ebc84a609a26d0d1b036e9bb7b10bf99.ppt
- Количество слайдов: 88

Visit our class website: Buttingerscience. weebly. com

Unit 1 – An Introduction to Science 1 – Define and explore the importance of Science 2 – Discuss the relationship between Science and Technology 3 – Define the Steps of the Scientific Method 4 – Explore the Scientific Process and Types of Observations 5 – Differentiate between a Theory, Blackbox and Model 6 – Recognize and interpret Domestic Hazardous symbols 7 – Recognize and interpret WHMIS symbols and labels (3 types) 8 – Lab Safety 9 – Metric System

Homework Assignment: A) Have the following supplies in 4 days and bring them from then on to EVERY Science class this semester: 1 - 3 ring binder and looseleaf 2 - 1 Duotang labeled “LAB REPORTS” 3 - Pencil, Pen, Eraser, Ruler Due: 4 class days B) Please read the article, “The Plight of the Honeybee. ” (Time Magazine) -Paper copy or on our class website: Buttingerscience. weebly. com – Intro Unit Page Answer all questions on the handout. Due: In 1 week

IS THIS SCIENCE? • • Hunt was working in the Tahoe Donner Subdivision in Truckee, standing atop a six-foot ladder while drilling over his head, when he gave the drill an extra push to bore a hole. As he felt the ladder begin to wobble out from under him, he tried tossing his power drill aside (a standard practice in the construction industry, intended to prevent workers from injuring themselves attempting to regain grips on out-of-control power tools) before falling to the ground. Unfortunately, he wasn't able to throw the drill far enough away, and he landed upon it face-first. The 18 -inch-long, 1. 5 -inch diameter chip auger drill bit pierced Hunt's right eye and exited through the side of his skull. As Hunt described the mishap: By the time I was falling, and I let the drill go down, I was already on top of it. The drill was facing up but it was off. When the drill hit, it just exploded my eye. It skewered me. I ran my hands up the drill bit, up to my eye, and put my other hand in the back of my head and felt it coming through the back of my head, and that's where pretty much the shock set in. The first thing I thought was 'Am I going to die? ' I knew it was serious. I was scared. I didn't know if it was in my brain or not. doctors pondered their options for treating the bizarre injury. Miraculously, although the drill bit tunneled between Hunt's scalp and his skull as it came out of the side of his head, it pushed his brain aside rather than pushing into it, sparing him from death, brain damage, or paralysis. We had to either cut down on it, which meant making a rather long incision through a lot of muscle, or just unscrew it - twist it all the way through and out. We would have cut it off, but after a few minutes of drilling, we noticed that it was loose. And so we just put down our blade and twisted the bit.

The Nature of Science 1. What is Science? (Draw a concept web to illustrate your ideas – leave space to add other ideas to your own) 2. Why do we study Science? Provide at least 5 reasons. 3. Is there a standard process that scientists follow which leads them to new discoveries? Name the process and list the key steps involved as best you can… 4. Would you consider yourself a scientist? Give reasons to support your claims. 5. List 5 scientific discoveries and 5 technological accomplishments over the last century that have had an effect on the human condition. 6. Is there a need to regulate scientific research? Who should decide on these regulations and how should such regulations be enforced?

Obj 1. What is Science? (Activities) -Concept Web Activity -“The Nature of Science” Activity -Group share Homework articles and decide on most “Scientific” -What is Science Flashcards Activity

What is Science? • Can be defined in several ways: 1 - An organized activity; the Scientific Process 2 - A body of knowledge; all the ideas of all the scientists who have worked before 3 - A human endeavor; done by people like you and me 4 - Puzzle-solving; the most creative people are the most successful usually 5 - Amoral; does not look at things as good or evil. (Evolution an argument between a faith view and a secular, scientific view) 6 - Sceptical; never takes things at face value, always looks below the surface. (Prove it or lose it. ) Intro to Zika Virus Outbreak Jan 2016

Main Branches of Science • Physics (Physical Science) -is the study of energy, force, and the motion of matter. • Chemistry (Physical Science)is the study of the properties and reactions of matter. • Biology (Health Science)is the study of living things • New names = Environmental Science, Physical Science and Health Science

