d6d8c92f4f77ee0ad32e58ae7bbc4ce4.ppt
- Количество слайдов: 26
 VA Drug Terminology Projects Update: NDF-RT, the New Drug Transaction, And Beyond Presented to HL 7 Vocabulary TC Meeting VA: Steve Brown, Mike Lincoln Apelon: Mark Tuttle, John Carter, Mark Erlbaum October 3, 2002 10/3/2002 HL 7 Baltimore
VA Drug Terminology Projects Update: NDF-RT, the New Drug Transaction, And Beyond Presented to HL 7 Vocabulary TC Meeting VA: Steve Brown, Mike Lincoln Apelon: Mark Tuttle, John Carter, Mark Erlbaum October 3, 2002 10/3/2002 HL 7 Baltimore
 Overview NDF-RT: Current state and plans • New Drug Transaction: Introduction • Interagency cooperation update • 10/3/2002 HL 7 Baltimore 2
Overview NDF-RT: Current state and plans • New Drug Transaction: Introduction • Interagency cooperation update • 10/3/2002 HL 7 Baltimore 2
 Background: VA National Drug File (NDF) • Centrally maintained and distributed, locally modified and deployed. Uses include: • Vist. A POE Decision Support • Mail out pharmacy (57 million in 2001) • Single-inheritance hierarchy including • VA Drug Classes (anti-hypertensives, beta-blockers) • Products (Ampicillin 250 mg tab) • Ingredients (carbidopa, codeine phosphate) • NDCs (acetaminophen 325 mg tab, bottle of 100) 10/3/2002 HL 7 Baltimore 3
Background: VA National Drug File (NDF) • Centrally maintained and distributed, locally modified and deployed. Uses include: • Vist. A POE Decision Support • Mail out pharmacy (57 million in 2001) • Single-inheritance hierarchy including • VA Drug Classes (anti-hypertensives, beta-blockers) • Products (Ampicillin 250 mg tab) • Ingredients (carbidopa, codeine phosphate) • NDCs (acetaminophen 325 mg tab, bottle of 100) 10/3/2002 HL 7 Baltimore 3
 NDF Reference Terminology (NDF-RT) • Explicit, multi-hierarchical model • Centered on drug ingredients for function, maintenance and economies of scale Semi-algorithmic initialization followed by human review • Authoritative, collaborative content (FDA, NLM, HL 7, others) • VA’s ERT strategy • • Subject Matter Experts use COTS tools to develop, modify, & maintain enterprise standards • Abstracted terminology services 10/3/2002 HL 7 Baltimore 4
NDF Reference Terminology (NDF-RT) • Explicit, multi-hierarchical model • Centered on drug ingredients for function, maintenance and economies of scale Semi-algorithmic initialization followed by human review • Authoritative, collaborative content (FDA, NLM, HL 7, others) • VA’s ERT strategy • • Subject Matter Experts use COTS tools to develop, modify, & maintain enterprise standards • Abstracted terminology services 10/3/2002 HL 7 Baltimore 4
