39db0e404e8535ddd270575b4cc2ba0b.ppt
- Количество слайдов: 85
Volumetric Analysis: Acid-Base Chpt. 13
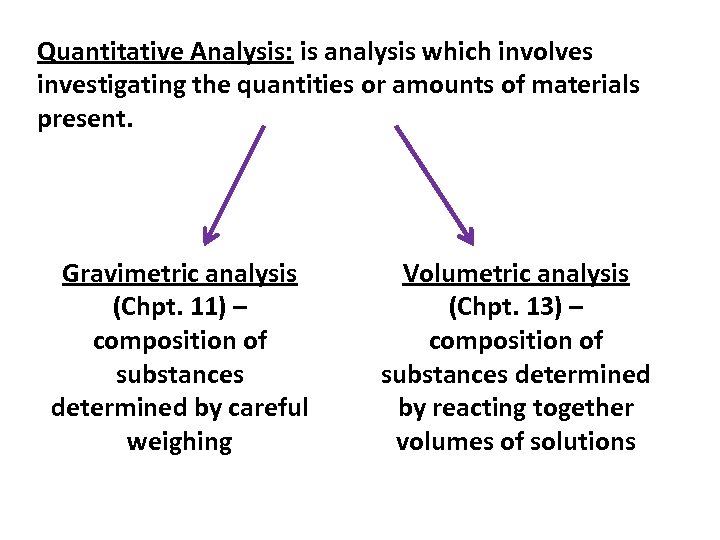
Quantitative Analysis: is analysis which involves investigating the quantities or amounts of materials present. Gravimetric analysis (Chpt. 11) – composition of substances determined by careful weighing Volumetric analysis (Chpt. 13) – composition of substances determined by reacting together volumes of solutions
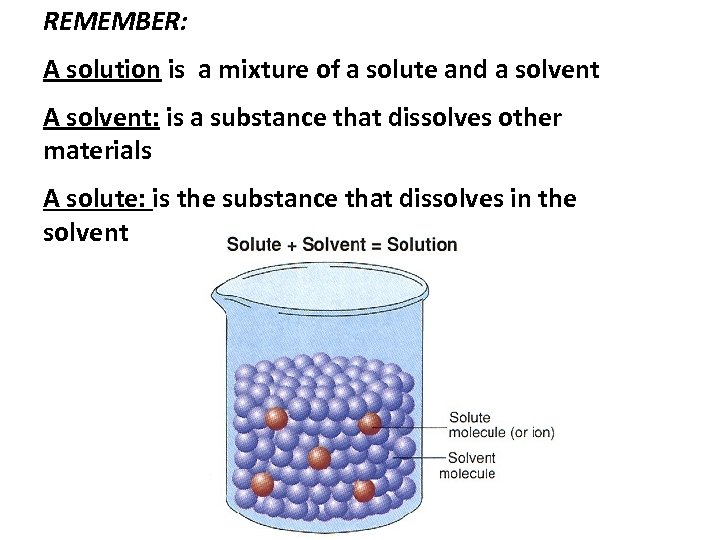
REMEMBER: A solution is a mixture of a solute and a solvent A solvent: is a substance that dissolves other materials A solute: is the substance that dissolves in the solvent
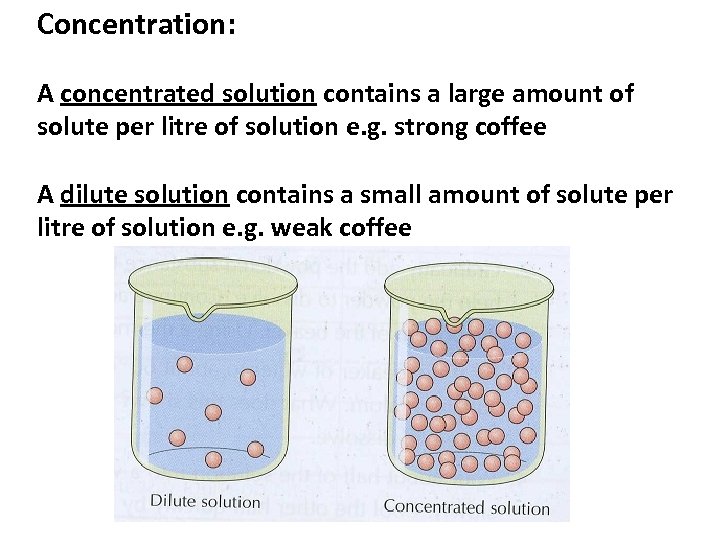
Concentration: A concentrated solution contains a large amount of solute per litre of solution e. g. strong coffee A dilute solution contains a small amount of solute per litre of solution e. g. weak coffee

Concentration The concentration of a solution is the amount of solute that is dissolved in a given volume of solution

There are several ways of expressing the concentration of a solution: 1) Percentage of solute – 3 forms 2) Parts per million (ppm) 3) Moles of solute per litre of solution (MOLARITY)
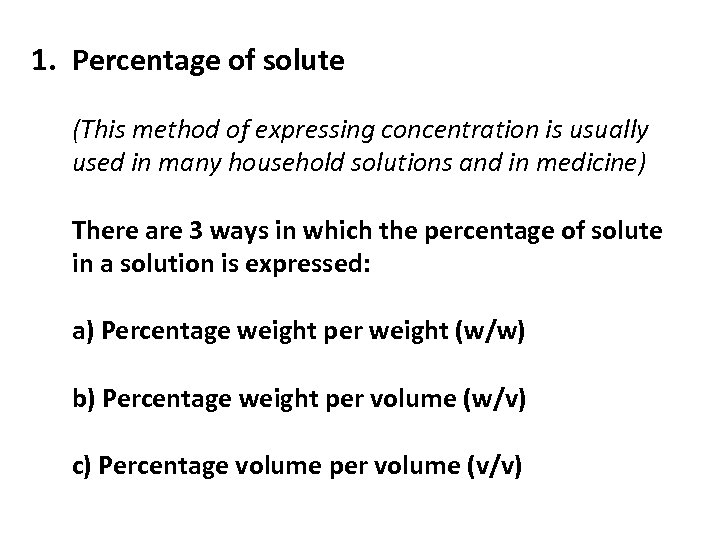
1. Percentage of solute (This method of expressing concentration is usually used in many household solutions and in medicine) There are 3 ways in which the percentage of solute in a solution is expressed: a) Percentage weight per weight (w/w) b) Percentage weight per volume (w/v) c) Percentage volume per volume (v/v)

a) Percentage weight per weight (w/w): This is the number of grams of solute per 100 g of solution e. g. 10% w/w Na. Cl → 10 g of sodium chloride per 100 g of solution 2% w/w Arnica ointment → 2 g of arnica per 100 g of ointment