THE IMPORTANT AND CHANGEABLE NATURE OF SCIENCE • Scientific investigation and explanations are what have allowed mankind to learn about their planet and world. Without Science we would be unable to survive in and improve our condition ( a definite human attribute) • The scientific community accepts and uses such explanations until displaced by better scientific ones when solving mysteries. • When such displacement occurs, the explanations change and science advances.
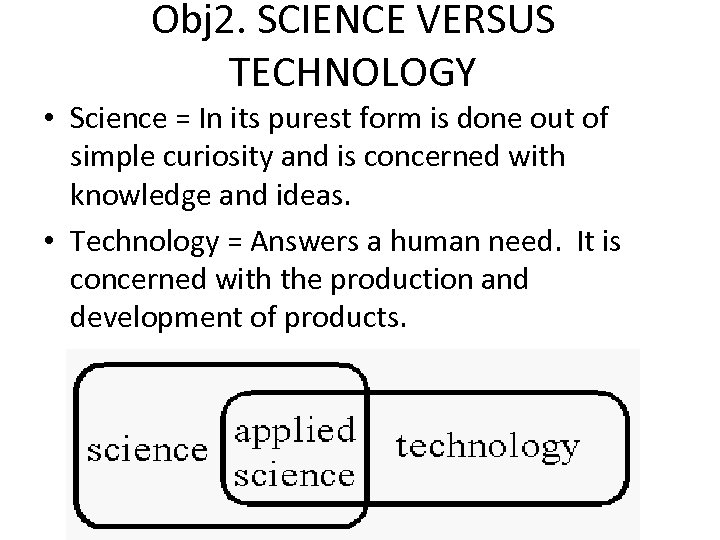
Obj 2. SCIENCE VERSUS TECHNOLOGY • Science = In its purest form is done out of simple curiosity and is concerned with knowledge and ideas. • Technology = Answers a human need. It is concerned with the production and development of products.
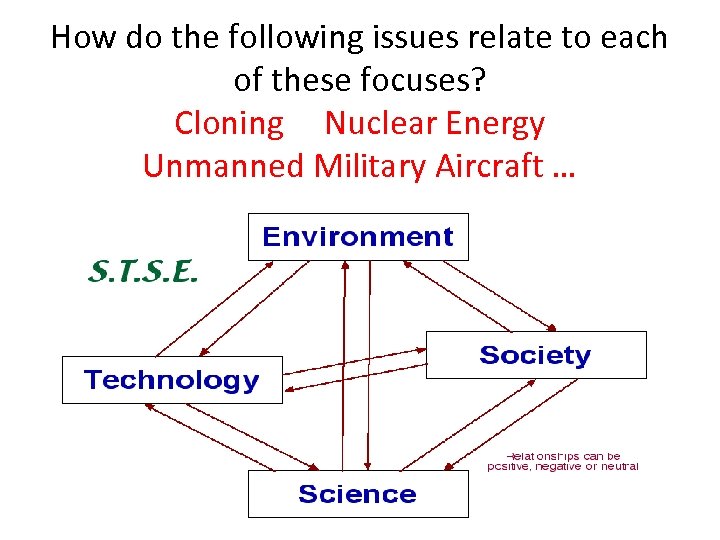
How do the following issues relate to each of these focuses? Cloning Nuclear Energy Unmanned Military Aircraft …

Video of Pop Bottle Lighting in Slum

Obj 3 -5. THE SCIENTIFIC METHOD, THE SCIENTIFIC PROCESS, THEORIES, MODELS AND BLACK BOXES -Students complete handout • 7 Steps of the Scientific Method, Process, Theories, Models, Black Boxes, and Types of Observations (4) • Activities: -Ambiguous Figures (Accurate Observations) -Loch Ness (Scientific Models) Scientists are expected to publish their discoveries in journals or scientific periodicals. • Other scientists check the facts in an article before it is published. • Even after publication, new scientific ideas are not generally accepted until the work has been replicated.


7 Steps of the Scientific Method • • 1. Find a Problem 2. Research the Problem 3. Create an Hypothesis 4. Experiment 5. Record Data 6. Conclusion 7. Repeat as Necessary Solar Freakin Roadways Example

• Why do we need to follow scientific procedures so strictly? • Because humans get strange ideas! • A - Medical Bleedings: • B- Earth is flat • C- Phrenology • Tests For Guilt at the Salem Witch Trials - Listverse • Is She a Witch? Experiment - Monty Python

Obj 6. DOMESTIC HAZARDOUS SYMBOLS • System of Precautionary symbols for use on products commonly available to the average consumer and found in average households. • The symbols represent the type of chemicals within a container, the possible risks associated with these chemicals and their containers.
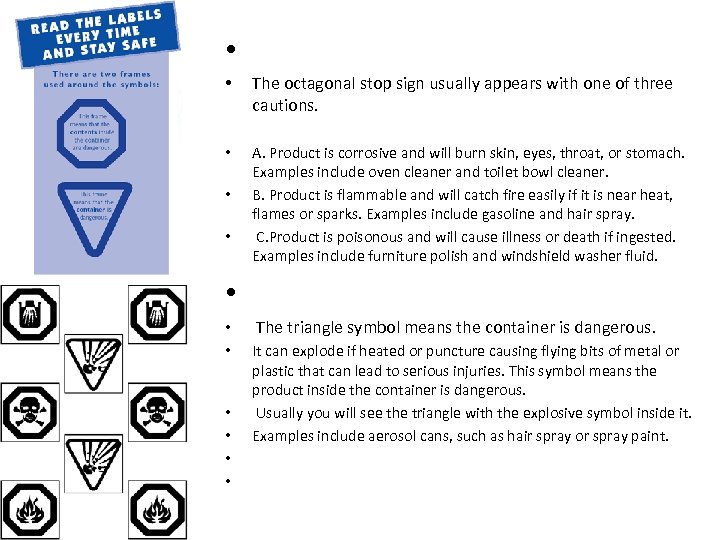
• • The octagonal stop sign usually appears with one of three cautions. • A. Product is corrosive and will burn skin, eyes, throat, or stomach. Examples include oven cleaner and toilet bowl cleaner. B. Product is flammable and will catch fire easily if it is near heat, flames or sparks. Examples include gasoline and hair spray. C. Product is poisonous and will cause illness or death if ingested. Examples include furniture polish and windshield washer fluid. • • • The triangle symbol means the container is dangerous. It can explode puncture causing flying bits of metal or if heated or plastic that can lead to serious injuries. This symbol means the product inside the container is dangerous. Usually you will see the triangle with the explosive symbol inside it. Examples include aerosol cans, such as hair spray or spray paint.

