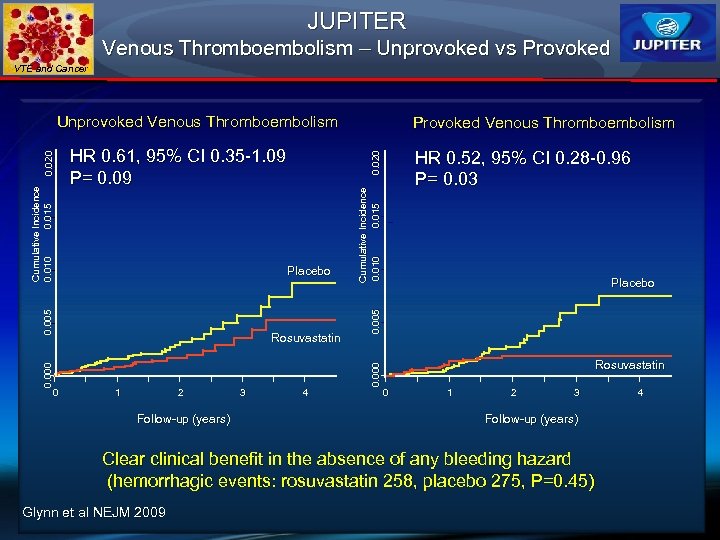 JUPITER Venous Thromboembolism – Unprovoked vs Provoked VTE and Cancer Rosuvastatin 1 2 Follow-up (years) 3 HR 0. 52, 95% CI 0. 28 -0. 96 P= 0. 03 4 Cumulative Incidence 0. 010 0. 015 Placebo 0. 000 0 Provoked Venous Thromboembolism 0. 020 HR 0. 61, 95% CI 0. 35 -1. 09 P= 0. 09 0. 005 Cumulative Incidence 0. 010 0. 015 0. 020 Unprovoked Venous Thromboembolism Rosuvastatin 0 1 2 3 Follow-up (years) Clear clinical benefit in the absence of any bleeding hazard (hemorrhagic events: rosuvastatin 258, placebo 275, P=0. 45) Glynn et al NEJM 2009 4
JUPITER Venous Thromboembolism – Unprovoked vs Provoked VTE and Cancer Rosuvastatin 1 2 Follow-up (years) 3 HR 0. 52, 95% CI 0. 28 -0. 96 P= 0. 03 4 Cumulative Incidence 0. 010 0. 015 Placebo 0. 000 0 Provoked Venous Thromboembolism 0. 020 HR 0. 61, 95% CI 0. 35 -1. 09 P= 0. 09 0. 005 Cumulative Incidence 0. 010 0. 015 0. 020 Unprovoked Venous Thromboembolism Rosuvastatin 0 1 2 3 Follow-up (years) Clear clinical benefit in the absence of any bleeding hazard (hemorrhagic events: rosuvastatin 258, placebo 275, P=0. 45) Glynn et al NEJM 2009 4
 VTE and Cancer Electronic and “Human” Prophylaxis Alerts Implications for Cancer Patients
VTE and Cancer Electronic and “Human” Prophylaxis Alerts Implications for Cancer Patients
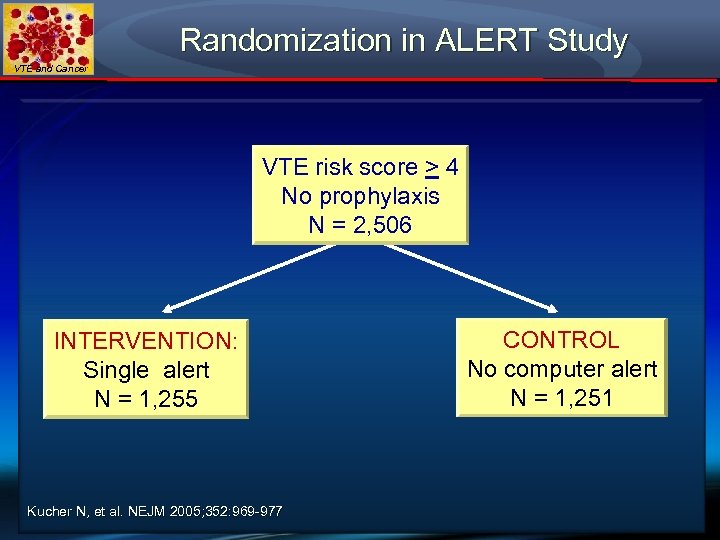 Randomization in ALERT Study VTE and Cancer VTE risk score > 4 No prophylaxis N = 2, 506 INTERVENTION: Single alert N = 1, 255 Kucher N, et al. NEJM 2005; 352: 969 -977 CONTROL No computer alert N = 1, 251
Randomization in ALERT Study VTE and Cancer VTE risk score > 4 No prophylaxis N = 2, 506 INTERVENTION: Single alert N = 1, 255 Kucher N, et al. NEJM 2005; 352: 969 -977 CONTROL No computer alert N = 1, 251
 VTE and Cancer
VTE and Cancer
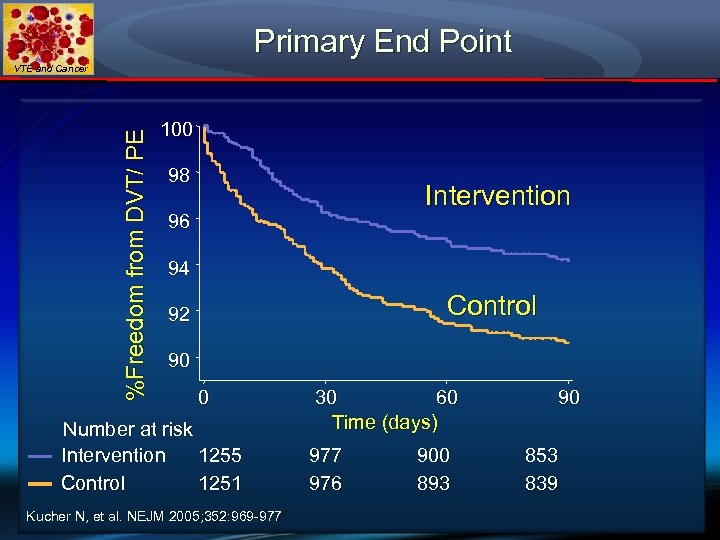 Primary End Point %Freedom from DVT/ PE VTE and Cancer 100 98 Intervention 96 94 Control 92 90 0 Number at risk Intervention 1255 Control 1251 Kucher N, et al. NEJM 2005; 352: 969 -977 30 60 Time (days) 977 976 900 893 90 853 839
Primary End Point %Freedom from DVT/ PE VTE and Cancer 100 98 Intervention 96 94 Control 92 90 0 Number at risk Intervention 1255 Control 1251 Kucher N, et al. NEJM 2005; 352: 969 -977 30 60 Time (days) 977 976 900 893 90 853 839
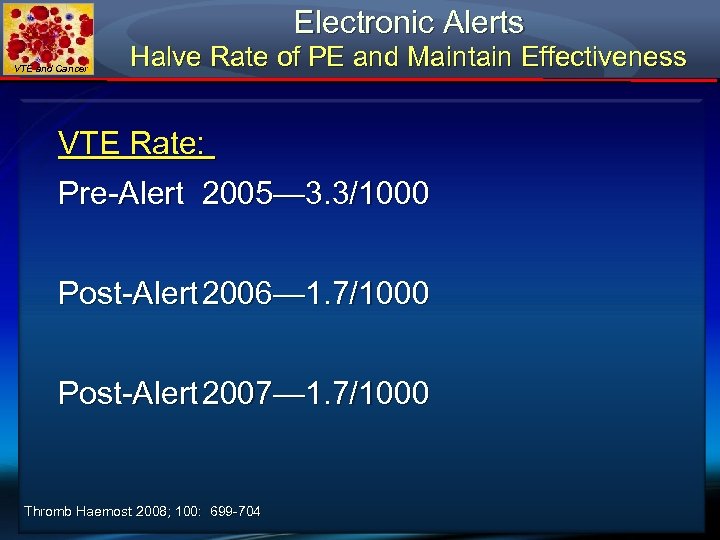 Electronic Alerts VTE and Cancer Halve Rate of PE and Maintain Effectiveness VTE Rate: Pre-Alert 2005— 3. 3/1000 Post-Alert 2006— 1. 7/1000 Post-Alert 2007— 1. 7/1000 Thromb Haemost 2008; 100: 699 -704