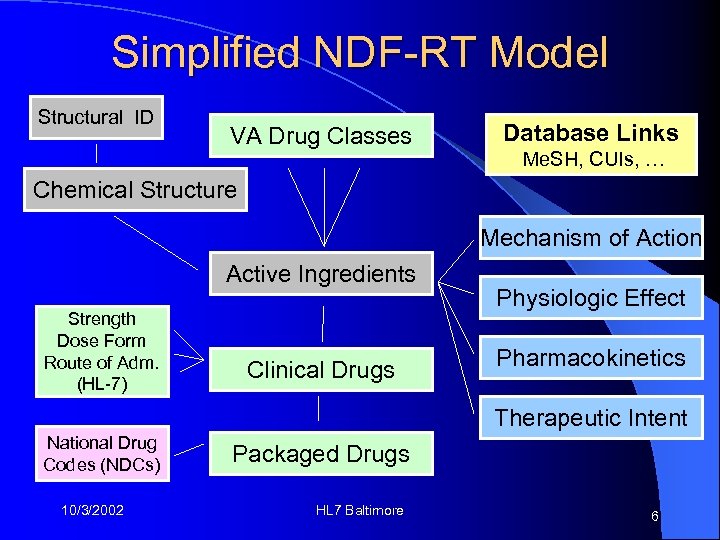 Simplified NDF-RT Model Structural ID VA Drug Classes Database Links Me. SH, CUIs, … Chemical Structure Mechanism of Action Active Ingredients Strength Dose Form Route of Adm. (HL-7) Clinical Drugs Physiologic Effect Pharmacokinetics Therapeutic Intent National Drug Codes (NDCs) 10/3/2002 Packaged Drugs HL 7 Baltimore 6
Simplified NDF-RT Model Structural ID VA Drug Classes Database Links Me. SH, CUIs, … Chemical Structure Mechanism of Action Active Ingredients Strength Dose Form Route of Adm. (HL-7) Clinical Drugs Physiologic Effect Pharmacokinetics Therapeutic Intent National Drug Codes (NDCs) 10/3/2002 Packaged Drugs HL 7 Baltimore 6
 NDF-RT Content • Drug Hierarchy • 3, 977 Active Ingredients (incl. salts & esters) • 11, 345 Orderable Drugs (= VA Products) • 87, 210 Packaged Drugs (NDCs) • Initialized from … • VA National Drug File (Sept 2001) • NLM Rx. Norm Drug Names (Dec 2001) 10/3/2002 HL 7 Baltimore 7
NDF-RT Content • Drug Hierarchy • 3, 977 Active Ingredients (incl. salts & esters) • 11, 345 Orderable Drugs (= VA Products) • 87, 210 Packaged Drugs (NDCs) • Initialized from … • VA National Drug File (Sept 2001) • NLM Rx. Norm Drug Names (Dec 2001) 10/3/2002 HL 7 Baltimore 7
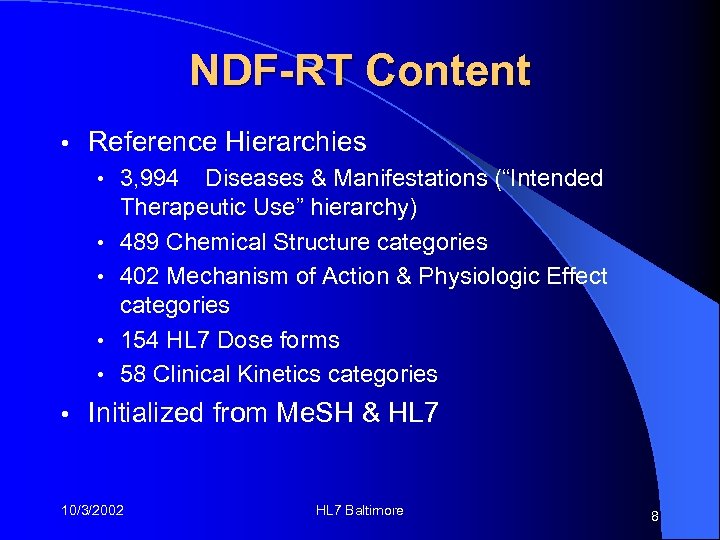 NDF-RT Content • Reference Hierarchies • 3, 994 • • • Diseases & Manifestations (“Intended Therapeutic Use” hierarchy) 489 Chemical Structure categories 402 Mechanism of Action & Physiologic Effect categories 154 HL 7 Dose forms 58 Clinical Kinetics categories Initialized from Me. SH & HL 7 10/3/2002 HL 7 Baltimore 8
NDF-RT Content • Reference Hierarchies • 3, 994 • • • Diseases & Manifestations (“Intended Therapeutic Use” hierarchy) 489 Chemical Structure categories 402 Mechanism of Action & Physiologic Effect categories 154 HL 7 Dose forms 58 Clinical Kinetics categories Initialized from Me. SH & HL 7 10/3/2002 HL 7 Baltimore 8
 Modeling Active Ingredients 4000 active ingredients & 87, 000 NDC level products • Descriptive aspects inherited down the hierarchy to orderable drugs & packaged NDC drug products which contain the active ingredients modeled • Increases modeling efficiency & productivity via “inheritance” • 10/3/2002 HL 7 Baltimore 9
Modeling Active Ingredients 4000 active ingredients & 87, 000 NDC level products • Descriptive aspects inherited down the hierarchy to orderable drugs & packaged NDC drug products which contain the active ingredients modeled • Increases modeling efficiency & productivity via “inheritance” • 10/3/2002 HL 7 Baltimore 9
 Covering Drugs Ordered in One VAMC (June 2000) Extracted 488 active NDF-RT ingredient names from sample of ordered drugs • Modeling 488 (12. 3%) of all 3977 active ingredients in NDF-RT would cover at least one ingredient in: • • 63. 7% (7228/11345) of all NDF-RT orderable drugs • 78. 2% (68194/87210) of all NDF-RT packaged drugs (NDCs) 10/3/2002 HL 7 Baltimore 10