7. WHMIS / GHS Workplace Hazardous Materials Information System Globally Harmonized System (WHMIS 2. 0)

• WHMIS / GHS is a system of warning symbols and information sheets which detail the danger, safe handling and disposal of a variety of chemical substances in Canada and around the world. • All chemicals handled in Canada must be labelled using the WHMIS / GHS system.

SIGNAL WORDS • DANGER – indicates immediate and grave risk: • very toxic (acute toxicity) • flammable at low temperature • can cause blindness or skin damage in seconds.

SIGNAL WORDS (CONT. ) • WARNING – a cause for concern, but less immediate: • a chronic toxin, mutagen or carcinogen • flammable at high temperature • can cause irritation to eyes or skin

PHYSICAL HAZARDS PICTOGRAMS

EXPLOSIVE • symbol is an exploding bomb • unstable • self-reactive
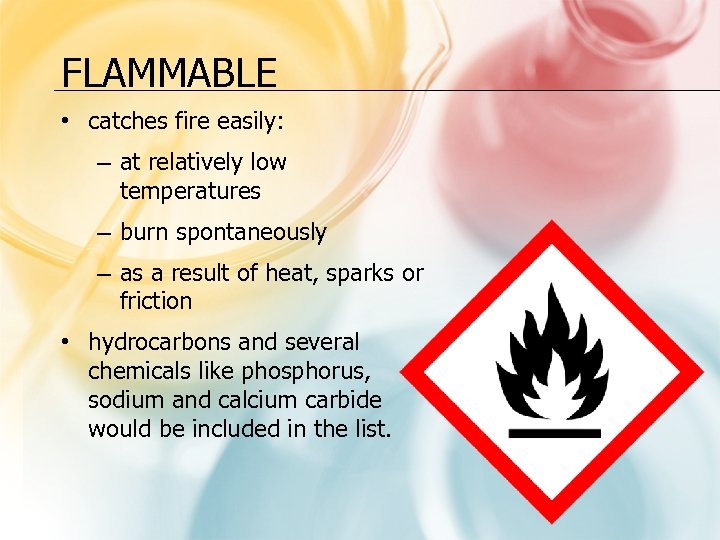
FLAMMABLE • catches fire easily: – at relatively low temperatures – burn spontaneously – as a result of heat, sparks or friction • hydrocarbons and several chemicals like phosphorus, sodium and calcium carbide would be included in the list.

OXIDIZER • may cause a fire, react violently or explode when it comes into contact with combustible materials such as wood. • an oxidizer supplies the oxygen for a chemical reaction.

COMPRESSED GAS • picture represents a gas cylinder • danger lies in the pressure, not the contents (this would add an extra symbol to the container).
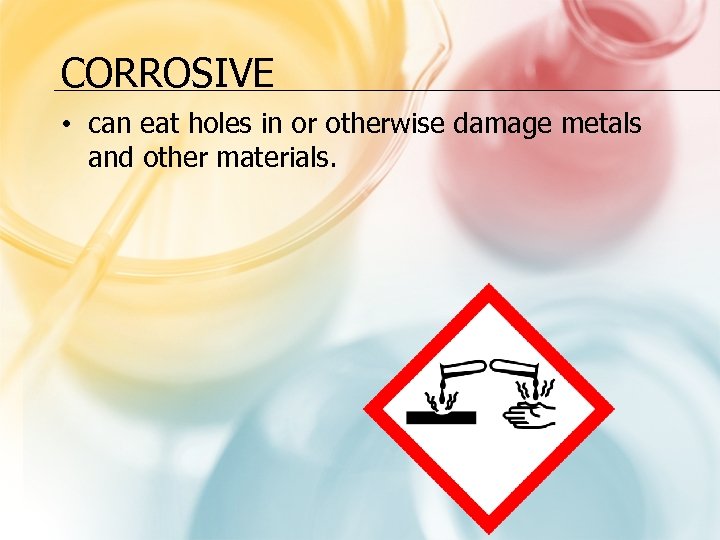
CORROSIVE • can eat holes in or otherwise damage metals and other materials.

HEALTH HAZARDS PICTOGRAMS

TOXIC • these substances have acute toxicity - refers to a substance which has immediate effects, usually within 24 hours

CORROSIVE • causes severe eye and skin irritation upon contact • causes severe tissue damage with prolonged exposure • may be harmful if inhaled • the effects are the same as under the household hazards
IRRITANT • used when “corrosive” is not required • can produce – skin sensitization – lung irritation – is toxic to specific organs • chronic effects

HEALTH HAZARD • Respiratory sensitization • Mutagenicity, Carcinogenicity • Reproductive toxicity • Specific target organ toxicity following single exposure • Aspiration hazard

ENVIRONMENTAL HAZARDS PICTOGRAMS
ENVIRONMENTALLY DAMAGING • acute or chronic effects • can affect terrestrial or aquatic ecosystems

OTHER SYMBOLS

BIOHAZARD • refers to an infectious agent (bacteria, virus or some other organism) which may spread disease if improperly handled, also called a biohazard • this symbol is common in hospital emergency rooms on containers where used needles and dressings are deposited

RADIOACTIVE • refers to ionizing radiation; can damage or destroy biological material, cause genetic mutations or cancer. • 3 classes of radioactivity: – alpha (α) • a helium nucleus • delivers energy to a surface. – beta (β) • an electron • delivers energy up to centimeter in depth

– gamma (γ) • high energy electromagnetic radiation • can go deep into tissue before releasing energy

RADIATION SYMBOL FOR DANGEROUS SOURCES CAPABLE OF DEATH OR SERIOUS INJURY. • includes traditional trefoil, with addition of wavy arrows to suggest radiation being emitted. • skull and crossbones emphasizes the health effects. • person running away suggests proper response.