Electronic Alerts VTE and Cancer Halve Rate of PE and Maintain Effectiveness VTE Rate: Pre-Alert 2005— 3. 3/1000 Post-Alert 2006— 1. 7/1000 Post-Alert 2007— 1. 7/1000 Thromb Haemost 2008; 100: 699 -704
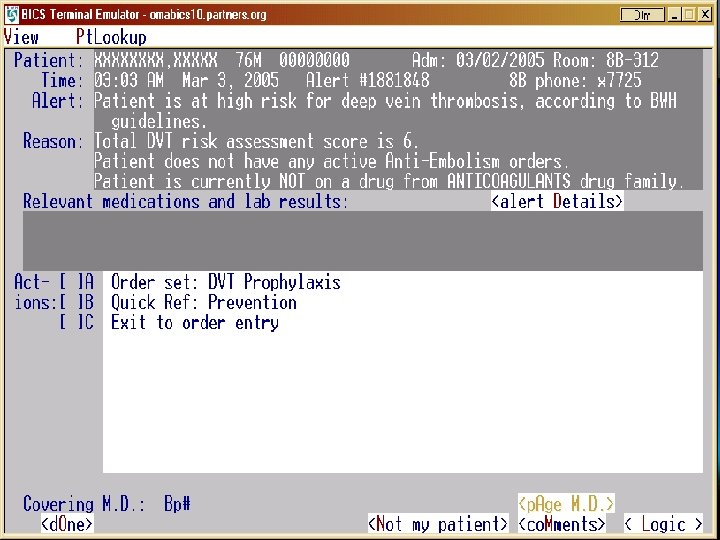
 “Human” Physician Alert VTE and Cancer ► As we planned a multicenter randomized trial applying the electronic alert strategy to a broad array of hospitals across the U. S. , we learned that replication of our electronic alert was not feasible. ► Therefore, we crafted a strategy that employed a “human” rather than electronic alerting system. ► The physician alert consisted of a direct page from a hospital staff member to the Attending Physician. ► The primary end point was reduction in symptomatic VTE within 90 days of randomization.
“Human” Physician Alert VTE and Cancer ► As we planned a multicenter randomized trial applying the electronic alert strategy to a broad array of hospitals across the U. S. , we learned that replication of our electronic alert was not feasible. ► Therefore, we crafted a strategy that employed a “human” rather than electronic alerting system. ► The physician alert consisted of a direct page from a hospital staff member to the Attending Physician. ► The primary end point was reduction in symptomatic VTE within 90 days of randomization.
 Physician Alert: Results VTE and Cancer ► 2493 patients (82% on Medical Services) from 25 study sites were randomized to the intervention (n=1238) versus the control group (n=1255). ► Patients whose physicians were alerted were more than twice as likely to receive VTE prophylaxis (46. 0% versus 20. 6%, p<0. 0001). ► The symptomatic VTE rate was lower in the intervention group (2. 7% versus 3. 4%; hazard ratio, 0. 79; 95% confidence interval, 0. 50 to 1. 25), but the difference did not achieve statistical significance. ► Major bleeding at 30 days in the alert group was similar to the control group.
Physician Alert: Results VTE and Cancer ► 2493 patients (82% on Medical Services) from 25 study sites were randomized to the intervention (n=1238) versus the control group (n=1255). ► Patients whose physicians were alerted were more than twice as likely to receive VTE prophylaxis (46. 0% versus 20. 6%, p<0. 0001). ► The symptomatic VTE rate was lower in the intervention group (2. 7% versus 3. 4%; hazard ratio, 0. 79; 95% confidence interval, 0. 50 to 1. 25), but the difference did not achieve statistical significance. ► Major bleeding at 30 days in the alert group was similar to the control group.
