477cfb805e4808f366fb9e2e6ee3524f.ppt
- Количество слайдов: 39
 VII. Human Factors Considerations in Medical Device Accident Investigation TRAINING SEMINAR ON MEDICAL DEVICE ACCIDENT INVESTIGATION for Kingdom of Saudi Arabia Saudi Food & Drug Authority Riyadh 11 -14 February, 2007 Presenter: Mark E. Bruley Vice President, Accident and Forensic Investigation ECRI 5200 Butler Pike, Plymouth Meeting, PA, 19642 USA Tel: +1 610 -825 -6000, ext. 5223 E-mail: mbruley@ecri. org Web Sites: www. ecri. org www. mdsr. ecri. org ©ECRI 2007 1
VII. Human Factors Considerations in Medical Device Accident Investigation TRAINING SEMINAR ON MEDICAL DEVICE ACCIDENT INVESTIGATION for Kingdom of Saudi Arabia Saudi Food & Drug Authority Riyadh 11 -14 February, 2007 Presenter: Mark E. Bruley Vice President, Accident and Forensic Investigation ECRI 5200 Butler Pike, Plymouth Meeting, PA, 19642 USA Tel: +1 610 -825 -6000, ext. 5223 E-mail: mbruley@ecri. org Web Sites: www. ecri. org www. mdsr. ecri. org ©ECRI 2007 1
 Human Factors Considerations in Surgical Accidents with Medical Devices u. ECRI u. Device Accidents: Causes u. User Error and Human Factors in Surgery u. Developments in Human Factors u. Examples and Case Histories © 2007 ECRI 2
Human Factors Considerations in Surgical Accidents with Medical Devices u. ECRI u. Device Accidents: Causes u. User Error and Human Factors in Surgery u. Developments in Human Factors u. Examples and Case Histories © 2007 ECRI 2
 ECRI and Human Factors (HF) • 30 years of HF application to: – Comparative medical device evaluations – Investigation of thousands of problem reports – Accident Investigations – Sponsored 1989 international symposium on Human Factors in Medical Devices © 2007 ECRI 3
ECRI and Human Factors (HF) • 30 years of HF application to: – Comparative medical device evaluations – Investigation of thousands of problem reports – Accident Investigations – Sponsored 1989 international symposium on Human Factors in Medical Devices © 2007 ECRI 3
 Human Factors • Means different things to different people – Visual Displays – Anthropometrics – Time-motion studies – Safe design that is not prone to causing misuse • Ergonomics ( User-Tool_Relationship) • Definition of Human Factors: Aggregate of design characteristics that determine the work relationship of product and user. © 2007 ECRI 4
Human Factors • Means different things to different people – Visual Displays – Anthropometrics – Time-motion studies – Safe design that is not prone to causing misuse • Ergonomics ( User-Tool_Relationship) • Definition of Human Factors: Aggregate of design characteristics that determine the work relationship of product and user. © 2007 ECRI 4
 Human Factors: Definitions • Error: – Actions or omissions leading to results that were neither foreseen or intended – Most errors are benign – Combinations of errors lead to accidents • Slip: Correct action done incorrectly • Mistake: Wrong action done correctly or incorrectly © 2007 ECRI 5
Human Factors: Definitions • Error: – Actions or omissions leading to results that were neither foreseen or intended – Most errors are benign – Combinations of errors lead to accidents • Slip: Correct action done incorrectly • Mistake: Wrong action done correctly or incorrectly © 2007 ECRI 5
 6
6
 Human Factors Considerations in Surgical Accidents with Medical Devices u. ECRI u. Device Accidents: Causes u. User Error and Human Factors in Surgery u. Developments in Human Factors u. Examples and Case Histories © 2007 ECRI 7
Human Factors Considerations in Surgical Accidents with Medical Devices u. ECRI u. Device Accidents: Causes u. User Error and Human Factors in Surgery u. Developments in Human Factors u. Examples and Case Histories © 2007 ECRI 7
