4fed42ad58b1c44a0350895d348958bd.ppt
- Количество слайдов: 72
 Using Statistical Innovation to Impact Regulatory Thinking Harry Yang, Ph. D. Senior Director, Head of Non-Clinical Biostatistics Med. Immune, LLC 2014 Nonclinical Biostatistics Conference
Using Statistical Innovation to Impact Regulatory Thinking Harry Yang, Ph. D. Senior Director, Head of Non-Clinical Biostatistics Med. Immune, LLC 2014 Nonclinical Biostatistics Conference
 What Roles Are We Playing in Regulatory Affairs? 2
What Roles Are We Playing in Regulatory Affairs? 2
 What Roles Are We Playing in Regulatory Affairs? u To think? 3
What Roles Are We Playing in Regulatory Affairs? u To think? 3
 What Roles Are We Playing in Regulatory Affairs? u To rule the world? 4
What Roles Are We Playing in Regulatory Affairs? u To rule the world? 4
 What Roles Are We Playing in Regulatory Affairs? u Or to influence? 5
What Roles Are We Playing in Regulatory Affairs? u Or to influence? 5
 The Answer Is… u TO INFLUENCE! 6
The Answer Is… u TO INFLUENCE! 6
 How Do We Influence Regulatory Thinking? 7
How Do We Influence Regulatory Thinking? 7
 An Old Tried and True Method u Throw statisticians at the deep end of regulatory interactions 8
An Old Tried and True Method u Throw statisticians at the deep end of regulatory interactions 8
 An Old Tried and True Method (Cont’d) u Throw statisticians at the deep end of regulatory interactions – Low success rate – Lost potential/opportunities 9
An Old Tried and True Method (Cont’d) u Throw statisticians at the deep end of regulatory interactions – Low success rate – Lost potential/opportunities 9
 A More Effective Approach to Influencing Regulatory Thinking u Identify opportunities Opportunities n Understand our own strengths n Influence thru collaboration 10
A More Effective Approach to Influencing Regulatory Thinking u Identify opportunities Opportunities n Understand our own strengths n Influence thru collaboration 10
 Areas Where Statistics Is Value-added n Design of experiment (DOE) 11
Areas Where Statistics Is Value-added n Design of experiment (DOE) 11
 Statistical Designs u Completely randomized designs u Randomized complete block designs u Split-plot designs u Cross-over designs u Latin square designs u Factorial designs u Analysis of variance designs 12
Statistical Designs u Completely randomized designs u Randomized complete block designs u Split-plot designs u Cross-over designs u Latin square designs u Factorial designs u Analysis of variance designs 12
 Too Many to Choose 13
Too Many to Choose 13
 How to Reduce Variability? 14
How to Reduce Variability? 14
 Should You Use Control? 15
Should You Use Control? 15
 Should You Be Blinded? u To reduce evaluator’s bias 16
Should You Be Blinded? u To reduce evaluator’s bias 16