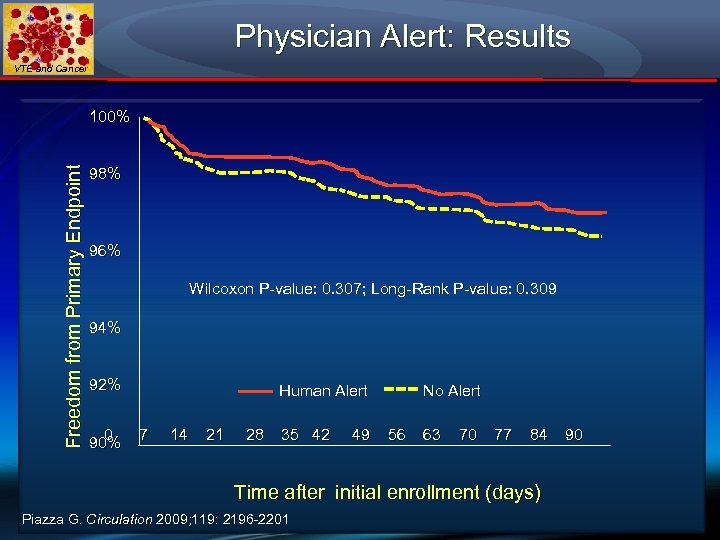 Physician Alert: Results VTE and Cancer Freedom from Primary Endpoint 100% 98% 96% Wilcoxon P-value: 0. 307; Long-Rank P-value: 0. 309 94% 92% 0 90% Human Alert 7 14 21 28 35 42 49 No Alert 56 63 70 77 84 Time after initial enrollment (days) Piazza G. Circulation 2009; 119: 2196 -2201 90
Physician Alert: Results VTE and Cancer Freedom from Primary Endpoint 100% 98% 96% Wilcoxon P-value: 0. 307; Long-Rank P-value: 0. 309 94% 92% 0 90% Human Alert 7 14 21 28 35 42 49 No Alert 56 63 70 77 84 Time after initial enrollment (days) Piazza G. Circulation 2009; 119: 2196 -2201 90
 VTE and Cancer Current Status of ASCO and NCCN Guidelines for VTE Prophylaxis in Cancer Patients
VTE and Cancer Current Status of ASCO and NCCN Guidelines for VTE Prophylaxis in Cancer Patients
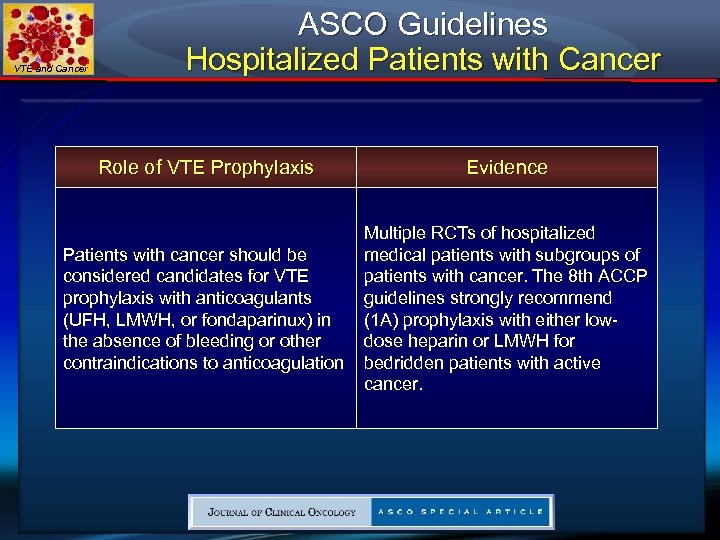 VTE and Cancer ASCO Guidelines Hospitalized Patients with Cancer Role of VTE Prophylaxis Evidence Patients with cancer should be considered candidates for VTE prophylaxis with anticoagulants (UFH, LMWH, or fondaparinux) in the absence of bleeding or other contraindications to anticoagulation Multiple RCTs of hospitalized medical patients with subgroups of patients with cancer. The 8 th ACCP guidelines strongly recommend (1 A) prophylaxis with either lowdose heparin or LMWH for bedridden patients with active cancer.
VTE and Cancer ASCO Guidelines Hospitalized Patients with Cancer Role of VTE Prophylaxis Evidence Patients with cancer should be considered candidates for VTE prophylaxis with anticoagulants (UFH, LMWH, or fondaparinux) in the absence of bleeding or other contraindications to anticoagulation Multiple RCTs of hospitalized medical patients with subgroups of patients with cancer. The 8 th ACCP guidelines strongly recommend (1 A) prophylaxis with either lowdose heparin or LMWH for bedridden patients with active cancer.
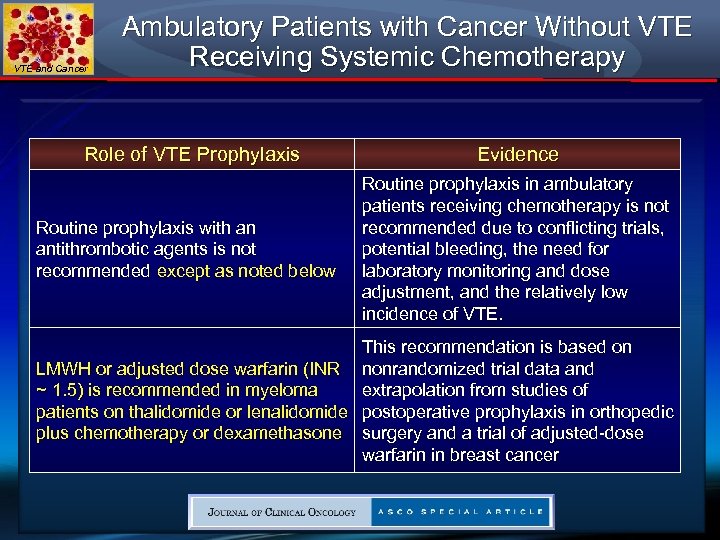 VTE and Cancer Ambulatory Patients with Cancer Without VTE Receiving Systemic Chemotherapy Role of VTE Prophylaxis Routine prophylaxis with an antithrombotic agents is not recommended except as noted below Evidence Routine prophylaxis in ambulatory patients receiving chemotherapy is not recommended due to conflicting trials, potential bleeding, the need for laboratory monitoring and dose adjustment, and the relatively low incidence of VTE. This recommendation is based on LMWH or adjusted dose warfarin (INR nonrandomized trial data and extrapolation from studies of ~ 1. 5) is recommended in myeloma patients on thalidomide or lenalidomide postoperative prophylaxis in orthopedic plus chemotherapy or dexamethasone surgery and a trial of adjusted-dose warfarin in breast cancer
VTE and Cancer Ambulatory Patients with Cancer Without VTE Receiving Systemic Chemotherapy Role of VTE Prophylaxis Routine prophylaxis with an antithrombotic agents is not recommended except as noted below Evidence Routine prophylaxis in ambulatory patients receiving chemotherapy is not recommended due to conflicting trials, potential bleeding, the need for laboratory monitoring and dose adjustment, and the relatively low incidence of VTE. This recommendation is based on LMWH or adjusted dose warfarin (INR nonrandomized trial data and extrapolation from studies of ~ 1. 5) is recommended in myeloma patients on thalidomide or lenalidomide postoperative prophylaxis in orthopedic plus chemotherapy or dexamethasone surgery and a trial of adjusted-dose warfarin in breast cancer