 Causes of Device Accidents (* Human Factors related) u. Device Factors u. External Factors u. Tampering and Sabotage u. Support System Failures u. User Factors (Error and Human Factors) If findings are undecided, consider idiosyncratic patient reaction to device or therapy. © 2007 ECRI 8
Causes of Device Accidents (* Human Factors related) u. Device Factors u. External Factors u. Tampering and Sabotage u. Support System Failures u. User Factors (Error and Human Factors) If findings are undecided, consider idiosyncratic patient reaction to device or therapy. © 2007 ECRI 8
 Accident Causes: Device Factors • • Device Failure Design or Labeling* Manufacturing Packaging* Software Random Component Failure of an Accessory Copyright ECRI 2000 9
Accident Causes: Device Factors • • Device Failure Design or Labeling* Manufacturing Packaging* Software Random Component Failure of an Accessory Copyright ECRI 2000 9
 Accident Causes: Device Factors (cont. ) • Invalid Device Foundation • Device Interactions • Improper maintenance, testing, repair, or lack or failure of pre-use incoming inspection • Device Interactions * • Improper Modification * © 2007 ECRI 10
Accident Causes: Device Factors (cont. ) • Invalid Device Foundation • Device Interactions • Improper maintenance, testing, repair, or lack or failure of pre-use incoming inspection • Device Interactions * • Improper Modification * © 2007 ECRI 10
 Accident Causes: Support System Failure • Poor device evaluation during tendering process • Lack or failure of incoming and pre-use inspections • Using inappropriate devices • Cleaning, sterilization, storage * © 2007 ECRI 11
Accident Causes: Support System Failure • Poor device evaluation during tendering process • Lack or failure of incoming and pre-use inspections • Using inappropriate devices • Cleaning, sterilization, storage * © 2007 ECRI 11
 Human Factors Considerations in Surgical Accidents with Medical Devices u. ECRI u. Device Accidents: Causes u. User Error and Human Factors in Surgery u. Developments in Human Factors u. Examples and Case Histories © 2007 ECRI 12
Human Factors Considerations in Surgical Accidents with Medical Devices u. ECRI u. Device Accidents: Causes u. User Error and Human Factors in Surgery u. Developments in Human Factors u. Examples and Case Histories © 2007 ECRI 12
 Device Accidents 13
Device Accidents 13
 Causes: User Error 50 - 70% of Device Accidents • • • Pre-use inspections Labeling * Mis-assembly * Mis-connection * Improper (“bad”) connection * • Incorrect clinical use • Incorrect control settings * • Incorrect programming * • Spills • Abuse * • Inappropriate reliance on automated features* • Failure to monitor • Maintenance or © 2007 incoming inspection ECRI 14
Causes: User Error 50 - 70% of Device Accidents • • • Pre-use inspections Labeling * Mis-assembly * Mis-connection * Improper (“bad”) connection * • Incorrect clinical use • Incorrect control settings * • Incorrect programming * • Spills • Abuse * • Inappropriate reliance on automated features* • Failure to monitor • Maintenance or © 2007 incoming inspection ECRI 14
 Human Factors and the O. R. Domain (Gaba 1994. In Bogner, ed. Human Error in Medicine, Hillsdale, NJ, Lawrence Erlbaum Assoc. ) • Complex and dynamic setting (ICU and Emergency Dept. also) • Multiple clinical specialists (surg, anes, neuro, nurse, perfusion) • Significant Risk • Uncertainty/ Fatigue • Limited space/time • EMI • Hundreds of devices/instruments © 2007 ECRI 15
Human Factors and the O. R. Domain (Gaba 1994. In Bogner, ed. Human Error in Medicine, Hillsdale, NJ, Lawrence Erlbaum Assoc. ) • Complex and dynamic setting (ICU and Emergency Dept. also) • Multiple clinical specialists (surg, anes, neuro, nurse, perfusion) • Significant Risk • Uncertainty/ Fatigue • Limited space/time • EMI • Hundreds of devices/instruments © 2007 ECRI 15