 Should You Randomize? 17
Should You Randomize? 17
 How to Minimize Chance of False Claim? 18
How to Minimize Chance of False Claim? 18
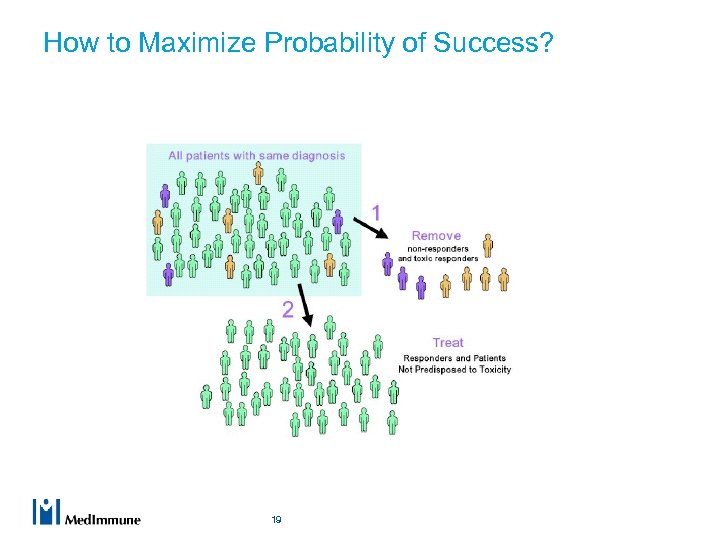 How to Maximize Probability of Success? 19
How to Maximize Probability of Success? 19
 Did You Use the Right Sample Size N? u A small N may miss biologically important effects u A large N wastes animals 20
Did You Use the Right Sample Size N? u A small N may miss biologically important effects u A large N wastes animals 20
 Facts Science “A collection of facts is no more a science than a heap of stones is a house. ” Henri Poincare (1854 – 1912) 21
Facts Science “A collection of facts is no more a science than a heap of stones is a house. ” Henri Poincare (1854 – 1912) 21
 How To Analyze Data with High Accuracy, Precision and Confidence? 22
How To Analyze Data with High Accuracy, Precision and Confidence? 22
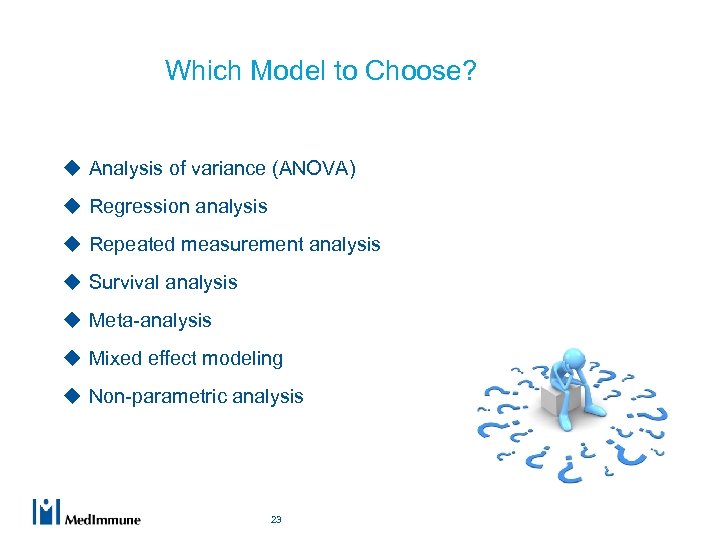 Which Model to Choose? u Analysis of variance (ANOVA) u Regression analysis u Repeated measurement analysis u Survival analysis u Meta-analysis u Mixed effect modeling u Non-parametric analysis 23
Which Model to Choose? u Analysis of variance (ANOVA) u Regression analysis u Repeated measurement analysis u Survival analysis u Meta-analysis u Mixed effect modeling u Non-parametric analysis 23
 Help Overcome Regulatory Hurdles 24
Help Overcome Regulatory Hurdles 24
 Be Bold and Innovative 25
Be Bold and Innovative 25
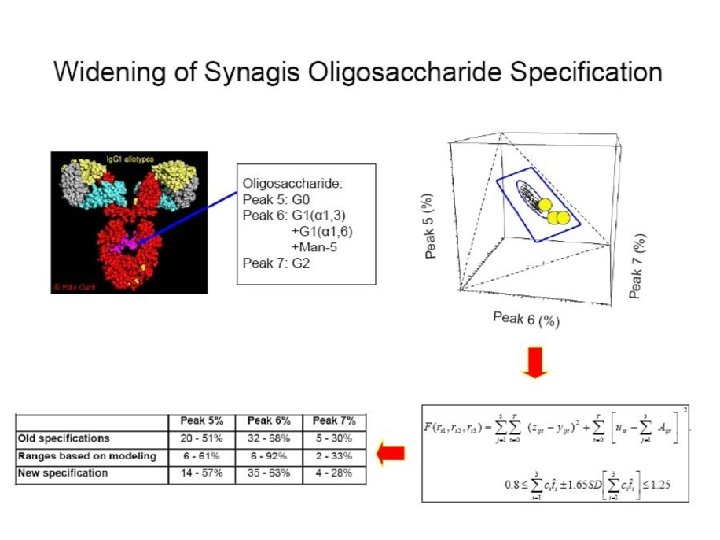
 Four Case Examples u Widening specification after OOS u Bridging assays as opposed to clinical studies u Acceptable limits of residual host cell DNA u Risk-based pre-filtration bio-burden limits 26
Four Case Examples u Widening specification after OOS u Bridging assays as opposed to clinical studies u Acceptable limits of residual host cell DNA u Risk-based pre-filtration bio-burden limits 26
 27 04/14/2008 – 6: 00 pm
27 04/14/2008 – 6: 00 pm
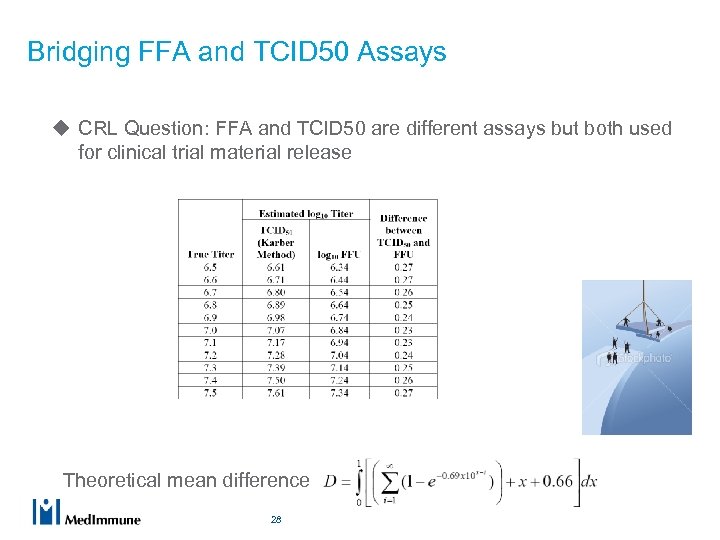 Bridging FFA and TCID 50 Assays u CRL Question: FFA and TCID 50 are different assays but both used for clinical trial material release Theoretical mean difference 28
Bridging FFA and TCID 50 Assays u CRL Question: FFA and TCID 50 are different assays but both used for clinical trial material release Theoretical mean difference 28
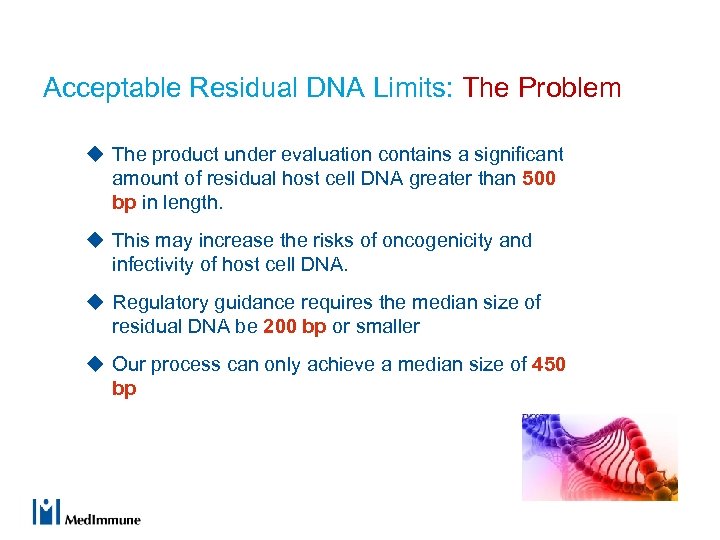 Acceptable Residual DNA Limits: The Problem u The product under evaluation contains a significant amount of residual host cell DNA greater than 500 bp in length. u This may increase the risks of oncogenicity and infectivity of host cell DNA. u Regulatory guidance requires the median size of residual DNA be 200 bp or smaller u Our process can only achieve a median size of 450 bp