b) Percentage weight per volume (w/v): This is the number of grams of solute per 100 cm 3 of solution e. g. 10% w/v Na. Cl → 10 g of sodium chloride per 100 cm 3 of solution 5% w/v Na. Cl → 5 g of Na. Cl in 100 cm 3 of solution *Note: usually the units are grams per litre (g/L) therefore you would have to bring it to a litre*

c) Percentage volume per volume (v/v): This is the number of cm 3 of solute per 100 cm 3 of solution e. g. 5% v/v vinegar solution → 5 cm 3 of ethanoic acid per 100 cm 3 of vinegar 13% v/v wine solution → 13 cm 3 of ethanol (alcohol) per 100 cm 3 of wine
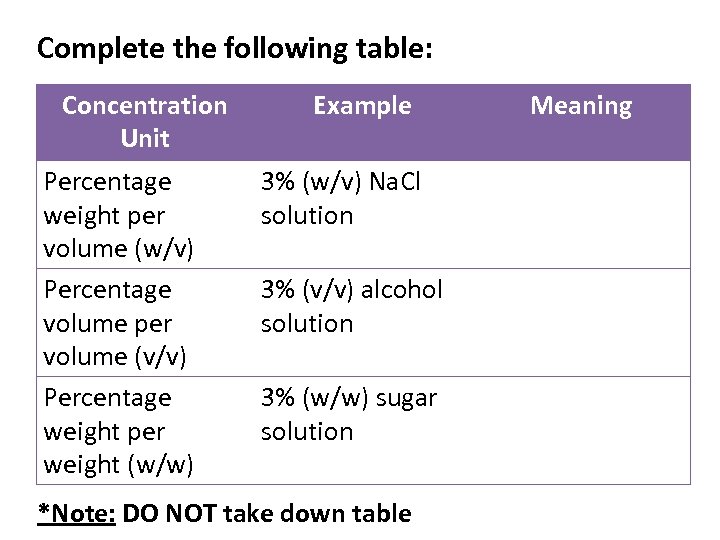
Complete the following table: Concentration Unit Percentage weight per volume (w/v) Percentage volume per volume (v/v) Percentage weight per weight (w/w) Example 3% (w/v) Na. Cl solution 3% (v/v) alcohol solution 3% (w/w) sugar solution *Note: DO NOT take down table Meaning

The following calculations involve working with percentages!!!! Make sure you understand the definitions!!!!!

Example 1: A solution contains 20 g of potassium hydroxide in 1 litre of solution. Express the concentration of the solution in % (w/v). Solution:

Example 2: A bottle of vinegar contains 25 cm 3 ethanoic acid in 500 cm 3 of solution. Express the concentration of ethanoic acid in the solution in % (v/v) Solution:

Example 3: A solution contains 10 g of sodium carbonate in 40 g of solution. Express the concentration of the solution in % (w/w). Solution:

Example 4: The label on a bottle of wine indicates that the concentration of alcohol in the wine is 9% (v/v). What volume of alcohol is there in 250 cm 3 of the wine? Solution:

Try the following: 1. A sample of sea water has a mass of 1. 3 kg. On evaporating the water, 148 g of salt was recovered from it. Express the concentration of the salt as % w/w. 2. Some illnesses can upset the salt balance in the body and it may be necessary to administer salt intravenously. The solution of salt that is injected is marked 0. 85% w/v. What weight of sodium chloride is needed to make up 250 cm 3 of this solution?

2. Parts per million (ppm) This method of expressing the concentration of a solution is only used for very dilute solutions i. e. when dealing with very low concentrations of substances. • This is the number of milligrams per litre (mg. L-1) *Note: 1 Litre of water has a mass of 1 million milligrams* • So, can say 1 mg/L = 1 mg per million mg = 1 ppm • Example: the concentration of chlorine in water is 2 ppm this means there are 2 mg of chlorine per litre of water

Example 1: 1 gram = 1000 milligrams
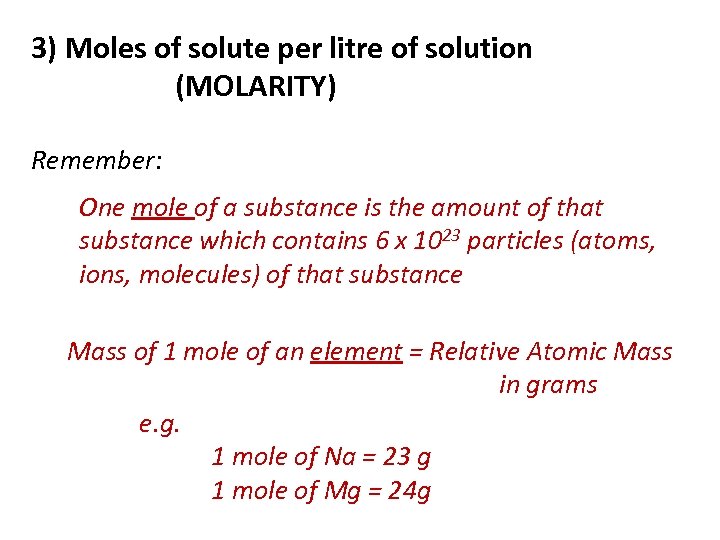
3) Moles of solute per litre of solution (MOLARITY) Remember: One mole of a substance is the amount of that substance which contains 6 x 1023 particles (atoms, ions, molecules) of that substance Mass of 1 mole of an element = Relative Atomic Mass in grams e. g. 1 mole of Na = 23 g 1 mole of Mg = 24 g