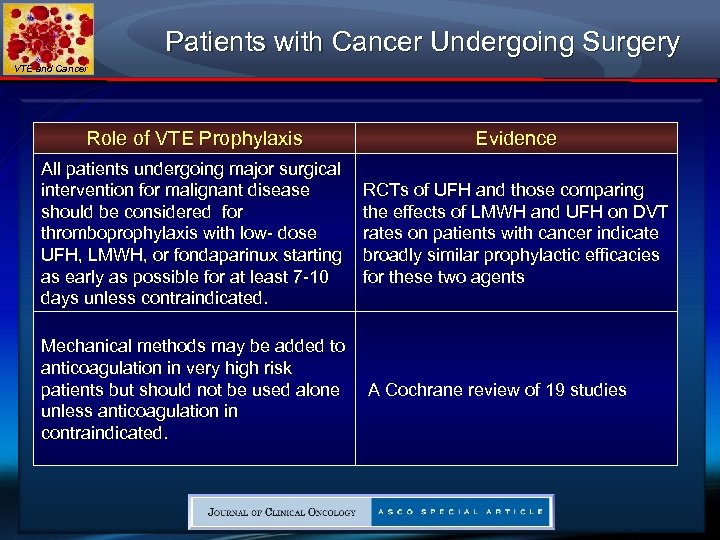 Patients with Cancer Undergoing Surgery VTE and Cancer Role of VTE Prophylaxis Evidence All patients undergoing major surgical intervention for malignant disease should be considered for thromboprophylaxis with low- dose UFH, LMWH, or fondaparinux starting as early as possible for at least 7 -10 days unless contraindicated. RCTs of UFH and those comparing the effects of LMWH and UFH on DVT rates on patients with cancer indicate broadly similar prophylactic efficacies for these two agents Mechanical methods may be added to anticoagulation in very high risk patients but should not be used alone unless anticoagulation in contraindicated. A Cochrane review of 19 studies
Patients with Cancer Undergoing Surgery VTE and Cancer Role of VTE Prophylaxis Evidence All patients undergoing major surgical intervention for malignant disease should be considered for thromboprophylaxis with low- dose UFH, LMWH, or fondaparinux starting as early as possible for at least 7 -10 days unless contraindicated. RCTs of UFH and those comparing the effects of LMWH and UFH on DVT rates on patients with cancer indicate broadly similar prophylactic efficacies for these two agents Mechanical methods may be added to anticoagulation in very high risk patients but should not be used alone unless anticoagulation in contraindicated. A Cochrane review of 19 studies
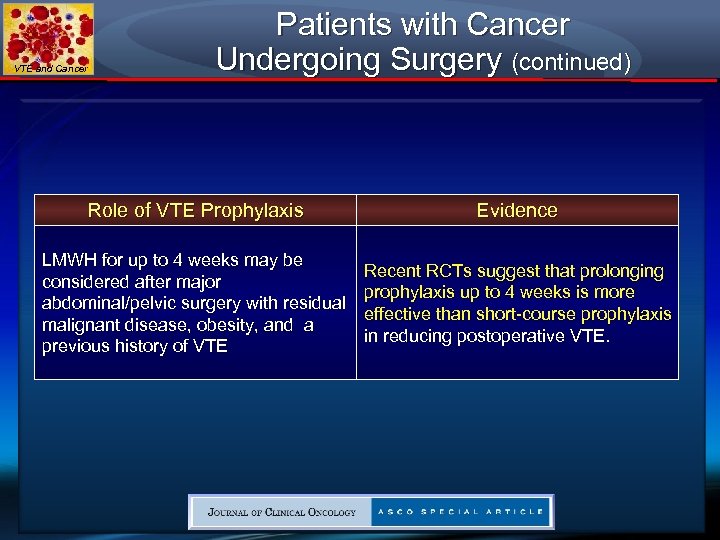 VTE and Cancer Patients with Cancer Undergoing Surgery (continued) Role of VTE Prophylaxis Evidence LMWH for up to 4 weeks may be considered after major abdominal/pelvic surgery with residual malignant disease, obesity, and a previous history of VTE Recent RCTs suggest that prolonging prophylaxis up to 4 weeks is more effective than short-course prophylaxis in reducing postoperative VTE.
VTE and Cancer Patients with Cancer Undergoing Surgery (continued) Role of VTE Prophylaxis Evidence LMWH for up to 4 weeks may be considered after major abdominal/pelvic surgery with residual malignant disease, obesity, and a previous history of VTE Recent RCTs suggest that prolonging prophylaxis up to 4 weeks is more effective than short-course prophylaxis in reducing postoperative VTE.
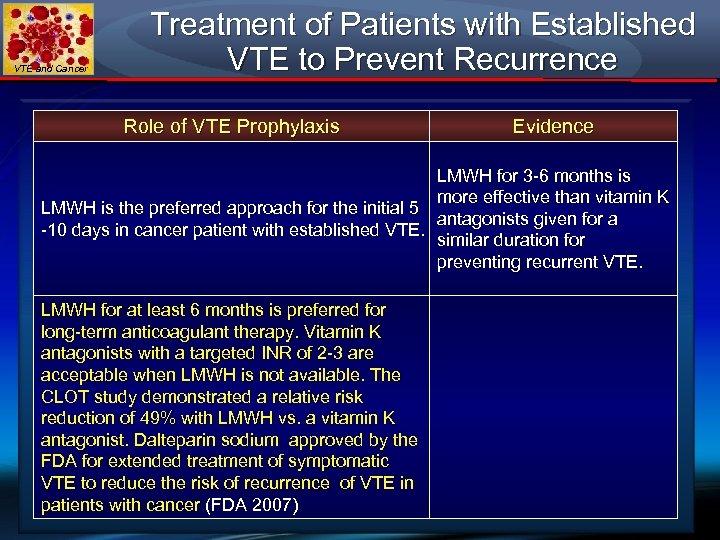 VTE and Cancer Treatment of Patients with Established VTE to Prevent Recurrence Role of VTE Prophylaxis Evidence LMWH for 3 -6 months is more effective than vitamin K LMWH is the preferred approach for the initial 5 antagonists given for a -10 days in cancer patient with established VTE. similar duration for preventing recurrent VTE. LMWH for at least 6 months is preferred for long-term anticoagulant therapy. Vitamin K antagonists with a targeted INR of 2 -3 are acceptable when LMWH is not available. The CLOT study demonstrated a relative risk reduction of 49% with LMWH vs. a vitamin K antagonist. Dalteparin sodium approved by the FDA for extended treatment of symptomatic VTE to reduce the risk of recurrence of VTE in patients with cancer (FDA 2007)
VTE and Cancer Treatment of Patients with Established VTE to Prevent Recurrence Role of VTE Prophylaxis Evidence LMWH for 3 -6 months is more effective than vitamin K LMWH is the preferred approach for the initial 5 antagonists given for a -10 days in cancer patient with established VTE. similar duration for preventing recurrent VTE. LMWH for at least 6 months is preferred for long-term anticoagulant therapy. Vitamin K antagonists with a targeted INR of 2 -3 are acceptable when LMWH is not available. The CLOT study demonstrated a relative risk reduction of 49% with LMWH vs. a vitamin K antagonist. Dalteparin sodium approved by the FDA for extended treatment of symptomatic VTE to reduce the risk of recurrence of VTE in patients with cancer (FDA 2007)