 Human Factors: The O. R. ’s Structure vs. Aviation Model • Useful in many respects, but limited in scope • Medical Devices vs. Aviation – 5, 000: 1 – Medical Devices - 5, 000 device types, plus subsystems and components – Aviation - 1 aircraft, plus subsystems and component © 2007 ECRI 16
Human Factors: The O. R. ’s Structure vs. Aviation Model • Useful in many respects, but limited in scope • Medical Devices vs. Aviation – 5, 000: 1 – Medical Devices - 5, 000 device types, plus subsystems and components – Aviation - 1 aircraft, plus subsystems and component © 2007 ECRI 16
 Human Factors: O. R. vs. Aviation Model • Both decentralized – 1000 s of operations/flights per day – 1000 s of hospitals vs. few air carriers • Aviation is highly regulated: O. R. s are not • Aircraft work setting (cockpits) are integrated systems when procured • O. R. equipment and systems are procured piecemeal © 2007 ECRI 17
Human Factors: O. R. vs. Aviation Model • Both decentralized – 1000 s of operations/flights per day – 1000 s of hospitals vs. few air carriers • Aviation is highly regulated: O. R. s are not • Aircraft work setting (cockpits) are integrated systems when procured • O. R. equipment and systems are procured piecemeal © 2007 ECRI 17
 Human Factors: Device Interfaces • Device - User • Device - Patient • Device - Accessories (Including disposable devices) • Device - Environment – Hospital, Ambulance, Home © 2007 ECRI 18
Human Factors: Device Interfaces • Device - User • Device - Patient • Device - Accessories (Including disposable devices) • Device - Environment – Hospital, Ambulance, Home © 2007 ECRI 18
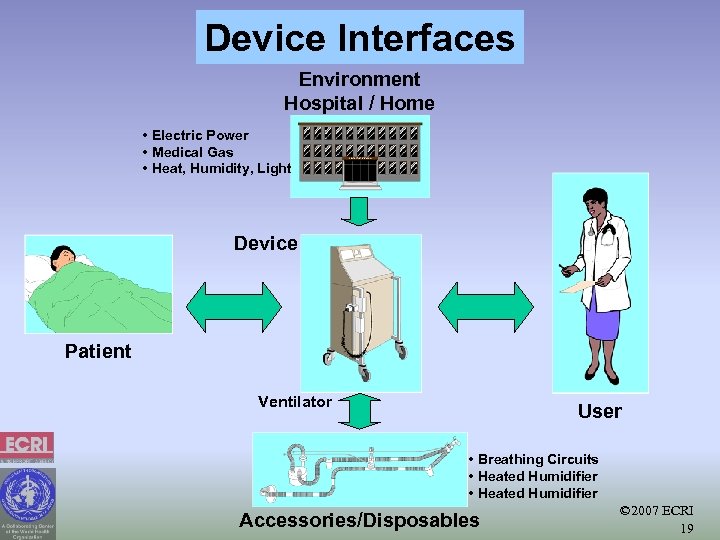 Device Interfaces Environment Hospital / Home • Electric Power • Medical Gas • Heat, Humidity, Light Device Patient Ventilator User • Breathing Circuits • Heated Humidifier Accessories/Disposables © 2007 ECRI 19
Device Interfaces Environment Hospital / Home • Electric Power • Medical Gas • Heat, Humidity, Light Device Patient Ventilator User • Breathing Circuits • Heated Humidifier Accessories/Disposables © 2007 ECRI 19
 Difficulties in Designing Ideal Medical Devices for O. R. (Gaba, ibid. ) • Users usually under great stress • Smart user’s false confidence in how to do with the device what they want to do. • Users unfamiliarity with devices limitations and subtleties of use is common • User training time is minimal • Few stds/regs on integrated HF performance • Existing stds/guidelines cover only sub -systems of HF problem © 2007 ECRI 20
Difficulties in Designing Ideal Medical Devices for O. R. (Gaba, ibid. ) • Users usually under great stress • Smart user’s false confidence in how to do with the device what they want to do. • Users unfamiliarity with devices limitations and subtleties of use is common • User training time is minimal • Few stds/regs on integrated HF performance • Existing stds/guidelines cover only sub -systems of HF problem © 2007 ECRI 20
 Human Factors: Common Conventions • Counter-intuitive designs • Oxygen regulator: Clockwise to increase flow (like volume control) • Oxygen cylinder tank valve: Counterclockwise to shut off © 2007 ECRI 21
Human Factors: Common Conventions • Counter-intuitive designs • Oxygen regulator: Clockwise to increase flow (like volume control) • Oxygen cylinder tank valve: Counterclockwise to shut off © 2007 ECRI 21
 Human Factors Considerations in Surgical Accidents with Medical Devices u. ECRI u. Device Accidents u. Mechanisms of Injury u. Causes of Accidents u. User Error and Human Factors in Surgery u. Developments in Human Factors u. Examples and Case Histories © 2007 ECRI 22