Acceptable Residual DNA Limits: The Problem u The product under evaluation contains a significant amount of residual host cell DNA greater than 500 bp in length. u This may increase the risks of oncogenicity and infectivity of host cell DNA. u Regulatory guidance requires the median size of residual DNA be 200 bp or smaller u Our process can only achieve a median size of 450 bp
 Anxiety Attack The Scream, by Edvard Munch, 1893
Anxiety Attack The Scream, by Edvard Munch, 1893
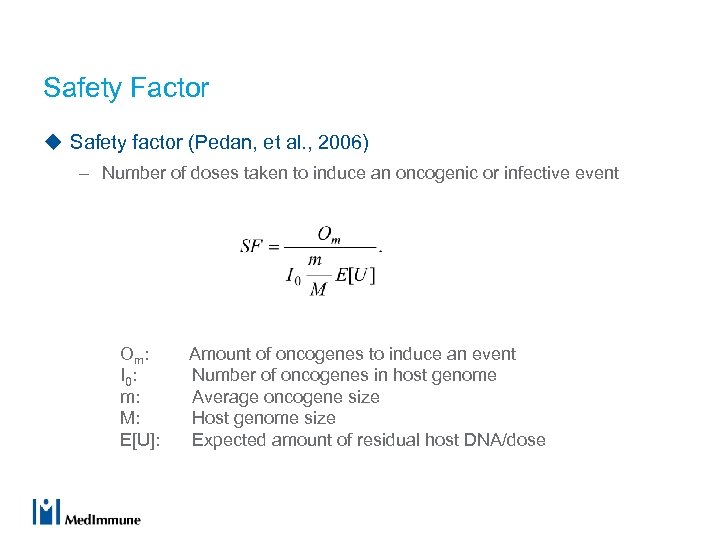 Safety Factor u Safety factor (Pedan, et al. , 2006) – Number of doses taken to induce an oncogenic or infective event Om: Amount of oncogenes to induce an event I 0 : Number of oncogenes in host genome m: Average oncogene size M: Host genome size E[U]: Expected amount of residual host DNA/dose
Safety Factor u Safety factor (Pedan, et al. , 2006) – Number of doses taken to induce an oncogenic or infective event Om: Amount of oncogenes to induce an event I 0 : Number of oncogenes in host genome m: Average oncogene size M: Host genome size E[U]: Expected amount of residual host DNA/dose
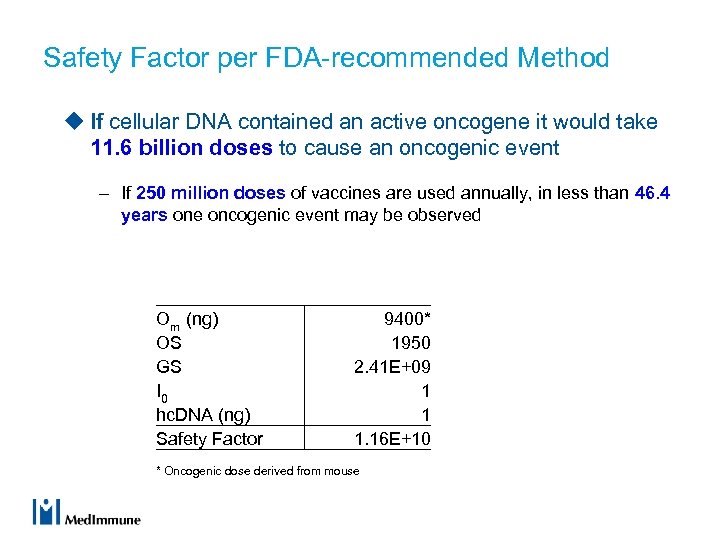 Safety Factor per FDA-recommended Method u If cellular DNA contained an active oncogene it would take 11. 6 billion doses to cause an oncogenic event – If 250 million doses of vaccines are used annually, in less than 46. 4 years one oncogenic event may be observed Om (ng) OS GS I 0 hc. DNA (ng) Safety Factor 9400* 1950 2. 41 E+09 1 1 1. 16 E+10 * Oncogenic dose derived from mouse
Safety Factor per FDA-recommended Method u If cellular DNA contained an active oncogene it would take 11. 6 billion doses to cause an oncogenic event – If 250 million doses of vaccines are used annually, in less than 46. 4 years one oncogenic event may be observed Om (ng) OS GS I 0 hc. DNA (ng) Safety Factor 9400* 1950 2. 41 E+09 1 1 1. 16 E+10 * Oncogenic dose derived from mouse
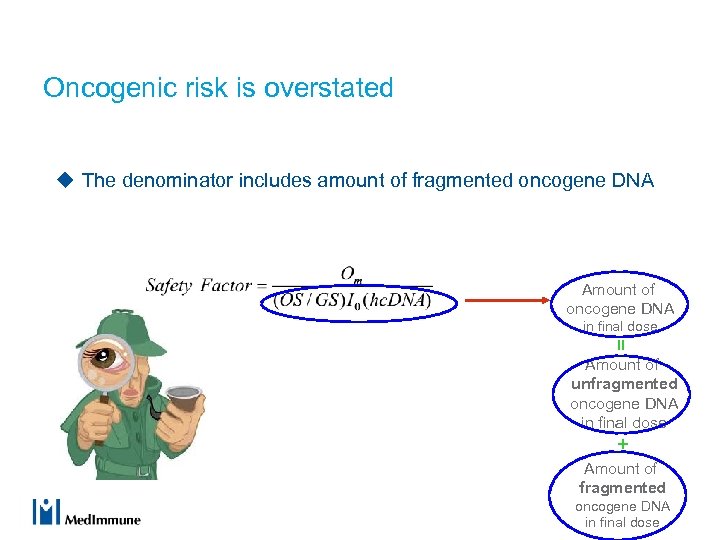 Oncogenic risk is overstated u The denominator includes amount of fragmented oncogene DNA Amount of oncogene DNA in final dose = Amount of unfragmented oncogene DNA in final dose + Amount of fragmented oncogene DNA in final dose
Oncogenic risk is overstated u The denominator includes amount of fragmented oncogene DNA Amount of oncogene DNA in final dose = Amount of unfragmented oncogene DNA in final dose + Amount of fragmented oncogene DNA in final dose
 DNA Inactivation
DNA Inactivation
 Enzymatic Degradation Inactivates DNA Benzonase and other ingredients
Enzymatic Degradation Inactivates DNA Benzonase and other ingredients
 Hope u This finding gives us hope that with median residual DNA size of 450 bp (albeit not quite up to the regulatory bar of 200 bp) perhaps the oncogenicity and infectivity risks are already reduced to an acceptable level.