Covering Drugs Ordered in One VAMC (June 2000) Extracted 488 active NDF-RT ingredient names from sample of ordered drugs • Modeling 488 (12. 3%) of all 3977 active ingredients in NDF-RT would cover at least one ingredient in: • • 63. 7% (7228/11345) of all NDF-RT orderable drugs • 78. 2% (68194/87210) of all NDF-RT packaged drugs (NDCs) 10/3/2002 HL 7 Baltimore 10
 Est. Time to Review NDF-RT • Time & motion study • Averaged 12 -13 minutes per concept, range 5 -30 minutes • Est. throughput = 4 concepts per hour • Approx. 1000 person hours for ~4000 active ingredient concepts in NDF-RT 10/3/2002 HL 7 Baltimore 11
Est. Time to Review NDF-RT • Time & motion study • Averaged 12 -13 minutes per concept, range 5 -30 minutes • Est. throughput = 4 concepts per hour • Approx. 1000 person hours for ~4000 active ingredient concepts in NDF-RT 10/3/2002 HL 7 Baltimore 11
 Upcoming Work VA Contract awarded 09/2002, to be completed Spring 2003 • NIGMS • • Includes support for reference hierarchy development and some human review • Focuses on meeting needs of pharmacogenomic research network 10/3/2002 HL 7 Baltimore 12
Upcoming Work VA Contract awarded 09/2002, to be completed Spring 2003 • NIGMS • • Includes support for reference hierarchy development and some human review • Focuses on meeting needs of pharmacogenomic research network 10/3/2002 HL 7 Baltimore 12
 Background: VA New Drug Transaction Thousands of NDC changes per year • Hundreds of clinical drug changes per year • Tens of New Molecular Entities per year • • How to maintain? 10/3/2002 HL 7 Baltimore 13
Background: VA New Drug Transaction Thousands of NDC changes per year • Hundreds of clinical drug changes per year • Tens of New Molecular Entities per year • • How to maintain? 10/3/2002 HL 7 Baltimore 13
 VA Formulary Updates: Current Situation • Manual system for review and entry into national formulary • Daily entry, quarterly publication • Multiple sources of data • Commercial KB’s, FDA, trade pubs, requests from field • Local hospitals also can add updates, these are then transmitted monthly to central office for review (workload dependent) 10/3/2002 HL 7 Baltimore 14
VA Formulary Updates: Current Situation • Manual system for review and entry into national formulary • Daily entry, quarterly publication • Multiple sources of data • Commercial KB’s, FDA, trade pubs, requests from field • Local hospitals also can add updates, these are then transmitted monthly to central office for review (workload dependent) 10/3/2002 HL 7 Baltimore 14