GHS LABELS


SOME DEFINITIONS • Acute Toxicity – immediate effects (minutes, hours). – Effects can include illness, organ damage or death. • Chronic Toxicity – repeated exposure over a long period (weeks, months or years – The effects can also include cancer, allergies or chronic diseases. • Biohazard – refers to an infectious agent (bacteria, virus or some other organism) which may spread disease if improperly handled. • Carcinogen – can cause cancer given either acute or chronic exposure. • Mutagen – can cause damage to cellular structures, including DNA.

8. SAFETY IN THE CLASSROOM

LAB PROCEDURES AND RULES 1. No eating or drinking. – food can be contaminated by chemicals in the lab. – liquids can be mistaken; you don’t want to drink acid. 2. Do not wear loose clothing. Tie long hair back. – equipment and chemicals can be knocked over – clothing and hair can be ignited by bunsen burners 3. Do not sit on the lab bench. – clothing and skin can be damaged by residual chemicals 4. Never perform unauthorized experiments. – this is a lab or class-ending activity

5. Treat all chemicals as if they were hazardous. – do not touch or taste – smell by wafting the vapour toward your nose 6. Report all accidents immediately. – it is important to get it cleaned up correctly 7. If you get a chemical solution in your eye, treat immediately. Do not wait for the teacher. – there is a window of less than 30 seconds before damage can occur

8. If you get chemicals on your clothes, wash the clothes thoroughly. – chemicals can stain or damage clothing – clothing can keep chemicals against skin 9. Follow directions concerning disposal of chemicals and solutions. – proper disposal protects others from the chemicals. – some chemicals can contaminate the environment.

10. Clean your lab station after a lab. Clean all equipment thoroughly and put it back where it belongs. – others will use the equipment. Don’t put them in harm’s way by passing on dirty equipment.

KNOW WHERE EQUIPMENT IS: • eye wash (portable and permanent) • fire extinguisher • fire blanket • nearest fire pull-station • AED (automated external defibrillator) • spill kit(s)

IN CASE OF AN ACCIDENT: • Inhaled Poison - Remove the patient to fresh air and apply artificial respiration if necessary. Keep the victim warm with blankets. • Contact of Poison with Skin or Eyes - Flood affected area with water, for at least 5 minutes. Remove contaminated clothing. DO NOT attempt to use chemical antidote. • Swallowed Poison - If the person is conscious and able to swallow, immediately dilute the poison by giving the victim 2 to 4 cups of milk or water. • Swallowed Corrosives - DO NOT INDUCE VOMITING. Give milk and water. If vomiting occurs naturally, hold head below hips to avoid choking.

LABORATORY EQUIPMENT • Be able to identify the items on the sheet given. • Note: the flask is an erlenmeyer flask

A SAFE LAB IS A HAPPY LAB

Safety Equipment What are 5 pieces of Safety Equipment in a Science Classroom? 1) Eye Wash – used to wash harmful chemicals from your eyes
2) First Aid Kit – used to help treat minor injuries

3) Water Taps – Can be used to wash off harmful chemicals - can also be used to extinguish fires

4) Fire Extinguisher – used to put out fires.

5) Fume Hood – used to remove harmful fumes from the area. These are usually vented directly outside.

Obj 9. The Metric System • Within the last half century, scientists worldwide have developed a system of measuring things (quantitative observations) that uses a universal system of measurement. Whether you are measuring distance (meters), mass (grams), time (seconds), volume (litres), energy (Joules), etc… • The metric system conveniently is based on the number 10 as a conversion factor(base-10) • We commonly use prefixes from the metric system as an easy way of writing very large or very small numbers. • Many calculations in science use different metric measurements in their units. • One metric tonne(t) is 1000 kilograms= 1 Mg • 1 m. L fits in a box 1 cm X 1 cm, so 1 cm 3 = 1 m. L • Intro Metric System - You. Tube

The Metric way to measure almost anything: Measurement Unit Symbol Length metre m Mass gram g Volume litre L Temperature degrees Celsius C Time second s Force Newton N Pressure Pascal Pa Heat energy Joule J Amount of matter mole mol Electric current Amperes A Light intensity Candella cd

History of Measurement • Usually parts of the human body were used for measuring lengths: – Cubit=_____ – Span=_____ – Palm=_____ – Digit=_____ – Foot=______ – Inch=______ – Yard=______ – Fathom=______ – Bill Cosby - Noah - You. Tube