Human Factors Considerations in Surgical Accidents with Medical Devices u. ECRI u. Device Accidents u. Mechanisms of Injury u. Causes of Accidents u. User Error and Human Factors in Surgery u. Developments in Human Factors u. Examples and Case Histories © 2007 ECRI 22
 Human Factors: Error Reduction Anesthesia • Long studied specialty in regard to error • Gaba, Cook, Cooper, Woods • Anesthesia Patient Safety Foundation (USA) • Simulators and training mannequins • 24% committed error leading to fatality (Mac. Donald JA. Anes 1985; 63: A 497. ) © 2007 ECRI 23
Human Factors: Error Reduction Anesthesia • Long studied specialty in regard to error • Gaba, Cook, Cooper, Woods • Anesthesia Patient Safety Foundation (USA) • Simulators and training mannequins • 24% committed error leading to fatality (Mac. Donald JA. Anes 1985; 63: A 497. ) © 2007 ECRI 23
 Databases on Problem Reports and/or Technology Assessment • ECRI’s Health Device Alerts (HDA) • ECRI’s Medical Device Safety Reports – www. mdsr. ecri. org • US FDA: MDR, PRP, and MAUDE – www. fda. gov/cdrh • Australia: Therapeutic Goods Admin. • European Union Medical Device Directive (MDD) (future) © 2007 ECRI 24
Databases on Problem Reports and/or Technology Assessment • ECRI’s Health Device Alerts (HDA) • ECRI’s Medical Device Safety Reports – www. mdsr. ecri. org • US FDA: MDR, PRP, and MAUDE – www. fda. gov/cdrh • Australia: Therapeutic Goods Admin. • European Union Medical Device Directive (MDD) (future) © 2007 ECRI 24
 Human Factors: Error Reduction Design Guidance • Human Factors Design Guides/Texts • AAMI Human Factors Engineering Guidelines and Preferred Practices for the Design of Medical Devices. ANSI/AAMI HE 48 1993. • FDA (USA) “Do It By Design: An Introduction to Human Factors in Medical Devices. ” www. fda. gov/cdrh/Human. Factors. html © 2007 ECRI 25
Human Factors: Error Reduction Design Guidance • Human Factors Design Guides/Texts • AAMI Human Factors Engineering Guidelines and Preferred Practices for the Design of Medical Devices. ANSI/AAMI HE 48 1993. • FDA (USA) “Do It By Design: An Introduction to Human Factors in Medical Devices. ” www. fda. gov/cdrh/Human. Factors. html © 2007 ECRI 25
 Human Factors: Error Reduction Realizations • Device Design: – Total error elimination by design is not possible. • Education has only a partial role • No “Big Fix” © 2007 ECRI 26
Human Factors: Error Reduction Realizations • Device Design: – Total error elimination by design is not possible. • Education has only a partial role • No “Big Fix” © 2007 ECRI 26
 Human Factors: Error Reduction. Training • • Frequently focused on problematic staff Tends to point blame Seldom has positive long-term effect Targeted Training – Some positive effect – Tool use – Effective staff communication © 2007 ECRI 27
Human Factors: Error Reduction. Training • • Frequently focused on problematic staff Tends to point blame Seldom has positive long-term effect Targeted Training – Some positive effect – Tool use – Effective staff communication © 2007 ECRI 27
 Human Factors: Error Reduction. Users • Users’ continuing awareness of hazards is essential • Hospital’s Responsibilities – Clinical Evaluations – Equipment Acquisition Tendering Process © 2007 ECRI 28
Human Factors: Error Reduction. Users • Users’ continuing awareness of hazards is essential • Hospital’s Responsibilities – Clinical Evaluations – Equipment Acquisition Tendering Process © 2007 ECRI 28
 Human Factors: Error Reduction. Vendors • Vendors’ Responsibilities – Technical design – The user’s role – The use environment – Instructions / labeling © 2007 ECRI 29
Human Factors: Error Reduction. Vendors • Vendors’ Responsibilities – Technical design – The user’s role – The use environment – Instructions / labeling © 2007 ECRI 29