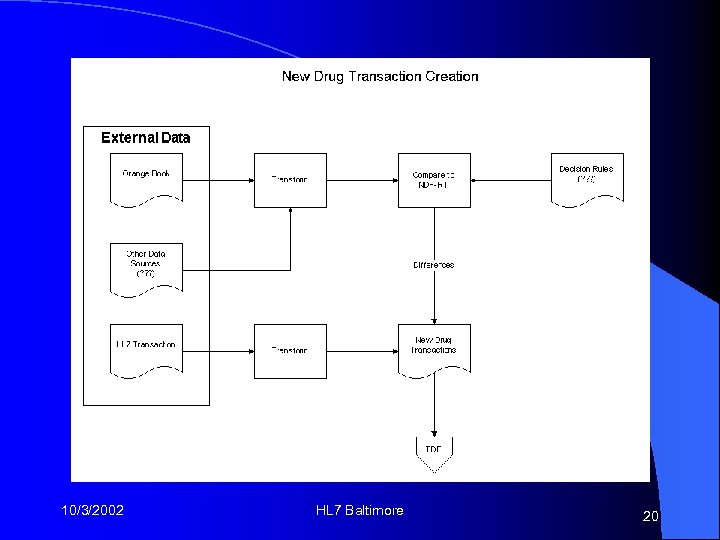
 New Drug Transaction Plan Create an XML message based on publicly available data sources to carry drug information • Develop a semi-automated method and software tool to review and accept new drugs into the formulary • 10/3/2002 HL 7 Baltimore 15
New Drug Transaction Plan Create an XML message based on publicly available data sources to carry drug information • Develop a semi-automated method and software tool to review and accept new drugs into the formulary • 10/3/2002 HL 7 Baltimore 15
 A Little Plan Detail Multiple “levels”: NDC, Clinical drug, NME • 3 “flavors”: new, change, retire • A single new NDC may cause an upward chain of updates • 10/3/2002 HL 7 Baltimore 16
A Little Plan Detail Multiple “levels”: NDC, Clinical drug, NME • 3 “flavors”: new, change, retire • A single new NDC may cause an upward chain of updates • 10/3/2002 HL 7 Baltimore 16
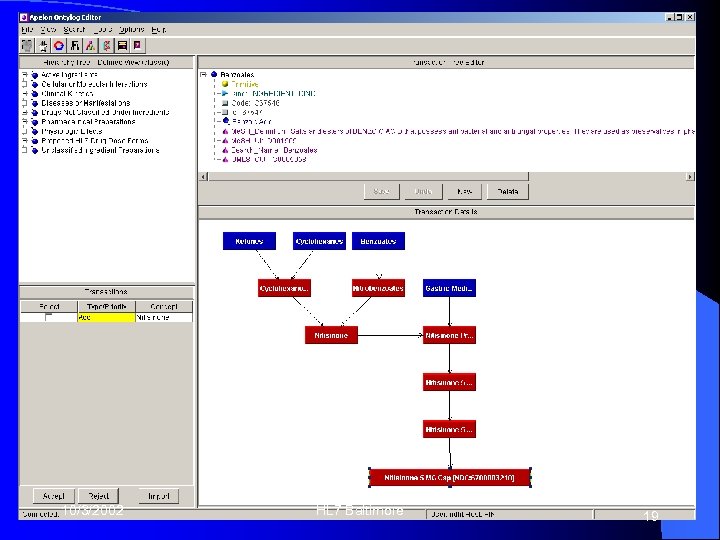
 New Dose Form Example: Copaxone Generic Name: Glatiramer Acetate • Treats: Multiple Sclerosis • Currently, the only GA packaged drug in NDFRT is: • • GLATIRAMER ACETATE 20 MG/ML SYR [NDC: 66914 - 1009 - 1] • Injectable solution • Add: • GLATIRAMER ACETATE 20 MG/VIL 84] • Powder, lyophilized for solution 10/3/2002 HL 7 Baltimore [NDC: 0480 -0126 - 17
New Dose Form Example: Copaxone Generic Name: Glatiramer Acetate • Treats: Multiple Sclerosis • Currently, the only GA packaged drug in NDFRT is: • • GLATIRAMER ACETATE 20 MG/ML SYR [NDC: 66914 - 1009 - 1] • Injectable solution • Add: • GLATIRAMER ACETATE 20 MG/VIL 84] • Powder, lyophilized for solution 10/3/2002 HL 7 Baltimore [NDC: 0480 -0126 - 17
 Necessary Transaction Steps “Clinical Drug” is the level of the dose form information • “Dose form” is different (? ? ? ) because existing NDC is already in solution • Potential new concepts: • • Clinical drug concept (? ? ? ) • NDC-level concept 10/3/2002 HL 7 Baltimore 18
Necessary Transaction Steps “Clinical Drug” is the level of the dose form information • “Dose form” is different (? ? ? ) because existing NDC is already in solution • Potential new concepts: • • Clinical drug concept (? ? ? ) • NDC-level concept 10/3/2002 HL 7 Baltimore 18
 10/3/2002 HL 7 Baltimore 19
10/3/2002 HL 7 Baltimore 19
 10/3/2002 HL 7 Baltimore 20
10/3/2002 HL 7 Baltimore 20
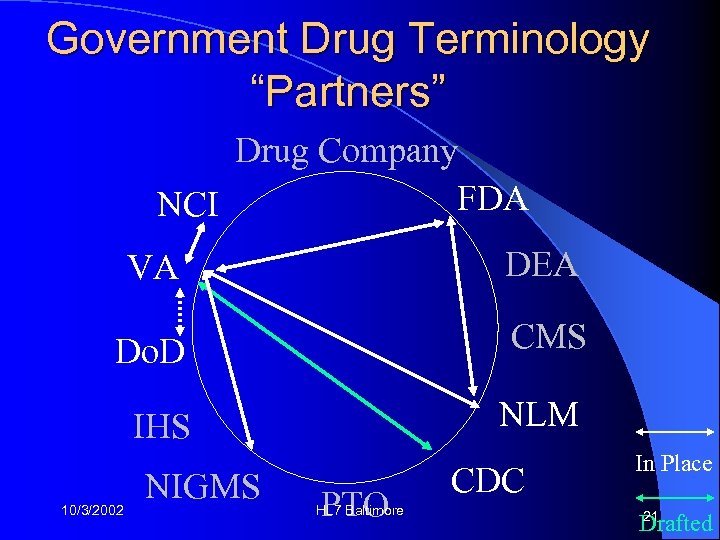 Government Drug Terminology “Partners” Drug Company FDA NCI VA DEA Do. D CMS IHS NLM 10/3/2002 NIGMS PTO HL 7 Baltimore CDC In Place 21 Drafted
Government Drug Terminology “Partners” Drug Company FDA NCI VA DEA Do. D CMS IHS NLM 10/3/2002 NIGMS PTO HL 7 Baltimore CDC In Place 21 Drafted