• This system of measurement created problems because their were no standards. • As the system evolved, new ways to measure were incorporated but the conversion between units became very complicated: • Examples: – 1 yard = _______ feet = ____inches – 1 mile = _______ feet = ____yards – (Goliath was 6 cubits and 1 span or about 9’ 8 in today’s standards!)
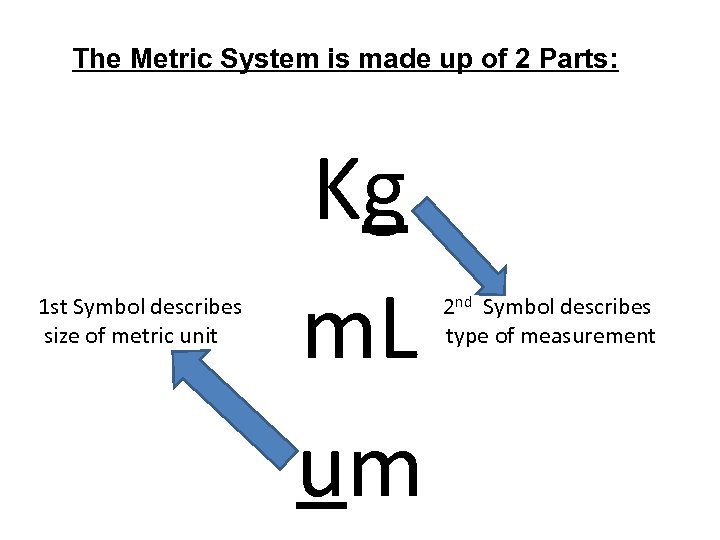
The Metric System is made up of 2 Parts: 1 st Symbol describes size of metric unit Kg m. L um 2 nd Symbol describes type of measurement
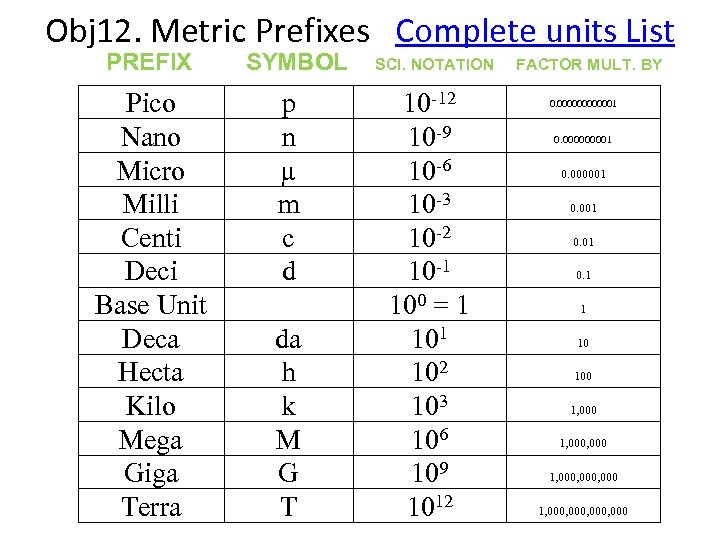
Obj 12. Metric Prefixes Complete units List PREFIX SYMBOL SCI. NOTATION FACTOR MULT. BY Pico Nano Micro Milli Centi Deci Base Unit Deca Hecta Kilo Mega Giga Terra p n µ m c d da h k M G T 10 -12 10 -9 10 -6 10 -3 10 -2 10 -1 100 = 1 102 103 106 109 1012 0. 0000001 0. 1 1 10 100 1, 000, 000, 000
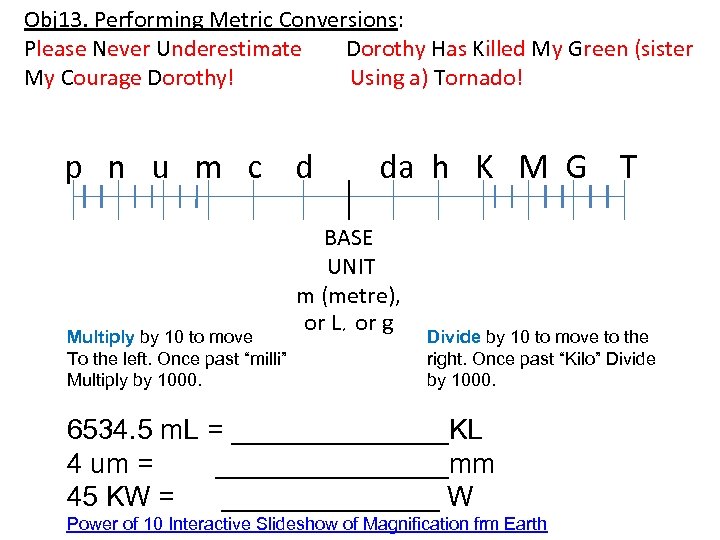
Obj 13. Performing Metric Conversions: Please Never Underestimate Dorothy Has Killed My Green (sister My Courage Dorothy! Using a) Tornado! p n u m c d Multiply by 10 to move To the left. Once past “milli” Multiply by 1000. da h K M G T BASE UNIT m (metre), or L, or g Divide by 10 to move to the right. Once past “Kilo” Divide by 1000. 6534. 5 m. L = _______KL 4 um = ________mm 45 KW = _______ W Power of 10 Interactive Slideshow of Magnification frm Earth

Scale of the Universe 2012 - One. More. Level. com
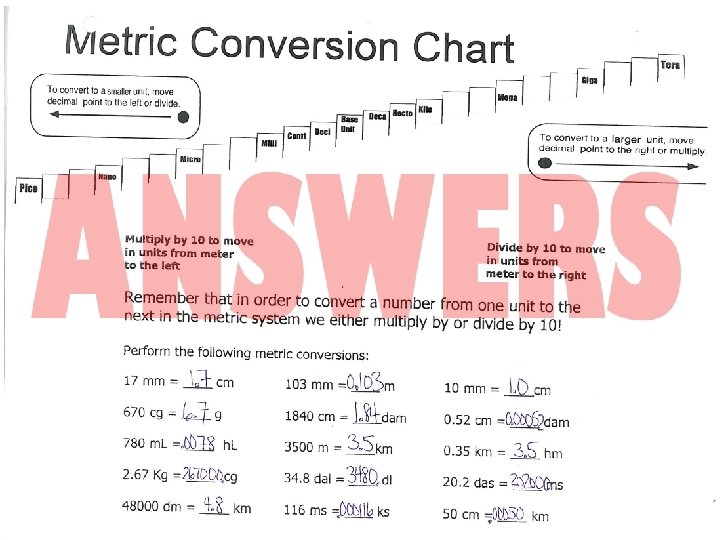