The most important way of expressing the concentration of a solution is in terms of moles per litre of solution (molarity) Definitions: • The Molarity of a solution is the number of moles of solute per litre of solution • A 1 molar solution is a solution which contains one mole of solute per litre of solution also, - a solution which contains 2 moles of solute in a litre of solution is said to be 2 molar (2 M) - a solution which contains 0. 5 moles of solute in a litre of solution is said to be 0. 5 molar (0. 5 M)
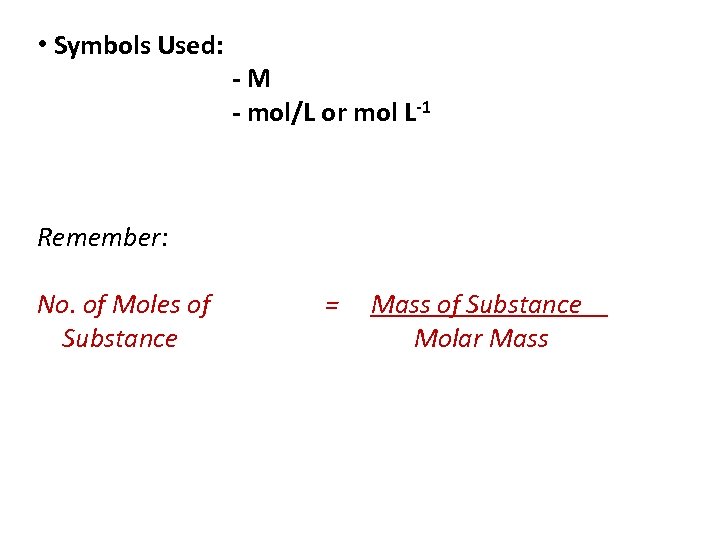
• Symbols Used: -M - mol/L or mol L-1 Remember: No. of Moles of Substance = Mass of Substance Molar Mass
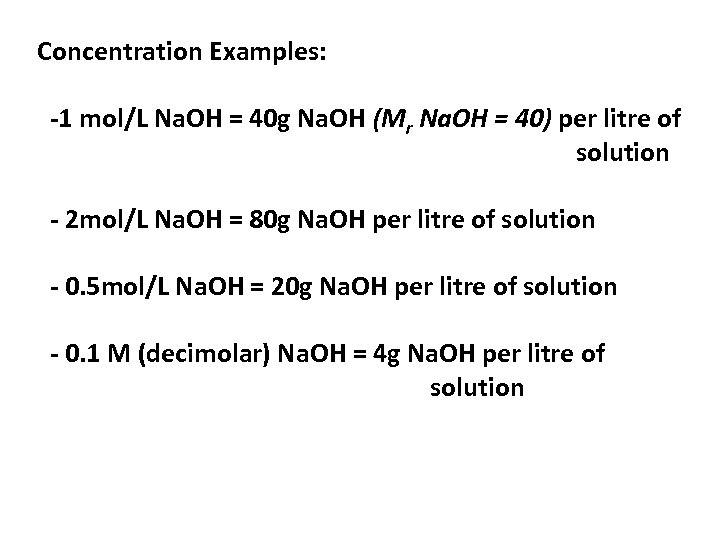
Concentration Examples: -1 mol/L Na. OH = 40 g Na. OH (Mr Na. OH = 40) per litre of solution - 2 mol/L Na. OH = 80 g Na. OH per litre of solution - 0. 5 mol/L Na. OH = 20 g Na. OH per litre of solution - 0. 1 M (decimolar) Na. OH = 4 g Na. OH per litre of solution

Complete the following: 1 M H 2 SO 4 = 0. 5 M H 2 SO 4 = 3 M H 2 SO 4 =
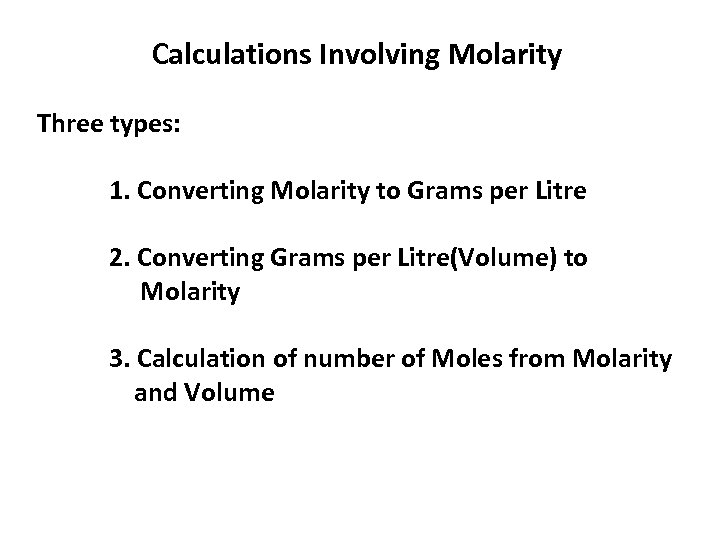
Calculations Involving Molarity Three types: 1. Converting Molarity to Grams per Litre 2. Converting Grams per Litre(Volume) to Molarity 3. Calculation of number of Moles from Molarity and Volume
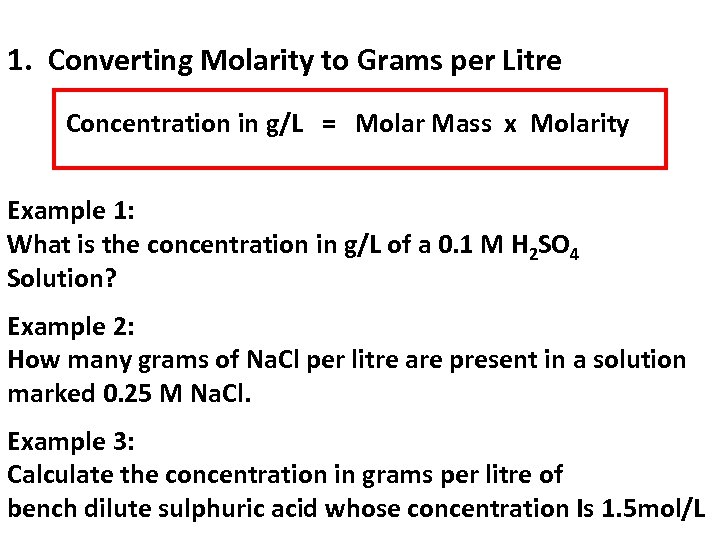
1. Converting Molarity to Grams per Litre Concentration in g/L = Molar Mass x Molarity Example 1: What is the concentration in g/L of a 0. 1 M H 2 SO 4 Solution? Example 2: How many grams of Na. Cl per litre are present in a solution marked 0. 25 M Na. Cl. Example 3: Calculate the concentration in grams per litre of bench dilute sulphuric acid whose concentration Is 1. 5 mol/L
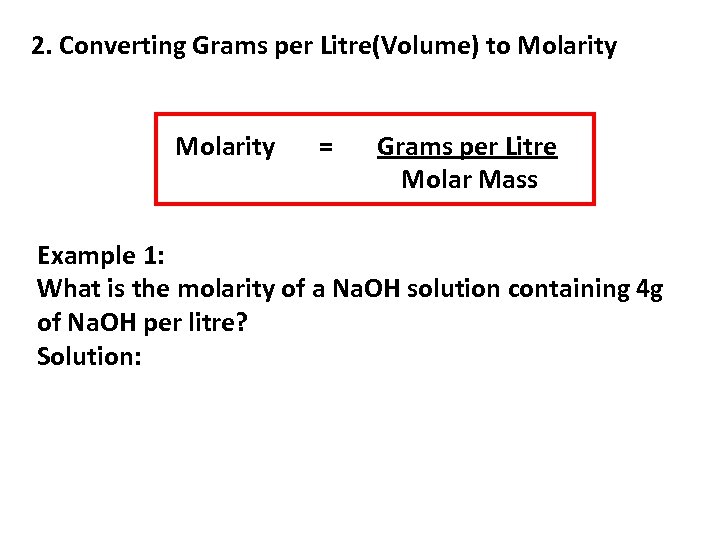
2. Converting Grams per Litre(Volume) to Molarity = Grams per Litre Molar Mass Example 1: What is the molarity of a Na. OH solution containing 4 g of Na. OH per litre? Solution:

Example 2: What is the molarity of a solution that contains 3. 68 g of Na. OH per litre of solution?