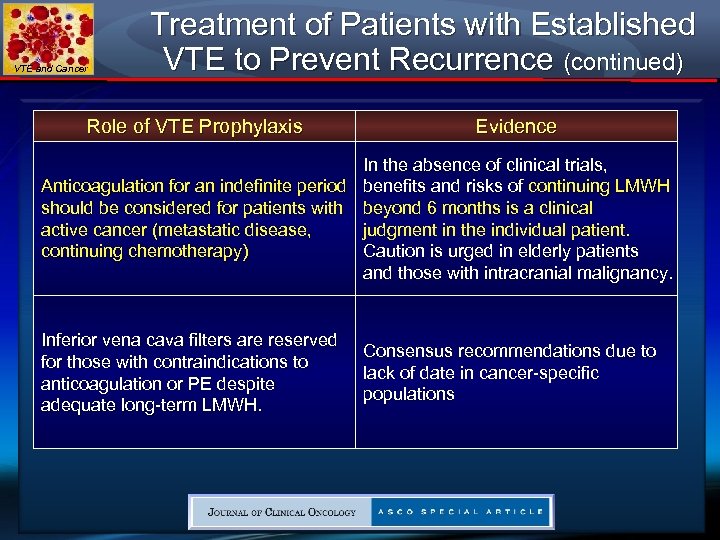 VTE and Cancer Treatment of Patients with Established VTE to Prevent Recurrence (continued) Role of VTE Prophylaxis Evidence In the absence of clinical trials, Anticoagulation for an indefinite period benefits and risks of continuing LMWH should be considered for patients with beyond 6 months is a clinical active cancer (metastatic disease, judgment in the individual patient. continuing chemotherapy) Caution is urged in elderly patients and those with intracranial malignancy. Inferior vena cava filters are reserved for those with contraindications to anticoagulation or PE despite adequate long-term LMWH. Consensus recommendations due to lack of date in cancer-specific populations
VTE and Cancer Treatment of Patients with Established VTE to Prevent Recurrence (continued) Role of VTE Prophylaxis Evidence In the absence of clinical trials, Anticoagulation for an indefinite period benefits and risks of continuing LMWH should be considered for patients with beyond 6 months is a clinical active cancer (metastatic disease, judgment in the individual patient. continuing chemotherapy) Caution is urged in elderly patients and those with intracranial malignancy. Inferior vena cava filters are reserved for those with contraindications to anticoagulation or PE despite adequate long-term LMWH. Consensus recommendations due to lack of date in cancer-specific populations
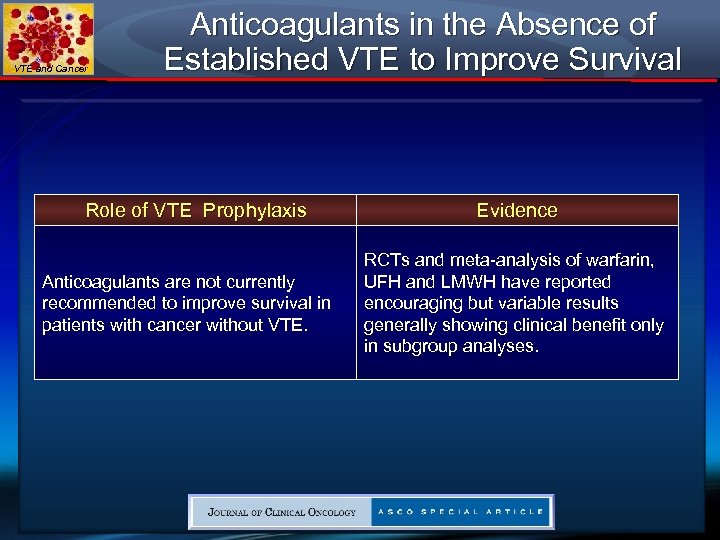 VTE and Cancer Anticoagulants in the Absence of Established VTE to Improve Survival Role of VTE Prophylaxis Anticoagulants are not currently recommended to improve survival in patients with cancer without VTE. Evidence RCTs and meta-analysis of warfarin, UFH and LMWH have reported encouraging but variable results generally showing clinical benefit only in subgroup analyses.
VTE and Cancer Anticoagulants in the Absence of Established VTE to Improve Survival Role of VTE Prophylaxis Anticoagulants are not currently recommended to improve survival in patients with cancer without VTE. Evidence RCTs and meta-analysis of warfarin, UFH and LMWH have reported encouraging but variable results generally showing clinical benefit only in subgroup analyses.
 Summary of the Guidelines Updates VTE and Cancer Summary of Major Changes in the 1. 2009 Version of the Venous Thromboembolic Disease Guidelines
Summary of the Guidelines Updates VTE and Cancer Summary of Major Changes in the 1. 2009 Version of the Venous Thromboembolic Disease Guidelines
 Changes in 2009 NCCN Guidelines VTE and Cancer Stage 1 Immediate: ► “Stage 1 Immediate: Concomitant with diagnosis or while diagnosis and risk assessment (heparin phase)” changed to “Stage 1 Immediate: At diagnosis or during diagnostic evaluation” ► Low –molecular-weight-heparin: New footnote “ 6” was added that states, “Although each of the low molecular weight heparins (LMWH), have been studies in randomized control trials in cancer patients, dalteparin’s efficacy in this population is supported by the highest quality evidence and it is the only LMWH approved by the FDA for this indication. ” ► Unfractionated heparin (IV): target a. PTT range changed from “ 2. 02. 9 x control) to “ 2. 0 -2. 5 x control…” (Also for VTE-H) in these patients.
Changes in 2009 NCCN Guidelines VTE and Cancer Stage 1 Immediate: ► “Stage 1 Immediate: Concomitant with diagnosis or while diagnosis and risk assessment (heparin phase)” changed to “Stage 1 Immediate: At diagnosis or during diagnostic evaluation” ► Low –molecular-weight-heparin: New footnote “ 6” was added that states, “Although each of the low molecular weight heparins (LMWH), have been studies in randomized control trials in cancer patients, dalteparin’s efficacy in this population is supported by the highest quality evidence and it is the only LMWH approved by the FDA for this indication. ” ► Unfractionated heparin (IV): target a. PTT range changed from “ 2. 02. 9 x control) to “ 2. 0 -2. 5 x control…” (Also for VTE-H) in these patients.