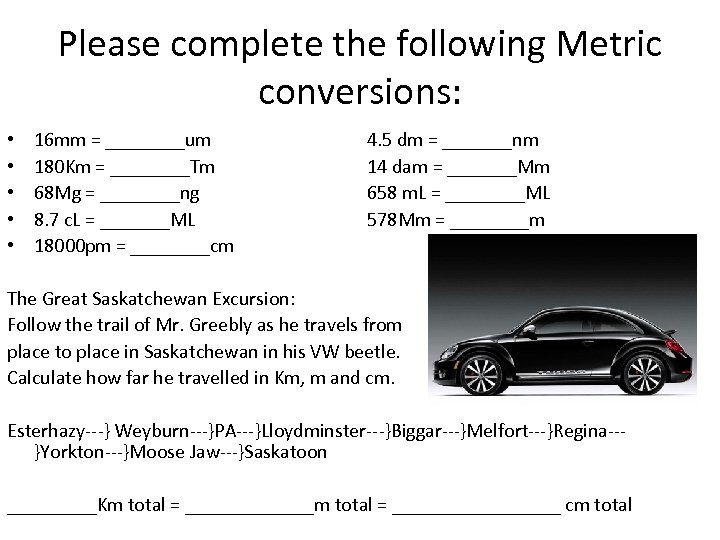
Please complete the following Metric conversions: • • • 16 mm = ____um 180 Km = ____Tm 68 Mg = ____ng 8. 7 c. L = _______ML 18000 pm = ____cm 4. 5 dm = _______nm 14 dam = _______Mm 658 m. L = ____ML 578 Mm = ____m The Great Saskatchewan Excursion: Follow the trail of Mr. Greebly as he travels from place to place in Saskatchewan in his VW beetle. Calculate how far he travelled in Km, m and cm. Esterhazy---} Weyburn---}PA---}Lloydminster---}Biggar---}Melfort---}Regina--}Yorkton---}Moose Jaw---}Saskatoon _____Km total = _________________ cm total

ANSWER • 253 • 479 • 339 • 230 • 268 • 279 • 187 • 258 + 226 2519 Km = 2, 519, 000. 00 m = 251, 900, 000. 00 cm

Scientific Notation • Allows us to express very large or very small numbers in a form that is easily understood and describes significant digits accurately. Examples: -The moon is 382 000 km away from the earth. - The sun is 149 600 000 meters away. -Mass of the sun= 1 989 000 000 000 g -Mass of 1 electron = 0. 000 000 000 911 g - Age of the Universe = 15 000 000 years In order to write out numbers in a more readable way we convert these large or small numbers into “Scientific Notation”. Before we do this however we must know what “Significant Figure’s” are:
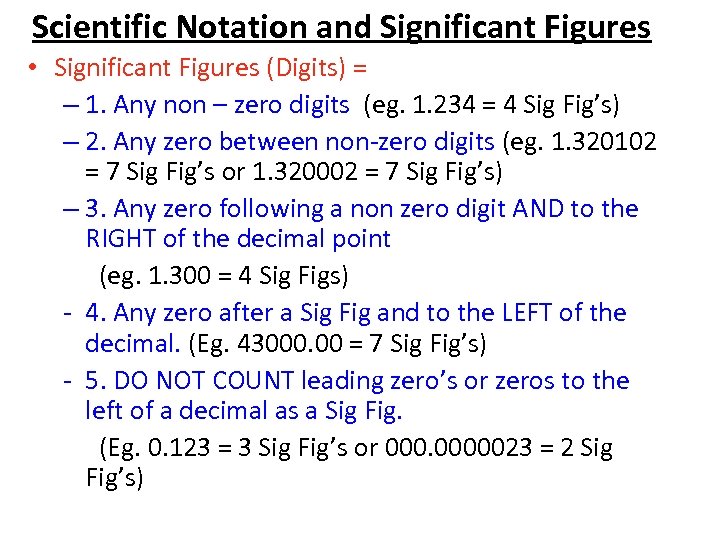
Scientific Notation and Significant Figures • Significant Figures (Digits) = – 1. Any non – zero digits (eg. 1. 234 = 4 Sig Fig’s) – 2. Any zero between non-zero digits (eg. 1. 320102 = 7 Sig Fig’s or 1. 320002 = 7 Sig Fig’s) – 3. Any zero following a non zero digit AND to the RIGHT of the decimal point (eg. 1. 300 = 4 Sig Figs) - 4. Any zero after a Sig Fig and to the LEFT of the decimal. (Eg. 43000. 00 = 7 Sig Fig’s) - 5. DO NOT COUNT leading zero’s or zeros to the left of a decimal as a Sig Fig. (Eg. 0. 123 = 3 Sig Fig’s or 0000023 = 2 Sig Fig’s)