Example 3: Calculate the concentration in moles per litre of a solution containing 45 grams of sulphuric acid per 250 cm 3 of solution.
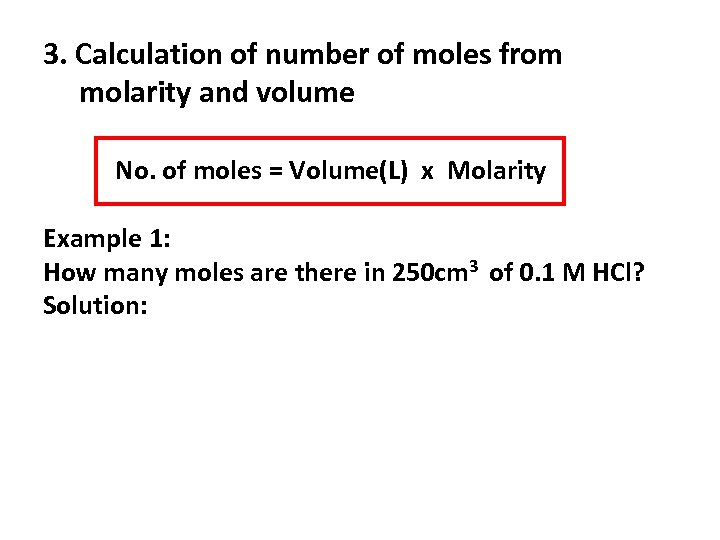
3. Calculation of number of moles from molarity and volume No. of moles = Volume(L) x Molarity Example 1: How many moles are there in 250 cm 3 of 0. 1 M HCl? Solution:

Example 2: How many moles of Na. OH are present in 25 cm 3 of 0. 55 M Na. OH

Example 3: How many moles of hydrochloric acid are present in 30 cm 3 of 0. 2 M HCl

Example 4: What mass of sodium hydroxide is contained in 25 cm 3 of a 0. 75 M solution of sodium hydroxide?

Example 5: What volume of 0. 15 M sodium hydroxide solution will contain 5 grams of sodium hydroxide?
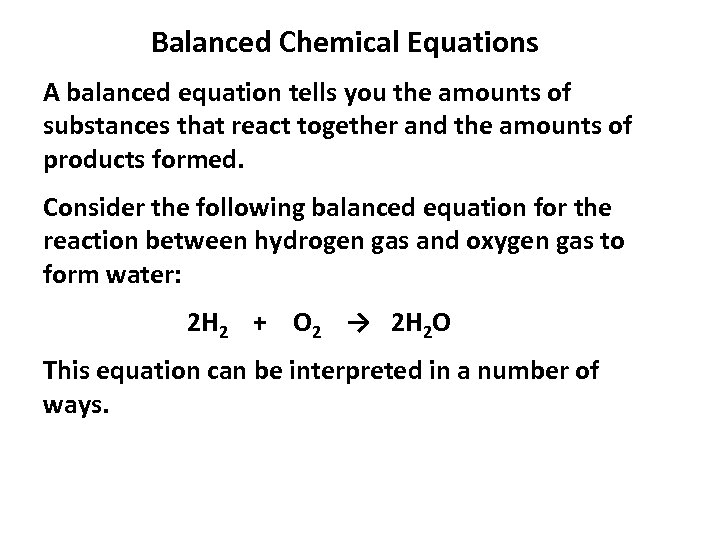
Balanced Chemical Equations A balanced equation tells you the amounts of substances that react together and the amounts of products formed. Consider the following balanced equation for the reaction between hydrogen gas and oxygen gas to form water: 2 H 2 + O 2 → 2 H 2 O This equation can be interpreted in a number of ways.
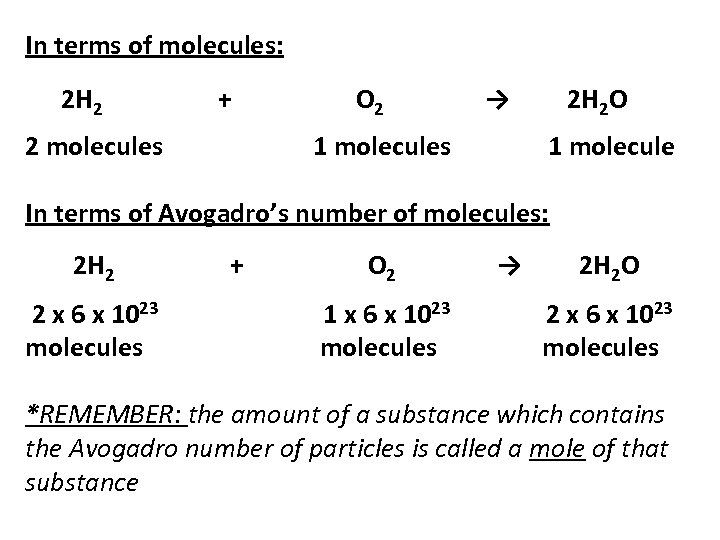
In terms of molecules: 2 H 2 + 2 molecules O 2 → 1 molecules 2 H 2 O 1 molecule In terms of Avogadro’s number of molecules: 2 H 2 2 x 6 x 1023 molecules + O 2 1 x 6 x 1023 molecules → 2 H 2 O 2 x 6 x 1023 molecules *REMEMBER: the amount of a substance which contains the Avogadro number of particles is called a mole of that substance
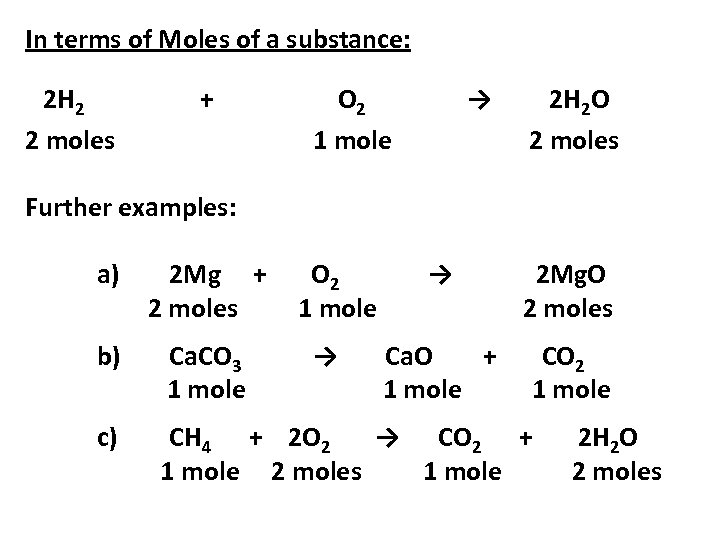
In terms of Moles of a substance: 2 H 2 2 moles + O 2 1 mole → 2 H 2 O 2 moles Further examples: a) 2 Mg + 2 moles O 2 1 mole b) Ca. CO 3 1 mole c) CH 4 + 2 O 2 → CO 2 + 1 mole 2 moles 1 mole → → 2 Mg. O 2 moles Ca. O + 1 mole CO 2 1 mole 2 H 2 O 2 moles
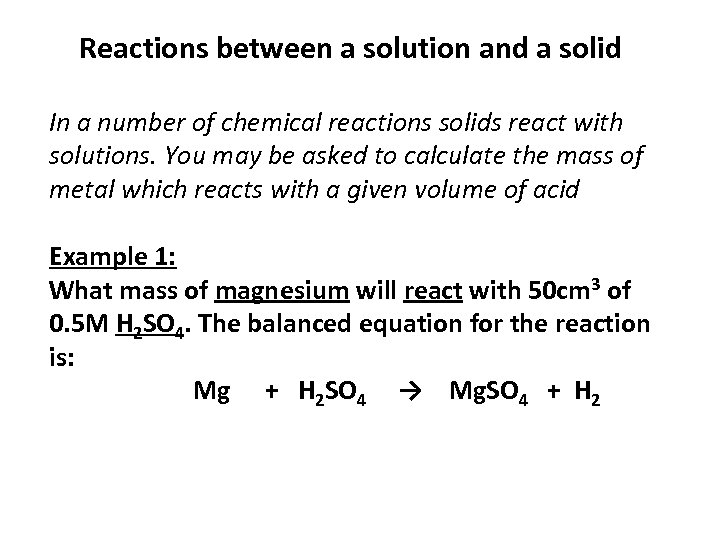
Reactions between a solution and a solid In a number of chemical reactions solids react with solutions. You may be asked to calculate the mass of metal which reacts with a given volume of acid Example 1: What mass of magnesium will react with 50 cm 3 of 0. 5 M H 2 SO 4. The balanced equation for the reaction is: Mg + H 2 SO 4 → Mg. SO 4 + H 2