 Changes in 2009 NCCN Guidelines VTE and Cancer Stage 3 Chronic: ► “Third bullet: “Consider indefinite anticoagulation…. ” changed to “Recommend indefinite anticoagulation…. ” ► Fourth bullet: “For catheter associated thrombosis, anticoagulate as long as catheter is in place and for at least 3 months after catheter removal”.
Changes in 2009 NCCN Guidelines VTE and Cancer Stage 3 Chronic: ► “Third bullet: “Consider indefinite anticoagulation…. ” changed to “Recommend indefinite anticoagulation…. ” ► Fourth bullet: “For catheter associated thrombosis, anticoagulate as long as catheter is in place and for at least 3 months after catheter removal”.
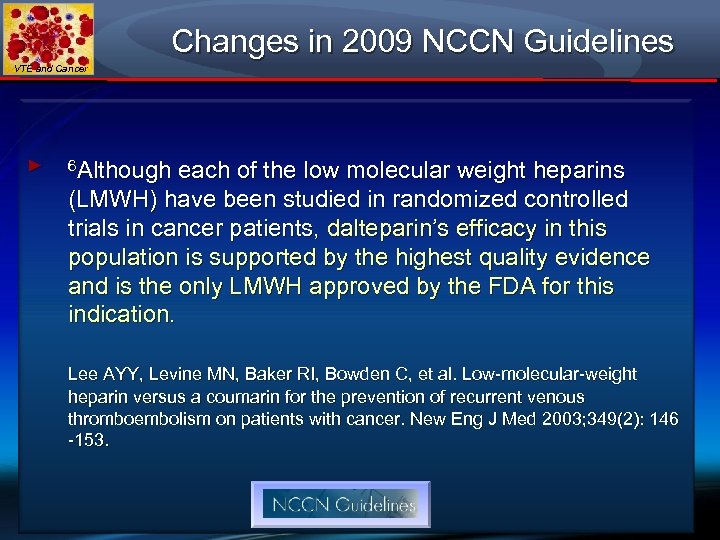 Changes in 2009 NCCN Guidelines VTE and Cancer ► 6 Although each of the low molecular weight heparins (LMWH) have been studied in randomized controlled trials in cancer patients, dalteparin’s efficacy in this population is supported by the highest quality evidence and is the only LMWH approved by the FDA for this indication. Lee AYY, Levine MN, Baker RI, Bowden C, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism on patients with cancer. New Eng J Med 2003; 349(2): 146 -153.
Changes in 2009 NCCN Guidelines VTE and Cancer ► 6 Although each of the low molecular weight heparins (LMWH) have been studied in randomized controlled trials in cancer patients, dalteparin’s efficacy in this population is supported by the highest quality evidence and is the only LMWH approved by the FDA for this indication. Lee AYY, Levine MN, Baker RI, Bowden C, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism on patients with cancer. New Eng J Med 2003; 349(2): 146 -153.
 VTE and Cancer (VTE-D): Therapeutic Anticoagulation Treatment for Venous. Thromboembolism ► The NCCN panel recommends VTE thromboprophylaxis for all hospitalized patients with cancer who do not have contraindications to such therapy, and the panel also emphasized that an increased level of clinical suspicion of VTE should be maintained for cancer patients. Following hospital discharge, it is recommended that patients at high-risk of VTE (e. g. cancer surgery patients) continue to receive VTE prophylaxis for up to 4 weeks post-operation. Careful evaluation and follow-up of cancer patients in whom VTE is suspected and prompt treatment and follow-up for patients diagnosed with VTE is recommended after the cancer status of the patient is assessed and the risks and benefits of treatment are considered.
VTE and Cancer (VTE-D): Therapeutic Anticoagulation Treatment for Venous. Thromboembolism ► The NCCN panel recommends VTE thromboprophylaxis for all hospitalized patients with cancer who do not have contraindications to such therapy, and the panel also emphasized that an increased level of clinical suspicion of VTE should be maintained for cancer patients. Following hospital discharge, it is recommended that patients at high-risk of VTE (e. g. cancer surgery patients) continue to receive VTE prophylaxis for up to 4 weeks post-operation. Careful evaluation and follow-up of cancer patients in whom VTE is suspected and prompt treatment and follow-up for patients diagnosed with VTE is recommended after the cancer status of the patient is assessed and the risks and benefits of treatment are considered.
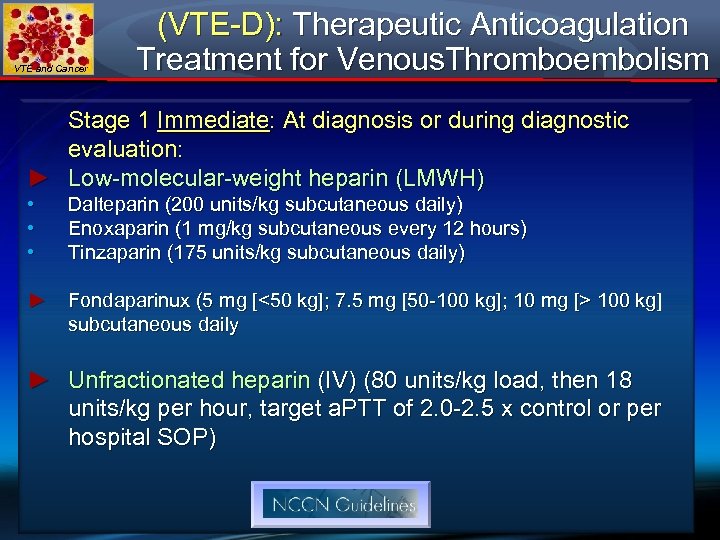 VTE and Cancer (VTE-D): Therapeutic Anticoagulation Treatment for Venous. Thromboembolism Stage 1 Immediate: At diagnosis or during diagnostic evaluation: ► Low-molecular-weight heparin (LMWH) • • • Dalteparin (200 units/kg subcutaneous daily) Enoxaparin (1 mg/kg subcutaneous every 12 hours) Tinzaparin (175 units/kg subcutaneous daily) ► Fondaparinux (5 mg [<50 kg]; 7. 5 mg [50 -100 kg]; 10 mg [> 100 kg] subcutaneous daily ► Unfractionated heparin (IV) (80 units/kg load, then 18 units/kg per hour, target a. PTT of 2. 0 -2. 5 x control or per hospital SOP)