How many Significant Digits are there in each of the following measured quantities? • • A) 37. 2 = B) 0. 000 076 s = C)301. 5 Kg = D) 56. 02 m = E) 5. 00 cm = F) 0. 000 000 97 m = G) 0. 05 bytes = H) 67 000 =

Process for Writing in Scientific Notation: • 1. Write the number out as a number between 1 and 9. 9999999. • 2. Count how many decimal places the decimal had to be moved to change the number into one between 1 and 9. 999999. • 3. Indicate this as an integer that is: • Positive (+) for numbers that were larger than 9. 99999 • Negative for numbers that were smaller than 1 Example 88 000 = 8. 8 x 10+4 0. 0000975 = 9. 75 x 10 -5
![Measurement Activity Information: Measuring the Size of: 1 Dimensional Object = Length = [----------------------] Measurement Activity Information: Measuring the Size of: 1 Dimensional Object = Length = [----------------------]](https://present5.com/presentation/ebc84a609a26d0d1b036e9bb7b10bf99/image-77.jpg)
Measurement Activity Information: Measuring the Size of: 1 Dimensional Object = Length = [----------------------] 2 Dimensional Object = Area Length Width 3 Dimensional Object = Volume = Height Length Width
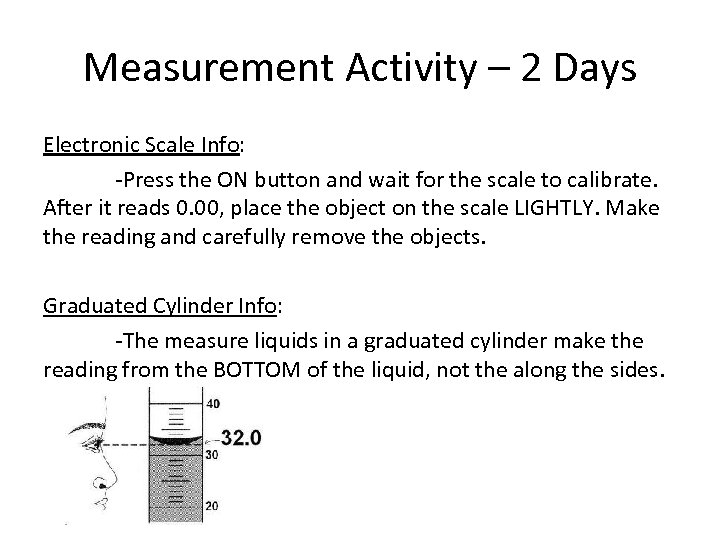
Measurement Activity – 2 Days Electronic Scale Info: -Press the ON button and wait for the scale to calibrate. After it reads 0. 00, place the object on the scale LIGHTLY. Make the reading and carefully remove the objects. Graduated Cylinder Info: -The measure liquids in a graduated cylinder make the reading from the BOTTOM of the liquid, not the along the sides.

Egg Drop Lab Potential Energy versus Kinetic Energy Purpose: 1. To learn about the transfer of energy; from potential to kinetic. 2. To design and test impact absorptive structures in an egg drop activity. 3. To learn about how the design of automobiles has changed over the course of the last 100 years. 4. To learn about the role that independant, dependant and controlled variables play in a science experiment. Materials: Hypothesis: I believe that ______’s structure will be the most effective because _______________. Procedure: Data: Discussion: Conclusion: Sources of Error:

Egg Drop Structure Rules: • The container must have dimensions no larger than 10”x 10”. • The container must have no water or liquid substance included in the design anywhere. • The structure may utilize any household materials found and should have a RAW egg within the structure at the time of the crash testing. • The egg can have no materials attached to it (glue, varnish) and must be a large, raw, unhampered egg. • The egg must be visible (or able to be checked on between trials drops. • The test structures will be tested on ________.

Objective 14 -16: Graphing • In order to understand how to complete a scientific graph we must first understand what a Science experiment’s 3 “VARIABLES” are: 1. Independent Variable: -Part of the experiment that you change on purpose. Eg. Airplane design 2. Dependant Variable: -Results of making the change in your experiment. Eg. Who’s plane went the furthest? 3. Controlled Variables: -Other factors in the experiment which need to be controlled so that they do not interfere with your results (dependant variable). Eg. Starting point, materials used, Way they threw, path of flight, air current, lack of damage btw. trials…
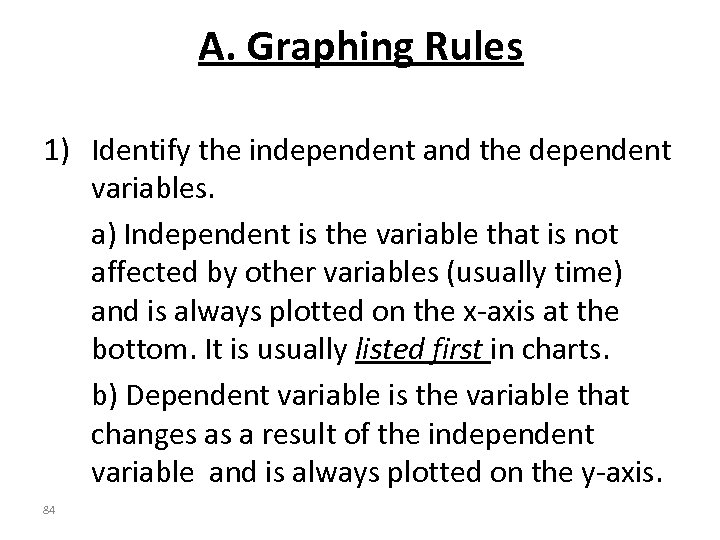
A. Graphing Rules 1) Identify the independent and the dependent variables. a) Independent is the variable that is not affected by other variables (usually time) and is always plotted on the x-axis at the bottom. It is usually listed first in charts. b) Dependent variable is the variable that changes as a result of the independent variable and is always plotted on the y-axis. 84