Example 1 Solution:

Example 2: Sodium carbonate, Na 2 CO 3, reacts with dilute hydrochloric acid according to the equation: Na 2 CO 3 + 2 HCl → 2 Na. Cl + CO 2 + H 2 O What volume of hydrochloric acid of concentration 0. 75 M would be needed to neutralise 7. 5 g of anhydrous sodium carbonate?

Example 2 Solution:

Concentration of Solutions 1 mole/L 1 mole/250 cm 3 1 mole/500 cm 3 1 mole/100 cm 3

If in each volumetric flask one mole of solute is dissolved then as the volume becomes smaller, the concentration increases. In the case of a coloured solution , as the concentration increases, the intensity of the colour also increases (see diagram pg. 148 book)

Dilution of Solutions To save space in our prep room we buy solutions in concentrated form, i. e. 18 M HCl (18 mol L-1). We call these stock solutions Definition: The process of adding more solvent to a solution is called dilution. A typical dilution involves determining how much water must be added to an amount of stock solution to achieve a solution of the desired concentration
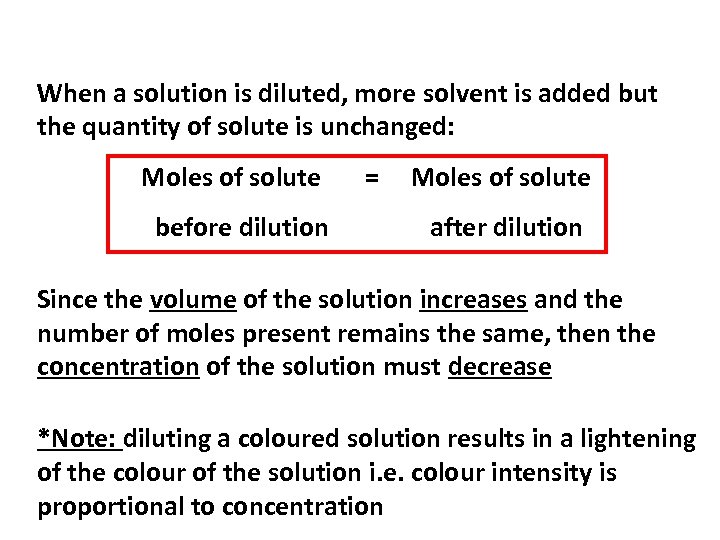
When a solution is diluted, more solvent is added but the quantity of solute is unchanged: Moles of solute before dilution = Moles of solute after dilution Since the volume of the solution increases and the number of moles present remains the same, then the concentration of the solution must decrease *Note: diluting a coloured solution results in a lightening of the colour of the solution i. e. colour intensity is proportional to concentration
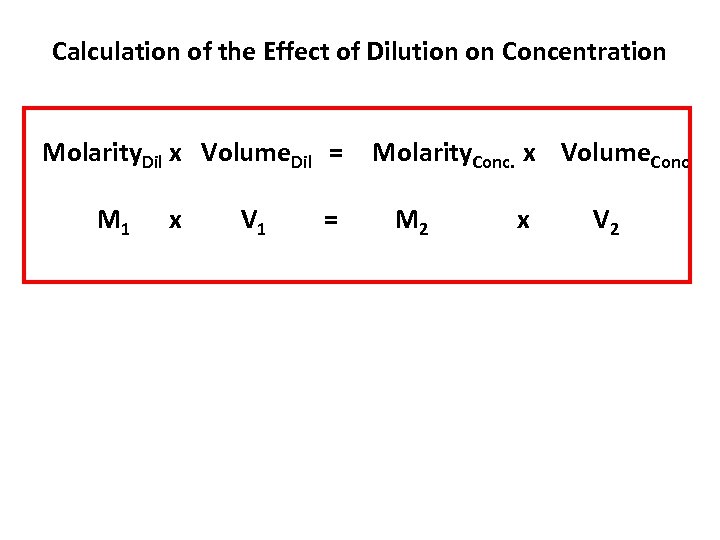
Calculation of the Effect of Dilution on Concentration Molarity. Dil x Volume. Dil = M 1 x V 1 = Molarity. Conc. x Volume. Conc. M 2 x V 2

Example 1: If 20 cm 3 of a 3 M hydrochloric acid solution is diluted to a volume of 1 L with water, what is the concentration of the diluted acid? Solution:

Example 2: What volume of a 2 M sodium hydroxide solution is needed to make up 100 cm 3 of a 0. 1 M sodium hydroxide solution

Student Questions: Question 1: If 12 cm 3 of a 0. 1 M sodium hydroxide solution is diluted to a volume of 500 cm 3 with water, what is the concentration of the diluted solution? Question 2: What volume of 1 M Na. OH solution is needed to make 300 cm 3 of 0. 05 M solution?

