e20e82c427b8fddaf962520deff4bb26.ppt
- Количество слайдов: 1
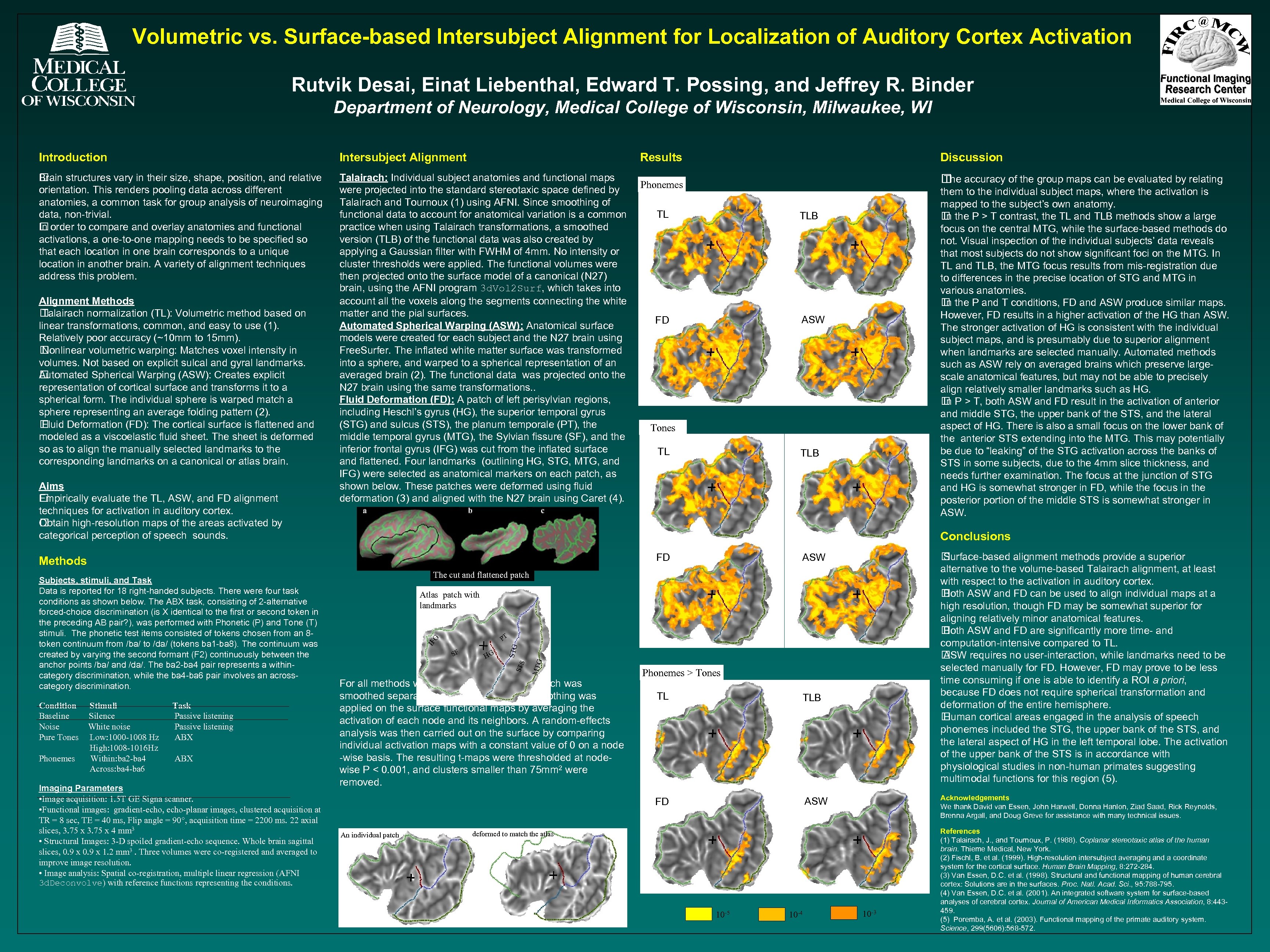
Volumetric vs. Surface-based Intersubject Alignment for Localization of Auditory Cortex Activation Rutvik Desai, Einat Liebenthal, Edward T. Possing, and Jeffrey R. Binder Department of Neurology, Medical College of Wisconsin, Milwaukee, WI Introduction Intersubject Alignment Brain structures vary in their size, shape, position, and relative orientation. This renders pooling data across different anatomies, a common task for group analysis of neuroimaging data, non-trivial. In order to compare and overlay anatomies and functional activations, a one-to-one mapping needs to be specified so that each location in one brain corresponds to a unique location in another brain. A variety of alignment techniques address this problem. Talairach: Individual subject anatomies and functional maps were projected into the standard stereotaxic space defined by Talairach and Tournoux (1) using AFNI. Since smoothing of functional data to account for anatomical variation is a common practice when using Talairach transformations, a smoothed version (TLB) of the functional data was also created by applying a Gaussian filter with FWHM of 4 mm. No intensity or cluster thresholds were applied. The functional volumes were then projected onto the surface model of a canonical (N 27) brain, using the AFNI program 3 d. Vol 2 Surf, which takes into account all the voxels along the segments connecting the white matter and the pial surfaces. Automated Spherical Warping (ASW): Anatomical surface models were created for each subject and the N 27 brain using Free. Surfer. The inflated white matter surface was transformed into a sphere, and warped to a spherical representation of an averaged brain (2). The functional data was projected onto the N 27 brain using the same transformations. . Fluid Deformation (FD): A patch of left perisylvian regions, including Heschl’s gyrus (HG), the superior temporal gyrus (STG) and sulcus (STS), the planum temporale (PT), the middle temporal gyrus (MTG), the Sylvian fissure (SF), and the inferior frontal gyrus (IFG) was cut from the inflated surface and flattened. Four landmarks (outlining HG, STG, MTG, and IFG) were selected as anatomical markers on each patch, as shown below. These patches were deformed using fluid deformation (3) and aligned with the N 27 brain using Caret (4). Alignment Methods Talairach normalization (TL): Volumetric method based on linear transformations, common, and easy to use (1). Relatively poor accuracy (~10 mm to 15 mm). Nonlinear volumetric warping: Matches voxel intensity in volumes. Not based on explicit sulcal and gyral landmarks. Automated Spherical Warping (ASW): Creates explicit representation of cortical surface and transforms it to a spherical form. The individual sphere is warped match a sphere representing an average folding pattern (2). Fluid Deformation (FD): The cortical surface is flattened and