 FDA Labeling Being created as a Structured Document using HL 7 Clinical Document Architecture • Label Warehouse System prototype in development • Selected data to be shared with NLM to help populate Rx. Norm • 10/3/2002 HL 7 Baltimore 22
FDA Labeling Being created as a Structured Document using HL 7 Clinical Document Architecture • Label Warehouse System prototype in development • Selected data to be shared with NLM to help populate Rx. Norm • 10/3/2002 HL 7 Baltimore 22
 NIGMS Helping develop reference hierarchies, especially pharmacokinetics • Tied to Pharm. GKB project at Stanford • Thanks to Chris Chute! • 10/3/2002 HL 7 Baltimore 24
NIGMS Helping develop reference hierarchies, especially pharmacokinetics • Tied to Pharm. GKB project at Stanford • Thanks to Chris Chute! • 10/3/2002 HL 7 Baltimore 24
 NCI Experimental Agents Approximately 300 agents, ranging from the common to the very experimental • A separate drug class hierarchy, focused on mechanisms of action and specialized antineoplastic categories • 10/3/2002 HL 7 Baltimore 25
NCI Experimental Agents Approximately 300 agents, ranging from the common to the very experimental • A separate drug class hierarchy, focused on mechanisms of action and specialized antineoplastic categories • 10/3/2002 HL 7 Baltimore 25
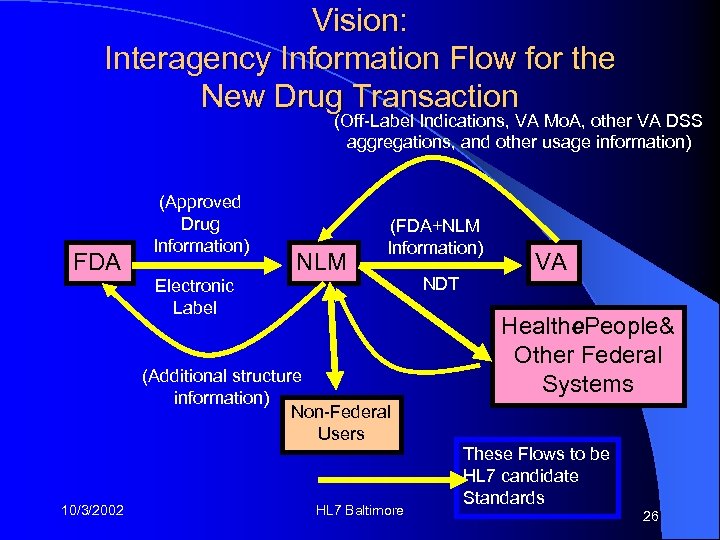 Vision: Interagency Information Flow for the New Drug Transaction (Off-Label Indications, VA Mo. A, other VA DSS aggregations, and other usage information) FDA (Approved Drug Information) NLM (FDA+NLM Information) Electronic Label (Additional structure information) Non-Federal Users 10/3/2002 HL 7 Baltimore NDT VA Healthe. People& Other Federal Systems These Flows to be HL 7 candidate Standards 26
Vision: Interagency Information Flow for the New Drug Transaction (Off-Label Indications, VA Mo. A, other VA DSS aggregations, and other usage information) FDA (Approved Drug Information) NLM (FDA+NLM Information) Electronic Label (Additional structure information) Non-Federal Users 10/3/2002 HL 7 Baltimore NDT VA Healthe. People& Other Federal Systems These Flows to be HL 7 candidate Standards 26
 Challenges Delivering drug definitions and reference hierarchies with “face validity” • Managing multi-agency, multi-institution collaboration • • Varying needs & expectations • Information model, terminology model, knowledge base interaction 10/3/2002 HL 7 Baltimore 27
Challenges Delivering drug definitions and reference hierarchies with “face validity” • Managing multi-agency, multi-institution collaboration • • Varying needs & expectations • Information model, terminology model, knowledge base interaction 10/3/2002 HL 7 Baltimore 27
 10/3/2002 HL 7 Baltimore 28
10/3/2002 HL 7 Baltimore 28