Standard Solutions Definition: A standard solution is a solution whose concentration is accurately known e. g. a solution containing 10 grams of Na. Cl per litre is a standard solution In the determination of the concentration of an acid a standard solution of an alkali is used and to determine the concentration of an alkali a standard acid would be used. However, before any determinations can be made a starting accurately standardised solution is required – from which to find the exact concentration of other solutions
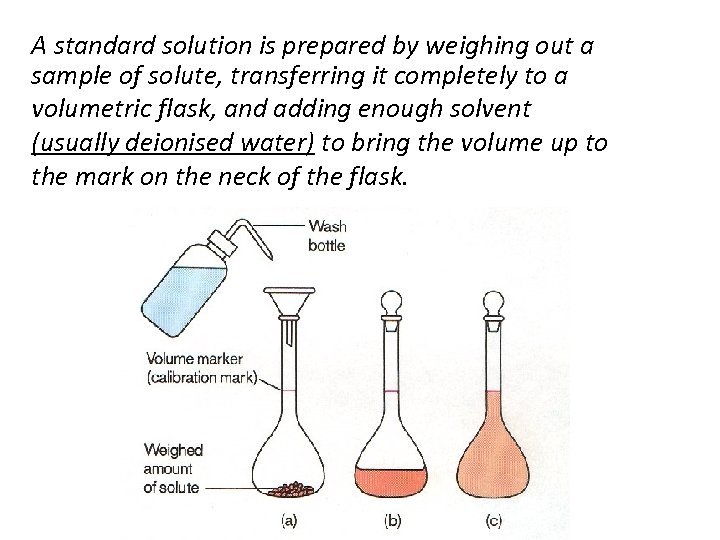
A standard solution is prepared by weighing out a sample of solute, transferring it completely to a volumetric flask, and adding enough solvent (usually deionised water) to bring the volume up to the mark on the neck of the flask.

Due to the fact that many substances can not be obtained in a high degree of purity standard solutions of common laboratory acids and bases cannot be prepared directly e. g. : - cannot weigh out 1 mole of sulphuric acid as it absorbs moisture from the air - cannot weigh out 1 mole of nitric acid as it is volatile - cannot weigh out 1 mole of iodine as it sublimes at room temperature In order to make up standard solutions substances which can be obtained in a highly pure state and which are stable in air are required

Primary Standard Definition: A primary standard is a substance which can be obtained in a stable, pure and soluble solid form so that it can be weighed out and dissolved in water to give a solution of accurately known concentration Primary Standard Solution = Pure 100% Soluble Stable once made up Examples of Primary Standards: - Anhydrous sodium carbonate Na 2 CO 3 - Sodium Chloride Na. Cl - Potassium Dichromate K 2 Cr 2 O 7

Mandatory Expt. 13. 1: To prepare a standard solution of sodium carbonate Note: You must have a clear understanding of all the steps you undertake in this experiment and be able to explain the importance of each step
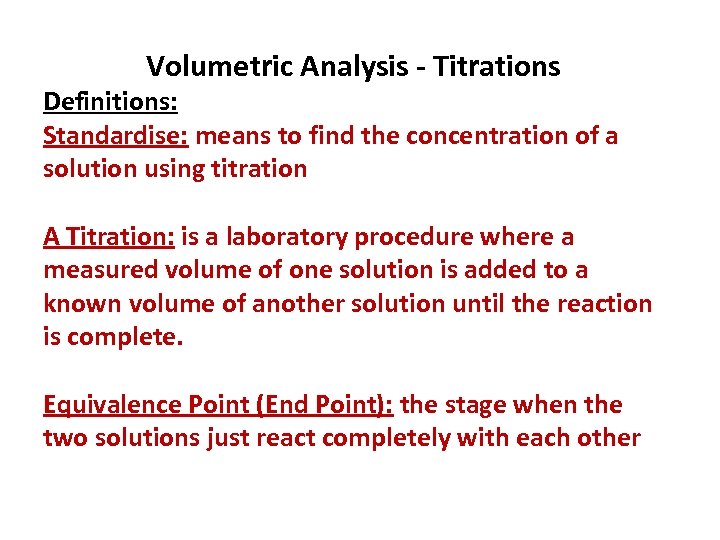
Volumetric Analysis - Titrations Definitions: Standardise: means to find the concentration of a solution using titration A Titration: is a laboratory procedure where a measured volume of one solution is added to a known volume of another solution until the reaction is complete. Equivalence Point (End Point): the stage when the two solutions just react completely with each other

Theory regarding apparatus and method involved in carrying out a titration on handout