modeled as a viscoelastic fluid sheet. The sheet is deformed so as to align the manually selected landmarks to the corresponding landmarks on a canonical or atlas brain. Aims Empirically evaluate the TL, ASW, and FD alignment techniques for activation in auditory cortex. Obtain high-resolution maps of the areas activated by categorical perception of speech sounds. Results Phonemes TL FD Stimuli Silence White noise Low: 1000 -1008 Hz High: 1008 -1016 Hz Within: ba 2 -ba 4 Across: ba 4 -ba 6 Task Passive listening ABX Imaging Parameters • Image acquisition: 1. 5 T GE Signa scanner. • Functional images: gradient-echo, echo-planar images, clustered acquisition at TR = 8 sec, TE = 40 ms, Flip angle = 90°, acquisition time = 2200 ms. 22 axial slices, 3. 75 x 4 mm 3 • Structural Images: 3 -D spoiled gradient-echo sequence. Whole brain sagittal slices, 0. 9 x 1. 2 mm 3. Three volumes were co-registered and averaged to improve image resolution. • Image analysis: Spatial co-registration, multiple linear regression (AFNI 3 d. Deconvolve) with reference functions representing the conditions. them to the individual subject maps, where the activation is mapped to the subject’s own anatomy. the P > T contrast, the TL and TLB methods show a large In focus on the central MTG, while the surface-based methods do not. Visual inspection of the individual subjects’ data reveals that most subjects do not show significant foci on the MTG. In TL and TLB, the MTG focus results from mis-registration due to differences in the precise location of STG and MTG in various anatomies. the P and T conditions, FD and ASW produce similar maps. In However, FD results in a higher activation of the HG than ASW. The stronger activation of HG is consistent with the individual subject maps, and is presumably due to superior alignment when landmarks are selected manually. Automated methods such as ASW rely on averaged brains which preserve largescale anatomical features, but may not be able to precisely align relatively smaller landmarks such as HG. P > T, both ASW and FD result in the activation of anterior In and middle STG, the upper bank of the STS, and the lateral aspect of HG. There is also a small focus on the lower bank of the anterior STS extending into the MTG. This may potentially be due to “leaking” of the STG activation across the banks of STS in some subjects, due to the 4 mm slice thickness, and needs further examination. The focus at the junction of STG and HG is somewhat stronger in FD, while the focus in the posterior portion of the middle STS is somewhat stronger in ASW. TLB ASW Tones TL TLB Surface-based alignment methods provide a superior alternative to the volume-based Talairach alignment, at least with respect to the activation in auditory cortex. Both ASW and FD can be used to align individual maps at a high resolution, though FD may be somewhat superior for aligning relatively minor anatomical features. Both ASW and FD are significantly more time- and