VTE and Cancer (VTE-D): Therapeutic Anticoagulation Treatment for Venous. Thromboembolism Stage 1 Immediate: At diagnosis or during diagnostic evaluation: ► Low-molecular-weight heparin (LMWH) • • • Dalteparin (200 units/kg subcutaneous daily) Enoxaparin (1 mg/kg subcutaneous every 12 hours) Tinzaparin (175 units/kg subcutaneous daily) ► Fondaparinux (5 mg [<50 kg]; 7. 5 mg [50 -100 kg]; 10 mg [> 100 kg] subcutaneous daily ► Unfractionated heparin (IV) (80 units/kg load, then 18 units/kg per hour, target a. PTT of 2. 0 -2. 5 x control or per hospital SOP)
 VTE and Cancer (VTE-D): Therapeutic Anticoagulation Treatment for Venous. Thromboembolism ► Additional VTE risk factors for surgical oncology patients with a previous episode of VTE include anesthesia times longer than 2 hours, advanced stage disease, bed rest, > 4 days and patients age 60 years or older. Extended prophylaxis out to 4 weeks post-surgery was associated with a greater than 50% reduction in venographic VTE
VTE and Cancer (VTE-D): Therapeutic Anticoagulation Treatment for Venous. Thromboembolism ► Additional VTE risk factors for surgical oncology patients with a previous episode of VTE include anesthesia times longer than 2 hours, advanced stage disease, bed rest, > 4 days and patients age 60 years or older. Extended prophylaxis out to 4 weeks post-surgery was associated with a greater than 50% reduction in venographic VTE
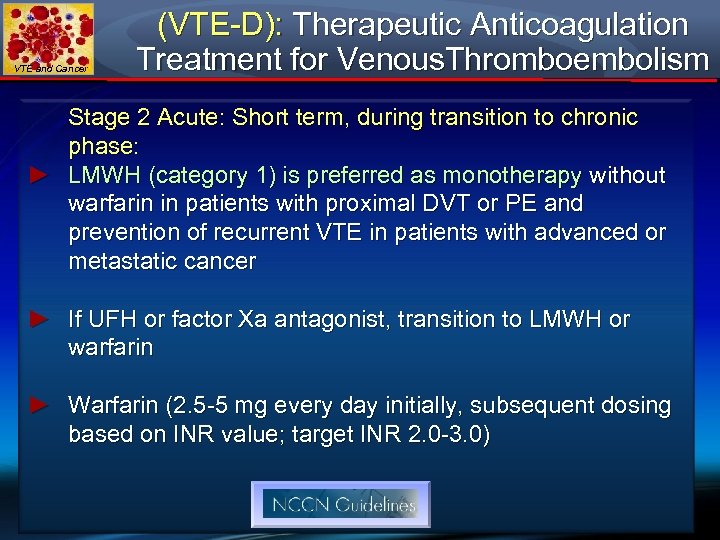 VTE and Cancer (VTE-D): Therapeutic Anticoagulation Treatment for Venous. Thromboembolism Stage 2 Acute: Short term, during transition to chronic phase: ► LMWH (category 1) is preferred as monotherapy without warfarin in patients with proximal DVT or PE and prevention of recurrent VTE in patients with advanced or metastatic cancer ► If UFH or factor Xa antagonist, transition to LMWH or warfarin ► Warfarin (2. 5 -5 mg every day initially, subsequent dosing based on INR value; target INR 2. 0 -3. 0)
VTE and Cancer (VTE-D): Therapeutic Anticoagulation Treatment for Venous. Thromboembolism Stage 2 Acute: Short term, during transition to chronic phase: ► LMWH (category 1) is preferred as monotherapy without warfarin in patients with proximal DVT or PE and prevention of recurrent VTE in patients with advanced or metastatic cancer ► If UFH or factor Xa antagonist, transition to LMWH or warfarin ► Warfarin (2. 5 -5 mg every day initially, subsequent dosing based on INR value; target INR 2. 0 -3. 0)
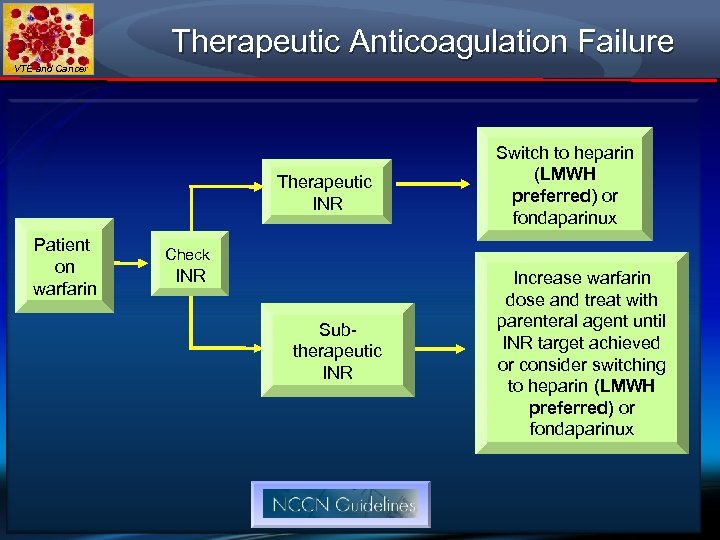 Therapeutic Anticoagulation Failure VTE and Cancer Therapeutic INR Patient on warfarin Switch to heparin (LMWH preferred) or fondaparinux Check INR Subtherapeutic INR Increase warfarin dose and treat with parenteral agent until INR target achieved or consider switching to heparin (LMWH preferred) or fondaparinux
Therapeutic Anticoagulation Failure VTE and Cancer Therapeutic INR Patient on warfarin Switch to heparin (LMWH preferred) or fondaparinux Check INR Subtherapeutic INR Increase warfarin dose and treat with parenteral agent until INR target achieved or consider switching to heparin (LMWH preferred) or fondaparinux
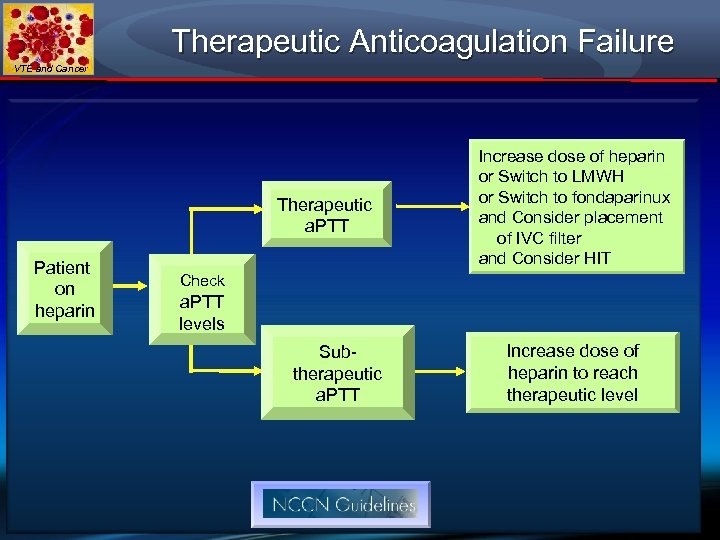 Therapeutic Anticoagulation Failure VTE and Cancer Therapeutic a. PTT Patient on heparin Increase dose of heparin or Switch to LMWH or Switch to fondaparinux and Consider placement of IVC filter and Consider HIT Check a. PTT levels Subtherapeutic a. PTT Increase dose of heparin to reach therapeutic level