Mandatory Expt. 13. 2: To use a standard solution of sodium carbonate to standardise a given hydrochloric acid solution Note 1: You must have a clear understanding of all the steps you undertake in this experiment and be able to explain the importance of each step Note 2: You must be able to carry out calculations on your results – see notes to follow *Need to calculate concentration of HCl in molL & g/L
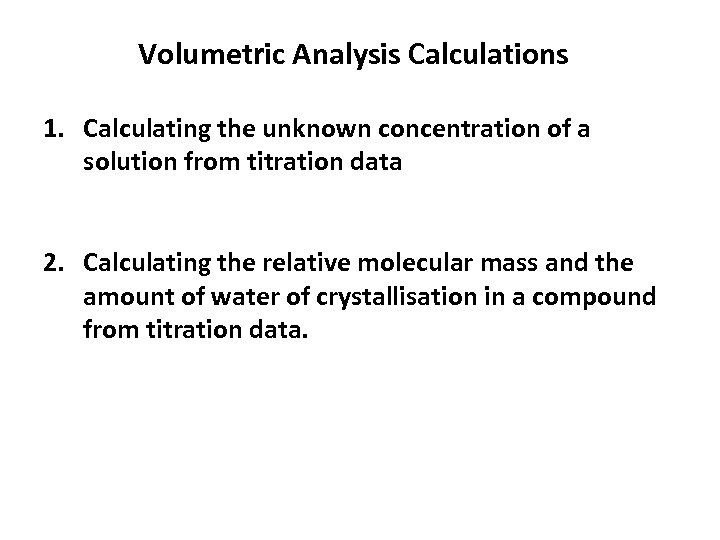
Volumetric Analysis Calculations 1. Calculating the unknown concentration of a solution from titration data 2. Calculating the relative molecular mass and the amount of water of crystallisation in a compound from titration data.
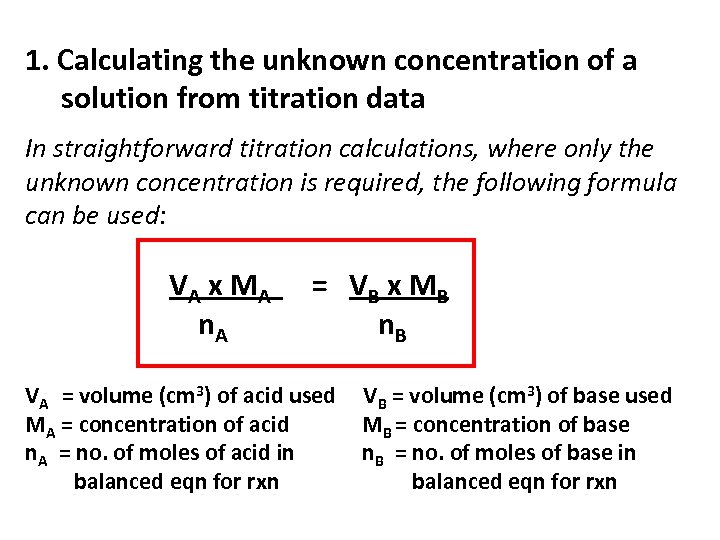
1. Calculating the unknown concentration of a solution from titration data In straightforward titration calculations, where only the unknown concentration is required, the following formula can be used: VA x M A n. A = VB x M B n. B VA = volume (cm 3) of acid used MA = concentration of acid n. A = no. of moles of acid in balanced eqn for rxn VB = volume (cm 3) of base used MB = concentration of base n. B = no. of moles of base in balanced eqn for rxn
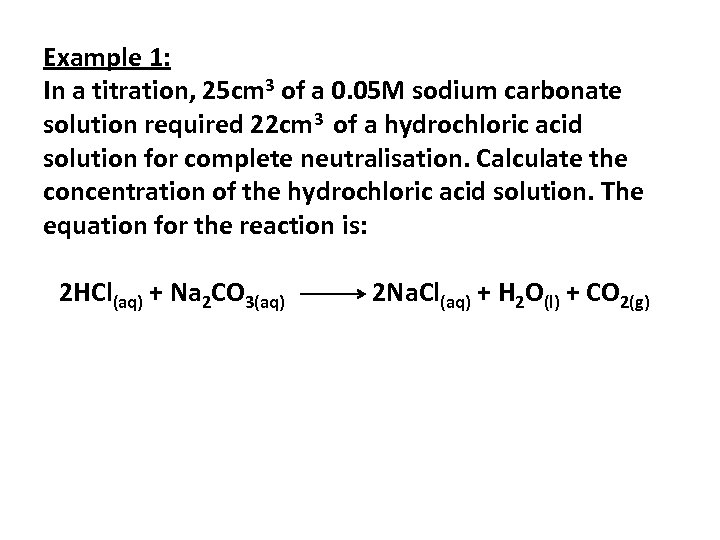
Example 1: In a titration, 25 cm 3 of a 0. 05 M sodium carbonate solution required 22 cm 3 of a hydrochloric acid solution for complete neutralisation. Calculate the concentration of the hydrochloric acid solution. The equation for the reaction is: 2 HCl(aq) + Na 2 CO 3(aq) 2 Na. Cl(aq) + H 2 O(l) + CO 2(g)

Example 1 Solution:
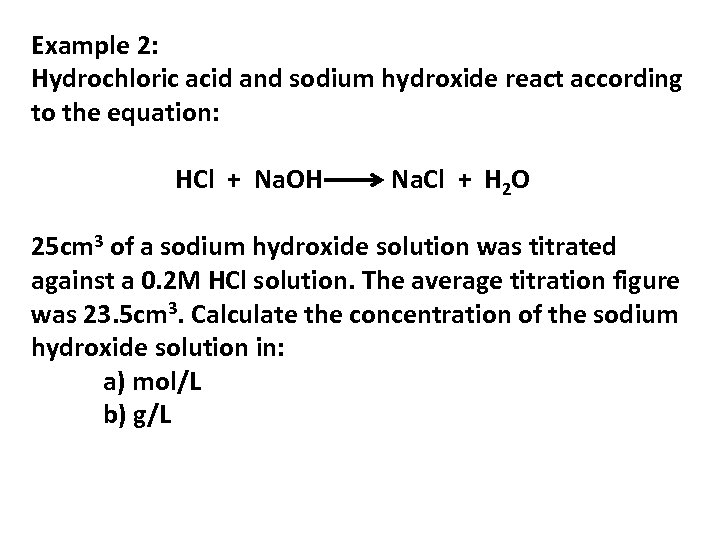
Example 2: Hydrochloric acid and sodium hydroxide react according to the equation: HCl + Na. OH Na. Cl + H 2 O 25 cm 3 of a sodium hydroxide solution was titrated against a 0. 2 M HCl solution. The average titration figure was 23. 5 cm 3. Calculate the concentration of the sodium hydroxide solution in: a) mol/L b) g/L

Example 2 Solution:

Using results from your experiment calculate the concentration of the given hydrochloric acid solution in mol/L and g/L *Note: The first titration you performed was a rough titration which gave you an idea of where the end point is and so this result should be neglected. The remaining two titration results should agree within 0. 1 cm 3 of each other. The average of these results should be used in your calculation of the concentration of HCl.
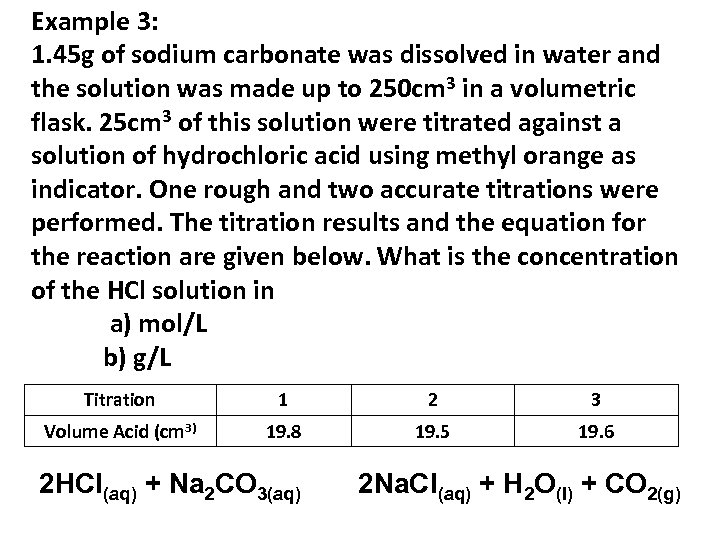
Example 3: 1. 45 g of sodium carbonate was dissolved in water and the solution was made up to 250 cm 3 in a volumetric flask. 25 cm 3 of this solution were titrated against a solution of hydrochloric acid using methyl orange as indicator. One rough and two accurate titrations were performed. The titration results and the equation for the reaction are given below. What is the concentration of the HCl solution in a) mol/L b) g/L Titration 1 2 3 Volume Acid (cm 3) 19. 8 19. 5 19. 6 2 HCl(aq) + Na 2 CO 3(aq) 2 Na. Cl(aq) + H 2 O(l) + CO 2(g)

Example 3 Solution:

Example 4: 10. 0 g of impure sodium hydroxide were weighed out, dissolved in water and solution made up to 250 cm 3 in a volumetric flask. 25 cm 3 of this solution, on being titrated with 1. 1 M HCl, required 21. 8 cm 3 of the acid for neutralisation. Calculate the % purity of the original sodium hydroxide.