computation-intensive compared to TL. ASW requires no user-interaction, while landmarks need to be selected manually for FD. However, FD may prove to be less time consuming if one is able to identify a ROI a priori, because FD does not require spherical transformation and deformation of the entire hemisphere. Human cortical areas engaged in the analysis of speech phonemes included the STG, the upper bank of the STS, and the lateral aspect of HG in the left temporal lobe. The activation of the upper bank of the STS is in accordance with physiological studies in non-human primates suggesting multimodal functions for this region (5). ASW FD The cut and flattened patch Atlas patch with landmarks MTG HG STS SF STG G PT IF Subjects, stimuli, and Task Data is reported for 18 right-handed subjects. There were four task conditions as shown below. The ABX task, consisting of 2 -alternative forced-choice discrimination (is X identical to the first or second token in the preceding AB pair? ), was performed with Phonetic (P) and Tone (T) stimuli. The phonetic test items consisted of tokens chosen from an 8 token continuum from /ba/ to /da/ (tokens ba 1 -ba 8). The continuum was created by varying the second formant (F 2) continuously between the anchor points /ba/ and /da/. The ba 2 -ba 4 pair represents a withincategory discrimination, while the ba 4 -ba 6 pair involves an acrosscategory discrimination. Phonemes accuracy of the group maps can be evaluated by relating The Conclusions Methods Condition Baseline Noise Pure Tones Discussion For all methods with the exception of TLB (which was smoothed separately), a small amount of smoothing was applied on the surface functional maps by averaging the activation of each node and its neighbors. A random-effects analysis was then carried out on the surface by comparing individual activation maps with a constant value of 0 on a node -wise basis. The resulting t-maps were thresholded at nodewise P < 0. 001, and clusters smaller than 75 mm 2 were removed. Phonemes > Tones TLB FD An individual patch TL ASW Acknowledgements We thank David van Essen, John Harwell, Donna Hanlon, Ziad Saad, Rick Reynolds, Brenna Argall, and Doug Greve for assistance with many technical issues. deformed to match the atlas 10 -5 10 -4 10 -3 References (1) Talairach, J. , and Tournoux, P. (1988). Coplanar stereotaxic atlas of the human brain. Thieme Medical, New York. (2) Fischl, B. et al. (1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8: 272 -284. (3) Van Essen, D. C. et al. (1998). Structural and functional mapping of human cerebral cortex: Solutions are in the surfaces. Proc. Natl. Acad. Sci. , 95: 788 -795. (4) Van Essen, D. C. et al. (2001). An integrated software system for surface-based analyses of cerebral cortex. Journal of American Medical Informatics Association, 8: 443459. (5) Poremba, A. et al. (2003). Functional mapping of the primate auditory system. Science, 299(5606): 568 -572.
e20e82c427b8fddaf962520deff4bb26.ppt