Hope u This finding gives us hope that with median residual DNA size of 450 bp (albeit not quite up to the regulatory bar of 200 bp) perhaps the oncogenicity and infectivity risks are already reduced to an acceptable level.
 Negotiation with FDA u Standard method overestimates risk u If DNA inactivation step is incorporated in the calculation, the risk might be adequately mitigated
Negotiation with FDA u Standard method overestimates risk u If DNA inactivation step is incorporated in the calculation, the risk might be adequately mitigated
 Burden of Proof
Burden of Proof
 How to Incorporate DNA Inactivation in the Risk Assessment? Enzymatic degradation of DNA Source: http: //1. bp. blogspot. com/_vg. EA 7 CHGLe 8/Sz. IAZHWs-v. I/AAAAAVc/v. Zcm. Dl. Rlx. SY/s 320/miracle. gif
How to Incorporate DNA Inactivation in the Risk Assessment? Enzymatic degradation of DNA Source: http: //1. bp. blogspot. com/_vg. EA 7 CHGLe 8/Sz. IAZHWs-v. I/AAAAAVc/v. Zcm. Dl. Rlx. SY/s 320/miracle. gif
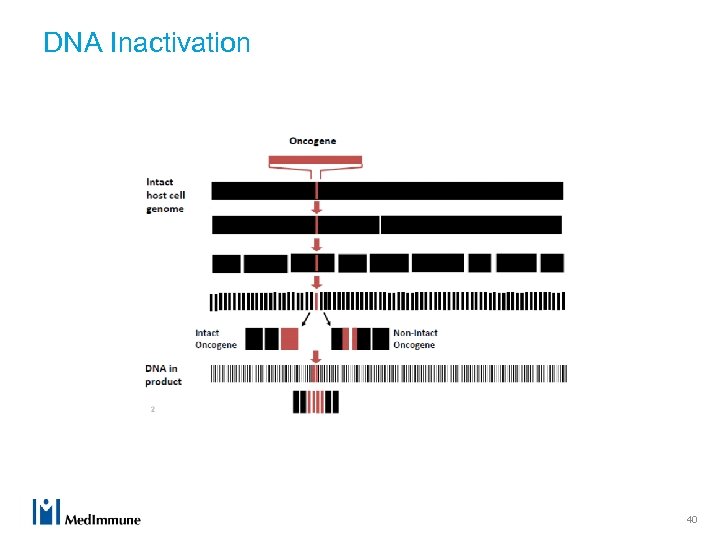 DNA Inactivation 40
DNA Inactivation 40
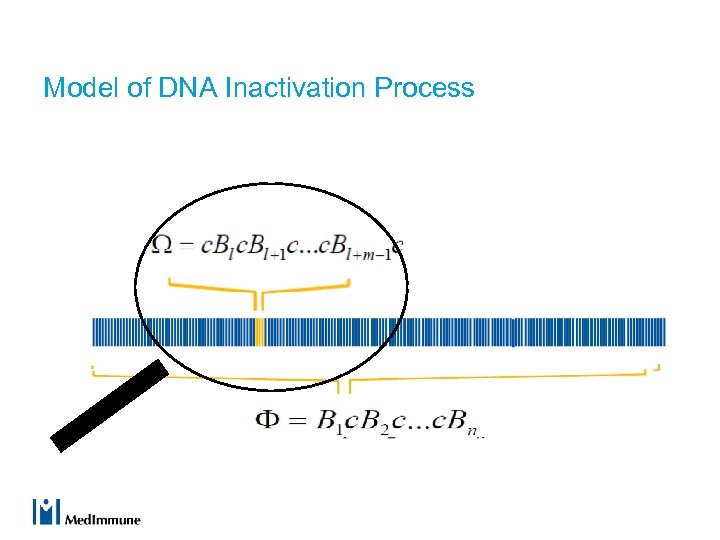 Model of DNA Inactivation Process
Model of DNA Inactivation Process
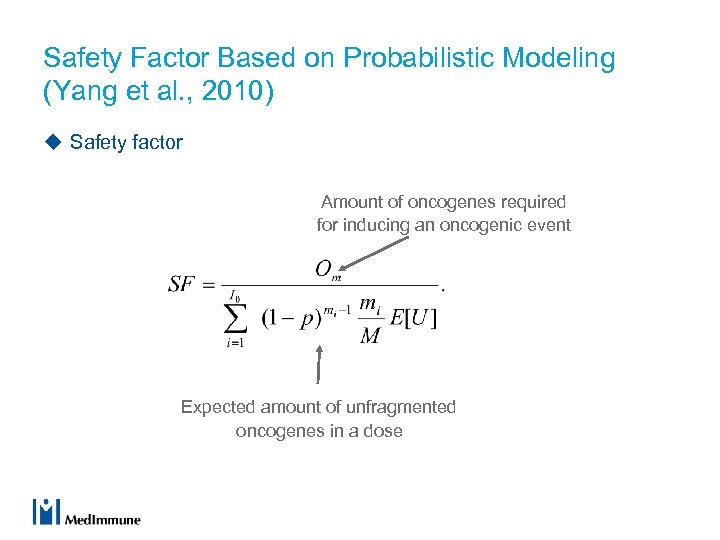 Safety Factor Based on Probabilistic Modeling (Yang et al. , 2010) u Safety factor Amount of oncogenes required for inducing an oncogenic event Expected amount of unfragmented oncogenes in a dose
Safety Factor Based on Probabilistic Modeling (Yang et al. , 2010) u Safety factor Amount of oncogenes required for inducing an oncogenic event Expected amount of unfragmented oncogenes in a dose
 Proof of the Theoretical Result u Trust me!
Proof of the Theoretical Result u Trust me!
 How to estimate enzyme cutting efficiency p?
How to estimate enzyme cutting efficiency p?