Therapeutic Anticoagulation Failure VTE and Cancer Therapeutic a. PTT Patient on heparin Increase dose of heparin or Switch to LMWH or Switch to fondaparinux and Consider placement of IVC filter and Consider HIT Check a. PTT levels Subtherapeutic a. PTT Increase dose of heparin to reach therapeutic level
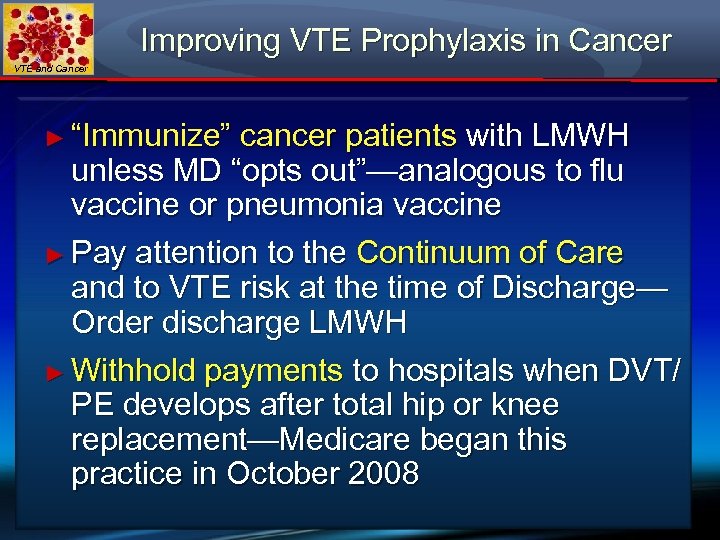 Improving VTE Prophylaxis in Cancer VTE and Cancer ► “Immunize” cancer patients with LMWH unless MD “opts out”—analogous to flu vaccine or pneumonia vaccine ► Pay attention to the Continuum of Care and to VTE risk at the time of Discharge— Order discharge LMWH ► Withhold payments to hospitals when DVT/ PE develops after total hip or knee replacement—Medicare began this practice in October 2008
Improving VTE Prophylaxis in Cancer VTE and Cancer ► “Immunize” cancer patients with LMWH unless MD “opts out”—analogous to flu vaccine or pneumonia vaccine ► Pay attention to the Continuum of Care and to VTE risk at the time of Discharge— Order discharge LMWH ► Withhold payments to hospitals when DVT/ PE develops after total hip or knee replacement—Medicare began this practice in October 2008
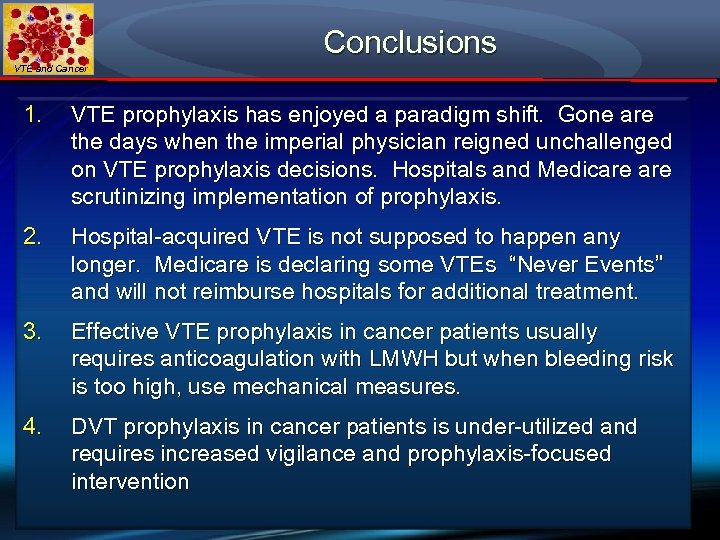 Conclusions VTE and Cancer 1. VTE prophylaxis has enjoyed a paradigm shift. Gone are the days when the imperial physician reigned unchallenged on VTE prophylaxis decisions. Hospitals and Medicare scrutinizing implementation of prophylaxis. 2. Hospital-acquired VTE is not supposed to happen any longer. Medicare is declaring some VTEs “Never Events” and will not reimburse hospitals for additional treatment. 3. Effective VTE prophylaxis in cancer patients usually requires anticoagulation with LMWH but when bleeding risk is too high, use mechanical measures. 4. DVT prophylaxis in cancer patients is under-utilized and requires increased vigilance and prophylaxis-focused intervention
Conclusions VTE and Cancer 1. VTE prophylaxis has enjoyed a paradigm shift. Gone are the days when the imperial physician reigned unchallenged on VTE prophylaxis decisions. Hospitals and Medicare scrutinizing implementation of prophylaxis. 2. Hospital-acquired VTE is not supposed to happen any longer. Medicare is declaring some VTEs “Never Events” and will not reimburse hospitals for additional treatment. 3. Effective VTE prophylaxis in cancer patients usually requires anticoagulation with LMWH but when bleeding risk is too high, use mechanical measures. 4. DVT prophylaxis in cancer patients is under-utilized and requires increased vigilance and prophylaxis-focused intervention
 Conclusions VTE and Cancer 5. DVT prophylaxis following cancer surgery for four weeks is recommended; longer periods may be necessary depending on risk assessment 6. DVT prophylaxis following established DVT in cancer for at least 6 months is recommended and for longer, indefinite periods with active cancer and/or chemotherapy. 7. Heart healthy lifestyle and statins reduce VTE risk 8. Electronic, computerized alerts can reduce symptomatic VTE by at least 40%. 9. When “human” alerts are used, symptomatic VTE is reduced by about 20%. 10. PE/ DVT patients with cancer warrant LMWH monotherapy.
Conclusions VTE and Cancer 5. DVT prophylaxis following cancer surgery for four weeks is recommended; longer periods may be necessary depending on risk assessment 6. DVT prophylaxis following established DVT in cancer for at least 6 months is recommended and for longer, indefinite periods with active cancer and/or chemotherapy. 7. Heart healthy lifestyle and statins reduce VTE risk 8. Electronic, computerized alerts can reduce symptomatic VTE by at least 40%. 9. When “human” alerts are used, symptomatic VTE is reduced by about 20%. 10. PE/ DVT patients with cancer warrant LMWH monotherapy.