Example 4 Solution:

Definition Secondary Standard: Make up a solution and then standardise this solution using a primary standard. This secondary standard can then be used to standardise other solutions e. g. HCl standardised and then used to standardise Na. OH

Mandatory Expt. 13. 3 (Ordinary Level): A Hydrochloric Acid/Sodium Hydroxide titration and the use of this titration in making the salt sodium chloride Note 1: You must have a clear understanding of all the steps you undertake in this experiment and be able to explain the importance of each step Note 2: You must be able to carry out calculations on your results *Calculate concentration of Na. OH in mol/L and g/L
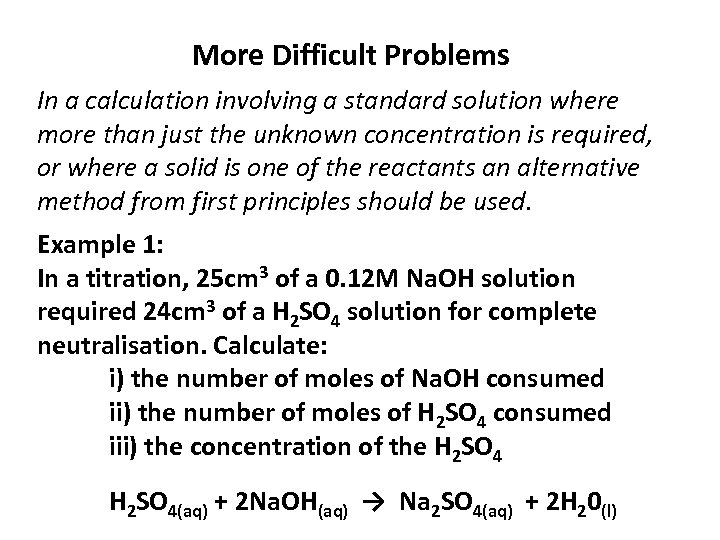
More Difficult Problems In a calculation involving a standard solution where more than just the unknown concentration is required, or where a solid is one of the reactants an alternative method from first principles should be used. Example 1: In a titration, 25 cm 3 of a 0. 12 M Na. OH solution required 24 cm 3 of a H 2 SO 4 solution for complete neutralisation. Calculate: i) the number of moles of Na. OH consumed ii) the number of moles of H 2 SO 4 consumed iii) the concentration of the H 2 SO 4(aq) + 2 Na. OH(aq) → Na 2 SO 4(aq) + 2 H 20(l)

Example 1 Solution:

Example 2: What mass of magnesium will react with 20 cm 3 of a 0. 09 M hydrochloric acid solution? The equation for the reaction is: 2 HCl(aq) + Mg(s) → Mg. Cl 2(aq) + H 2(g)

Example 2 Solution:
Applications of Acid-Base Titrations Mand. Expt. 13. 4: To determine the percentage of ethanoic acid in vinegar Mand. Expt. 13. 5: To determine the percentage of water of crystallisation in hydrated sodium carbonate (washing soda)
Mandatory Expt. 13. 4: To determine the percentage of ethanoic acid (acetic acid) in vinegar Note 1: You must have a clear understanding of all the steps you undertake in this experiment and be able to explain the importance of each step Note 3: You must understand the need for and the use of the dilution factor Note 2: You must be able to carry out calculations on your results *Calculate concentration of ethanoic acid in the original vinegar in mol/L, g/L and % w/v
Example 1: A sample of vinegar was diluted from 25 cm 3 to 250 cm 3 with water. In a titration, 25 cm 3 of a 0. 1 M Na. OH solution required 30 cm 3 of the diluted vinegar for complete neutralisation. Calculate the concentration of ethanoic acid (CH 3 COOH) in the vinegar in: i) mol L-1 ii) g/L iii) %w/v The equation for the reaction is CH 3 COOH(aq) + Na. OH(aq) → CH 3 COONa(aq) + H 2 O(l)

Example 1 Solution:
2. Calculating the amount of water of crystallisation in a compound and the relative molecular mass of the compound from the titration data Definition: • Water of crystallisation: is water that is chemically bound in the compound, which gives rise to the crystalline form. • Crystals that contain water of crystallisation are said to be hydrated.

Mandatory Expt. 13. 5: To determine the percentage of water of crystallisation in hydrated sodium carbonate (washing soda) • Hydrated sodium carbonate has the formula Na 2 CO 3. x. H 2 O ( where x = no. of molecules of water of crystallisation present) • The purpose of this experiment is to determine a value for x

Mandatory Expt. 13. 5: To determine the percentage of water of crystallisation in hydrated sodium carbonate (washing soda) Note 1: You must have a clear understanding of all the steps you undertake in this experiment and be able to explain the importance of each step Note 2: You must be able to carry out calculations on your results *Calculate the molar mass of hydrated sodium carbonate, the percentage of water of crystallisation and the value of X in the formula Na 2 CO 3. x. H 2 O

NOTE: Mandatory Expt. 13. 5: • Washing soda is composed of large translucent crystals of Na 2 CO 3. 10 H 2 O (form of hydrated sodium carbonate) • It is important to realise that hydrated sodium carbonate gradually looses water of crystallisation over a period of time. • Thus, if washing soda is used a lower value than the expected value of 10 for x in Na 2 CO 3. x. H 2 O will be obtained.

Example 1: Crystals of hydrated sodium carbonate (Na 2 CO 3. x. H 2 O) of mass 3. 15 g were dissolved in water and made up to 250 cm 3 in a volumetric flask. 25 cm 3 of this solution required 15 cm 3 of a 0. 15 M HCl solution for complete neutralisation. The equation for the reaction is: 2 HCl(aq) + Na 2 CO 3(aq) 2 Na. Cl(aq) + H 2 O(l) + CO 2(g) Find the: i) concentration of the sodium carbonate solution ii) the molar mass of the sodium carbonate solution iii) the value of x ion the formula Na 2 CO 3. x. H 2 O iv) the % of water of crystallisation in the hydrated sodium carbonate

Example 1 Solution:

Student Questions: Book: pg. 174 No’s 13. 15 & 3. 16
39db0e404e8535ddd270575b4cc2ba0b.ppt