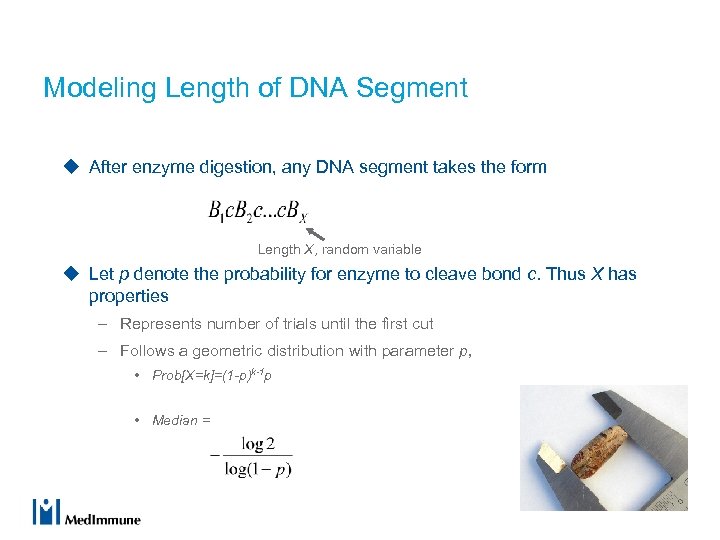 Modeling Length of DNA Segment u After enzyme digestion, any DNA segment takes the form Length X, random variable u Let p denote the probability for enzyme to cleave bond c. Thus X has properties – Represents number of trials until the first cut – Follows a geometric distribution with parameter p, • Prob[X=k]=(1 -p)k-1 p • Median =
Modeling Length of DNA Segment u After enzyme digestion, any DNA segment takes the form Length X, random variable u Let p denote the probability for enzyme to cleave bond c. Thus X has properties – Represents number of trials until the first cut – Follows a geometric distribution with parameter p, • Prob[X=k]=(1 -p)k-1 p • Median =
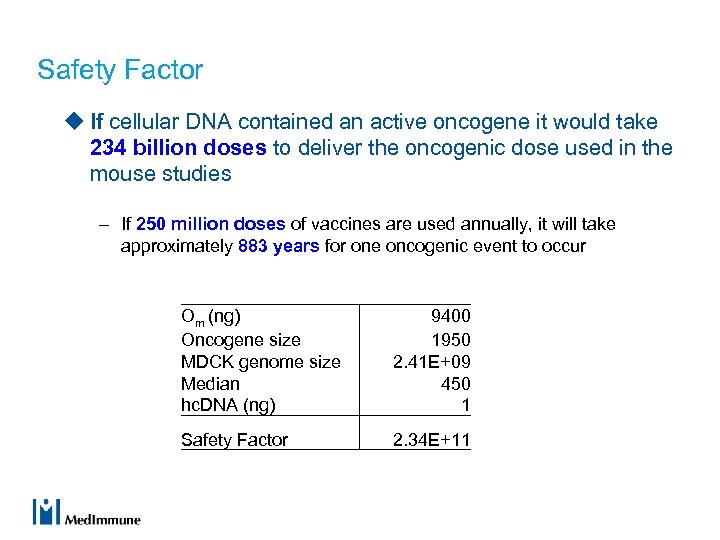 Safety Factor u If cellular DNA contained an active oncogene it would take 234 billion doses to deliver the oncogenic dose used in the mouse studies – If 250 million doses of vaccines are used annually, it will take approximately 883 years for one oncogenic event to occur Om (ng) Oncogene size MDCK genome size Median hc. DNA (ng) 9400 1950 2. 41 E+09 450 1 Safety Factor 2. 34 E+11
Safety Factor u If cellular DNA contained an active oncogene it would take 234 billion doses to deliver the oncogenic dose used in the mouse studies – If 250 million doses of vaccines are used annually, it will take approximately 883 years for one oncogenic event to occur Om (ng) Oncogene size MDCK genome size Median hc. DNA (ng) 9400 1950 2. 41 E+09 450 1 Safety Factor 2. 34 E+11
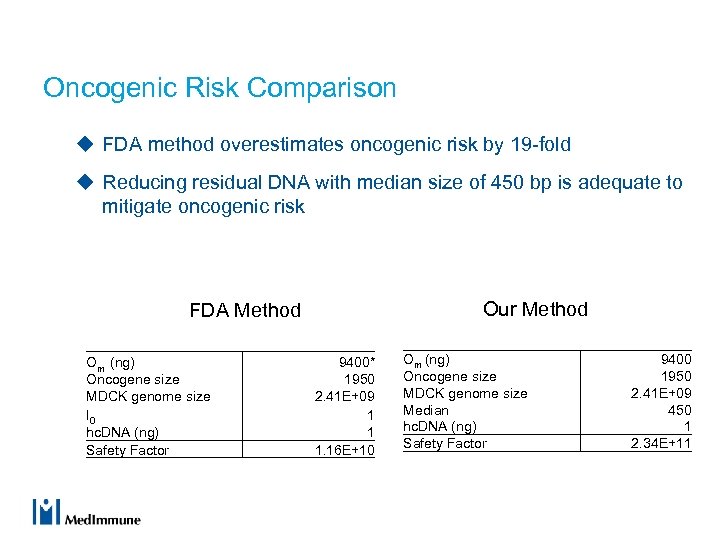 Oncogenic Risk Comparison u FDA method overestimates oncogenic risk by 19 -fold u Reducing residual DNA with median size of 450 bp is adequate to mitigate oncogenic risk Our Method FDA Method Om (ng) Oncogene size MDCK genome size I 0 hc. DNA (ng) Safety Factor 9400* 1950 2. 41 E+09 1 1 1. 16 E+10 Om (ng) Oncogene size MDCK genome size Median hc. DNA (ng) Safety Factor 9400 1950 2. 41 E+09 450 1 2. 34 E+11
Oncogenic Risk Comparison u FDA method overestimates oncogenic risk by 19 -fold u Reducing residual DNA with median size of 450 bp is adequate to mitigate oncogenic risk Our Method FDA Method Om (ng) Oncogene size MDCK genome size I 0 hc. DNA (ng) Safety Factor 9400* 1950 2. 41 E+09 1 1 1. 16 E+10 Om (ng) Oncogene size MDCK genome size Median hc. DNA (ng) Safety Factor 9400 1950 2. 41 E+09 450 1 2. 34 E+11