 Human Factors Considerations in Surgical Accidents with Medical Devices u. ECRI u. Device Accidents: Causes u. User Error and Human Factors in Surgery u. Developments in Human Factors u. Examples and Case Histories © 2007 ECRI 30
Human Factors Considerations in Surgical Accidents with Medical Devices u. ECRI u. Device Accidents: Causes u. User Error and Human Factors in Surgery u. Developments in Human Factors u. Examples and Case Histories © 2007 ECRI 30
 Examples and Case Histories • High Risk Devices – Surgical Diathermy (electrosurgery) /Lasers • Fires (NYU Med Cntr; Cedars-Sinai) – Nitrogen Powered Irrigation Systems • Misconnection and gas embolism – Arthroscopic Instruments • Breakage (Powered cutters; Small instruments) – Trocars • Hand Grip Technique – Cranial Perforators • Assembly and Pre-Use Checkout © 2007 ECRI 31
Examples and Case Histories • High Risk Devices – Surgical Diathermy (electrosurgery) /Lasers • Fires (NYU Med Cntr; Cedars-Sinai) – Nitrogen Powered Irrigation Systems • Misconnection and gas embolism – Arthroscopic Instruments • Breakage (Powered cutters; Small instruments) – Trocars • Hand Grip Technique – Cranial Perforators • Assembly and Pre-Use Checkout © 2007 ECRI 31
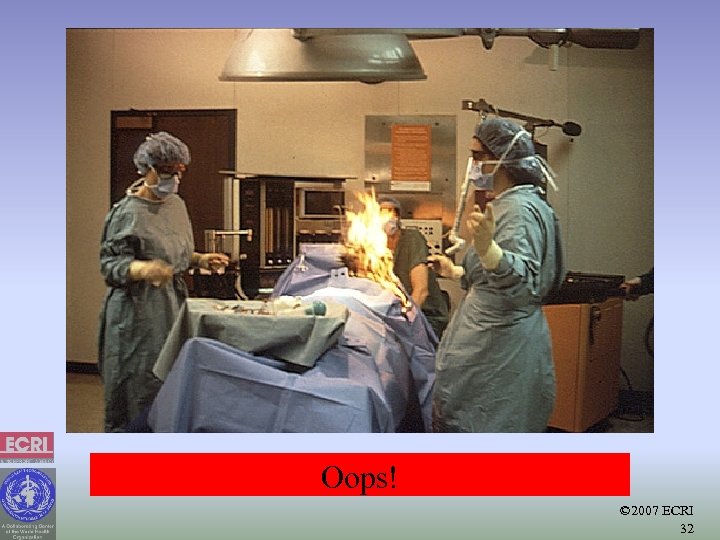 Oops! © 2007 ECRI 32
Oops! © 2007 ECRI 32
 M 005 XN 9 A-03 33
M 005 XN 9 A-03 33
 M 005 XN 9 A-43 © 2007 ECRI 34
M 005 XN 9 A-43 © 2007 ECRI 34
 M 005 XN 9 A-44 © 2007 ECRI 35
M 005 XN 9 A-44 © 2007 ECRI 35
 Examples and Case Histories • High Risk Devices – Surgical Diathermy (electrosurgery) /Lasers • Fires (NYU Med Cntr; Cedars-Sinai) – Nitrogen Powered Irrigation Systems • Misconnection and gas embolism – Arthroscopic Instruments • Breakage (Powered cutters; Small instruments) – Trocars • Hand Grip Technique – Cranial Perforators • Assembly and Pre-Use Checkout © 2007 ECRI 36
Examples and Case Histories • High Risk Devices – Surgical Diathermy (electrosurgery) /Lasers • Fires (NYU Med Cntr; Cedars-Sinai) – Nitrogen Powered Irrigation Systems • Misconnection and gas embolism – Arthroscopic Instruments • Breakage (Powered cutters; Small instruments) – Trocars • Hand Grip Technique – Cranial Perforators • Assembly and Pre-Use Checkout © 2007 ECRI 36
 Human Factors and Device Accidents in Surgery: The Future • Continuing research in HF • Need for ongoing user vigilance of device hazards • Design for error avoidance and system error tolerance • User preference in design and selection versus device performance (especially important in tendering process) © 2007 ECRI 37
Human Factors and Device Accidents in Surgery: The Future • Continuing research in HF • Need for ongoing user vigilance of device hazards • Design for error avoidance and system error tolerance • User preference in design and selection versus device performance (especially important in tendering process) © 2007 ECRI 37
 Again – Human Factors: Error Reduction Design Guidance • Human Factors Design Guides/Texts • AAMI Human Factors Engineering Guidelines and Preferred Practices for the Design of Medical Devices. ANSI/AAMI HE 48 1993. • FDA (USA) “Do It By Design: An Introduction to Human Factors in Medical Devices. ” www. fda. gov/cdrh/Human. Factors. html © 2007 ECRI 38
Again – Human Factors: Error Reduction Design Guidance • Human Factors Design Guides/Texts • AAMI Human Factors Engineering Guidelines and Preferred Practices for the Design of Medical Devices. ANSI/AAMI HE 48 1993. • FDA (USA) “Do It By Design: An Introduction to Human Factors in Medical Devices. ” www. fda. gov/cdrh/Human. Factors. html © 2007 ECRI 38
 VII. Human Factors Considerations in Accident Investigation QUESTIONS? © 2007 ECRI 39
VII. Human Factors Considerations in Accident Investigation QUESTIONS? © 2007 ECRI 39