2) Label each axis with the name of the variable and the unit. 3) Title your graph (ex. A Graph of Dependent vs. Independent) 4) Include your name, date, class, and period on the top right hand corner of your graph. 5) Choose your scale carefully and make your graph as large as possible. Use EQUAL increments and make the largest number on the scale just larger than the highest point to plot. 85
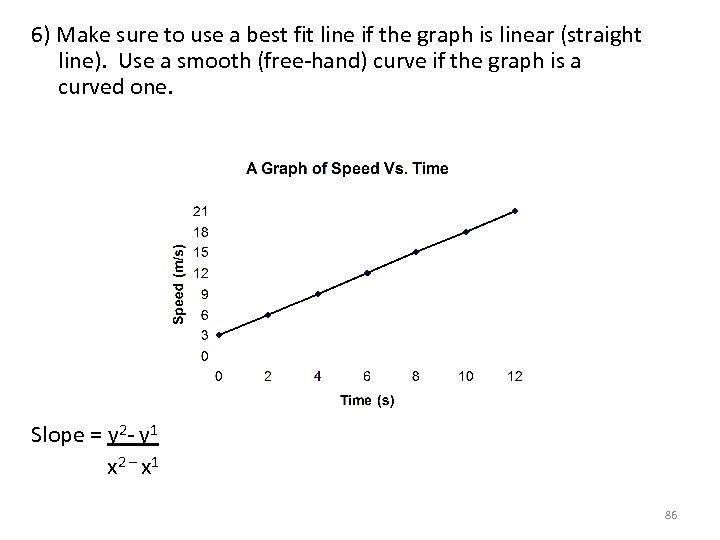
6) Make sure to use a best fit line if the graph is linear (straight line). Use a smooth (free-hand) curve if the graph is a curved one. Slope = y 2 - y 1 x 2 – x 1 86
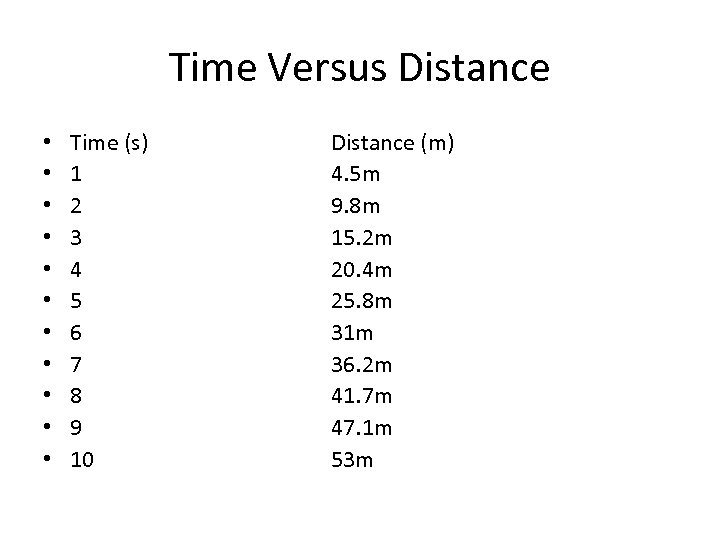
Time Versus Distance • • • Time (s) 1 2 3 4 5 6 7 8 9 10 Distance (m) 4. 5 m 9. 8 m 15. 2 m 20. 4 m 25. 8 m 31 m 36. 2 m 41. 7 m 47. 1 m 53 m
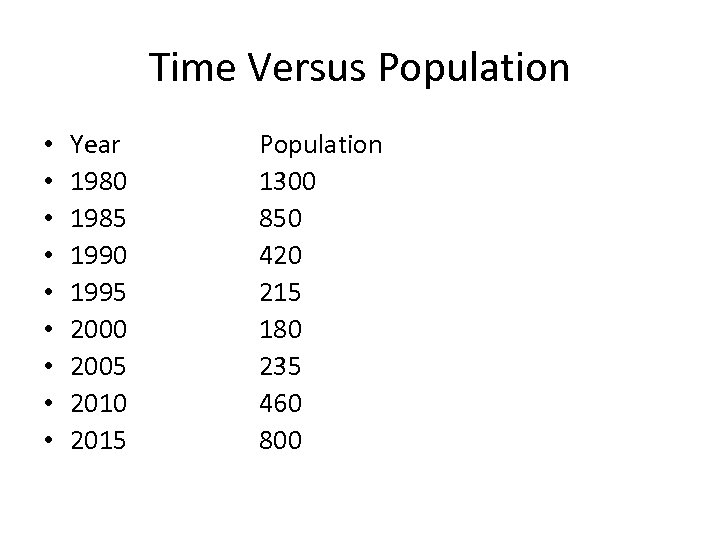
Time Versus Population • • • Year 1980 1985 1990 1995 2000 2005 2010 2015 Population 1300 850 420 215 180 235 460 800
ebc84a609a26d0d1b036e9bb7b10bf99.ppt