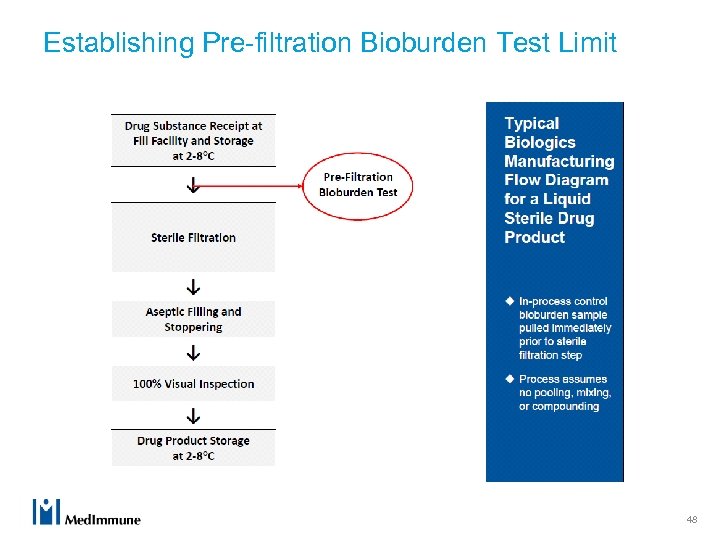 Establishing Pre-filtration Bioburden Test Limit 48
Establishing Pre-filtration Bioburden Test Limit 48
 Manufacture of a Sterile Drug Product u Microbial control during manufacturing is critical for ensuring product quality and safety. u Sterile biologic drug products (finished dosage forms) are typically manufactured by sterile filtration followed by aseptic filling and processing. u Control of microbial load at the sterile filtration step is an essential and required component of the overall microbial control strategy. 49
Manufacture of a Sterile Drug Product u Microbial control during manufacturing is critical for ensuring product quality and safety. u Sterile biologic drug products (finished dosage forms) are typically manufactured by sterile filtration followed by aseptic filling and processing. u Control of microbial load at the sterile filtration step is an essential and required component of the overall microbial control strategy. 49
 Measures to Mitigate Bioburden Risk u Pre-filtration testing u Filtration u Minimization of manufacturing hold times between process steps u Utilization of refrigerated storage for intermediates 50
Measures to Mitigate Bioburden Risk u Pre-filtration testing u Filtration u Minimization of manufacturing hold times between process steps u Utilization of refrigerated storage for intermediates 50
 51
51
 52
52
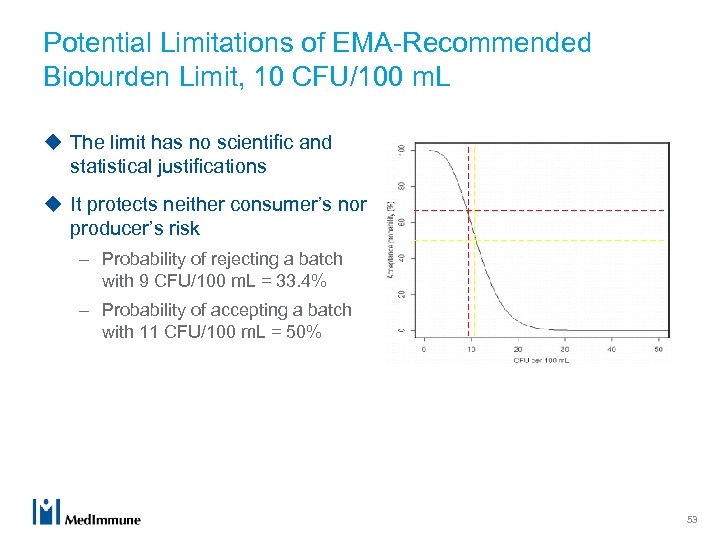 Potential Limitations of EMA-Recommended Bioburden Limit, 10 CFU/100 m. L u The limit has no scientific and statistical justifications u It protects neither consumer’s nor producer’s risk – Probability of rejecting a batch with 9 CFU/100 m. L = 33. 4% – Probability of accepting a batch with 11 CFU/100 m. L = 50% 53
Potential Limitations of EMA-Recommended Bioburden Limit, 10 CFU/100 m. L u The limit has no scientific and statistical justifications u It protects neither consumer’s nor producer’s risk – Probability of rejecting a batch with 9 CFU/100 m. L = 33. 4% – Probability of accepting a batch with 11 CFU/100 m. L = 50% 53
 Additional Limitations of 10 CFU/100 m. L Bioburden Limit u It does not take into account assay variability and the fact that microorganisms are not homogeneously distributed u Meeting or failing 10 CFU/100 m. L acceptance limit may not provide adequate assurance that the true biobruden level is below or above 10 CFU/100 m. L 54
Additional Limitations of 10 CFU/100 m. L Bioburden Limit u It does not take into account assay variability and the fact that microorganisms are not homogeneously distributed u Meeting or failing 10 CFU/100 m. L acceptance limit may not provide adequate assurance that the true biobruden level is below or above 10 CFU/100 m. L 54
 A Risk-based Approach to Development of Bioburden Control and Pre-filtration Testing Strategy u Driven by product and process knowledge u Identification of types of risks, their associations with testing method and process parameters u Development of control strategy 55
A Risk-based Approach to Development of Bioburden Control and Pre-filtration Testing Strategy u Driven by product and process knowledge u Identification of types of risks, their associations with testing method and process parameters u Development of control strategy 55
 Two Types of Risk Associated with Sterile Filtration Process u Drug solution with an unacceptable bioburden level passes the prefiltration test u Breakthrough of bioburden through the final sterile filter u Both types of risk can be characterized thru probabilities of occurrence 56
Two Types of Risk Associated with Sterile Filtration Process u Drug solution with an unacceptable bioburden level passes the prefiltration test u Breakthrough of bioburden through the final sterile filter u Both types of risk can be characterized thru probabilities of occurrence 56
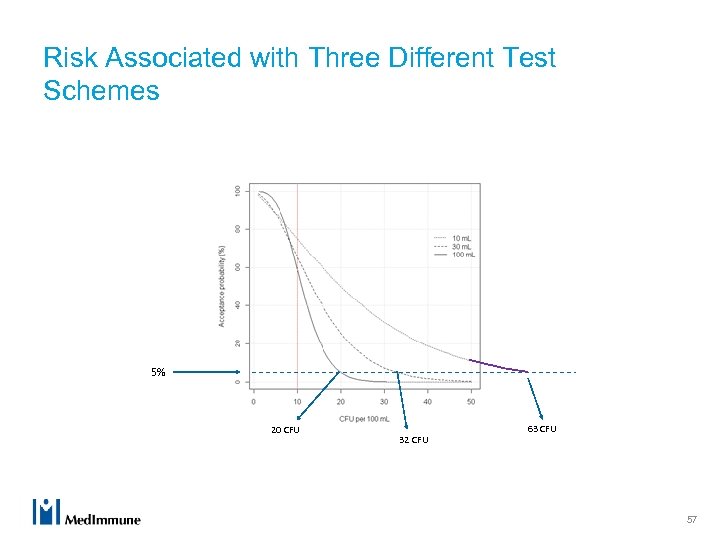 Risk Associated with Three Different Test Schemes 5% 20 CFU 32 CFU 63 CFU 57
Risk Associated with Three Different Test Schemes 5% 20 CFU 32 CFU 63 CFU 57
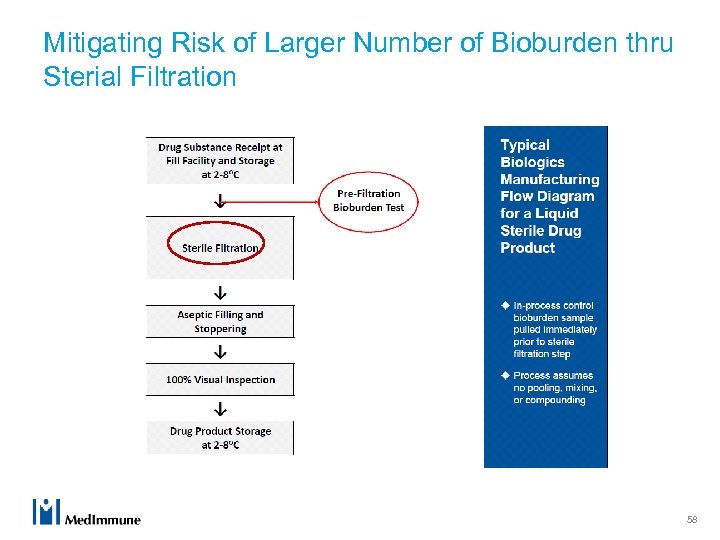 Mitigating Risk of Larger Number of Bioburden thru Sterial Filtration 58
Mitigating Risk of Larger Number of Bioburden thru Sterial Filtration 58
 Sterile Filtration u FDA guidance requires that filters used for the final filtration should be validated to reproducibly remove microorganisms from a carrier solution containing bioburden of a high concentration of at least 107 CFU/cm 2 of effective filter area (EFA) 59
Sterile Filtration u FDA guidance requires that filters used for the final filtration should be validated to reproducibly remove microorganisms from a carrier solution containing bioburden of a high concentration of at least 107 CFU/cm 2 of effective filter area (EFA) 59
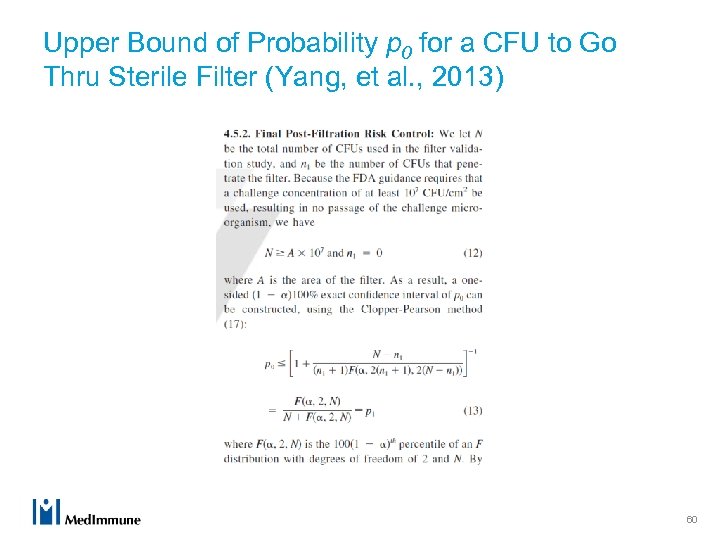 Upper Bound of Probability p 0 for a CFU to Go Thru Sterile Filter (Yang, et al. , 2013) 60
Upper Bound of Probability p 0 for a CFU to Go Thru Sterile Filter (Yang, et al. , 2013) 60
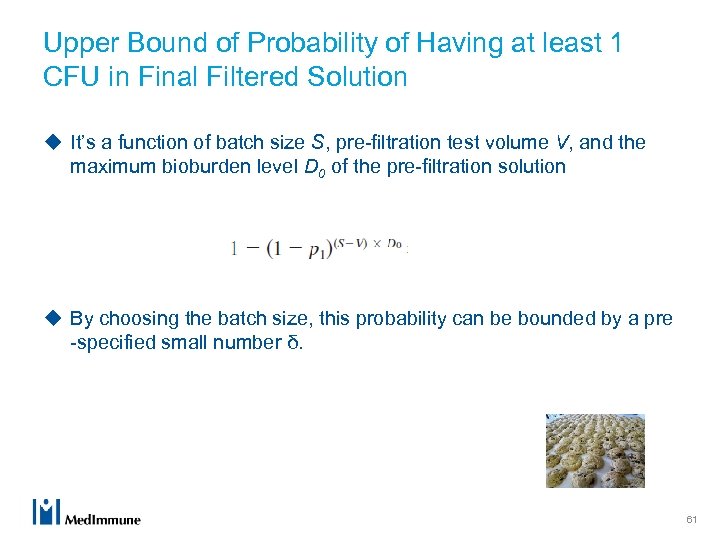 Upper Bound of Probability of Having at least 1 CFU in Final Filtered Solution u It’s a function of batch size S, pre-filtration test volume V, and the maximum bioburden level D 0 of the pre-filtration solution u By choosing the batch size, this probability can be bounded by a pre -specified small number δ. 61
Upper Bound of Probability of Having at least 1 CFU in Final Filtered Solution u It’s a function of batch size S, pre-filtration test volume V, and the maximum bioburden level D 0 of the pre-filtration solution u By choosing the batch size, this probability can be bounded by a pre -specified small number δ. 61
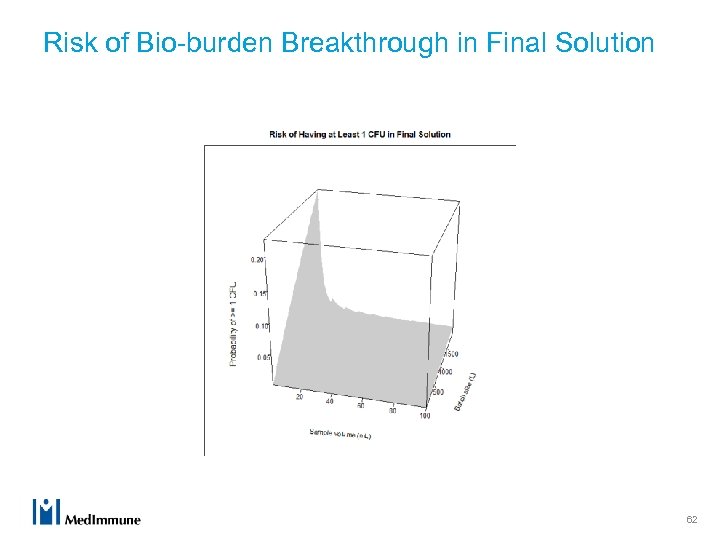 Risk of Bio-burden Breakthrough in Final Solution 62
Risk of Bio-burden Breakthrough in Final Solution 62
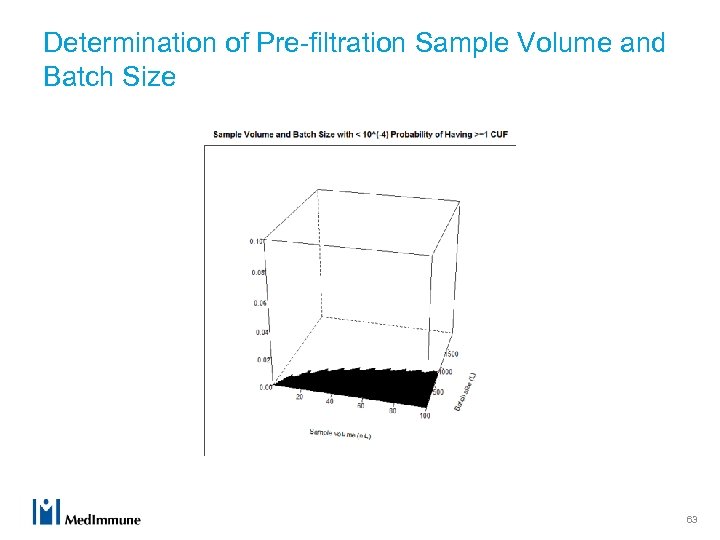 Determination of Pre-filtration Sample Volume and Batch Size 63
Determination of Pre-filtration Sample Volume and Batch Size 63
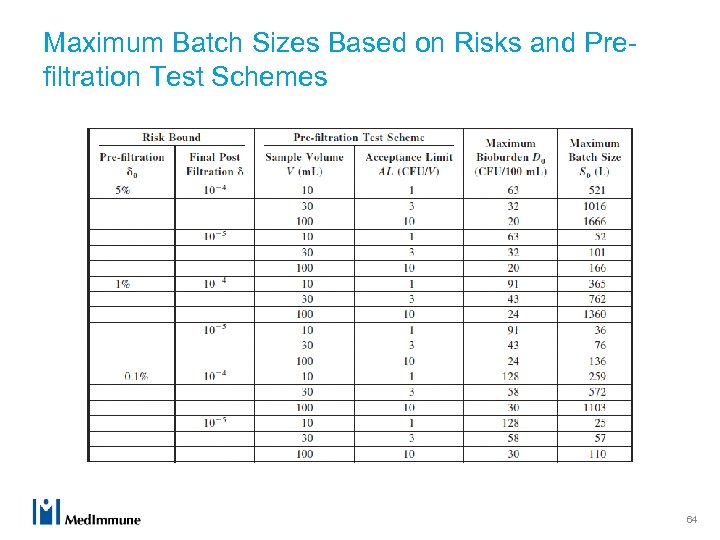 Maximum Batch Sizes Based on Risks and Prefiltration Test Schemes 64
Maximum Batch Sizes Based on Risks and Prefiltration Test Schemes 64
 A Few Additional Thoughts 65
A Few Additional Thoughts 65
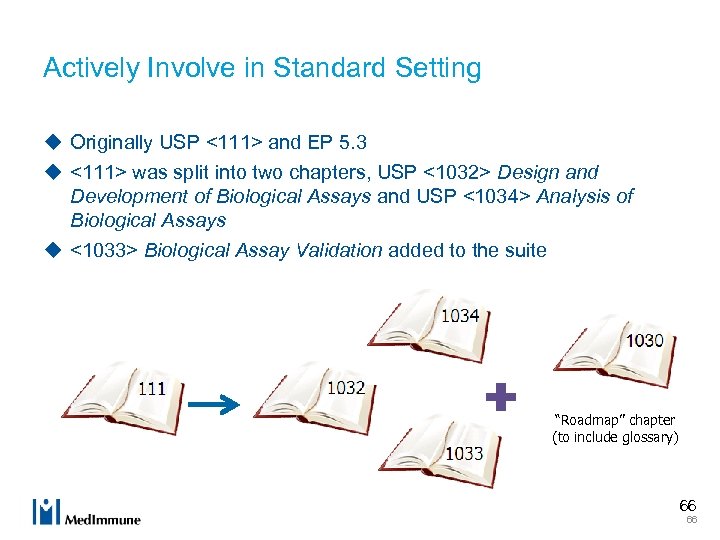 Actively Involve in Standard Setting u Originally USP <111> and EP 5. 3 u <111> was split into two chapters, USP <1032> Design and Development of Biological Assays and USP <1034> Analysis of Biological Assays u <1033> Biological Assay Validation added to the suite “Roadmap” chapter (to include glossary) 66 66
Actively Involve in Standard Setting u Originally USP <111> and EP 5. 3 u <111> was split into two chapters, USP <1032> Design and Development of Biological Assays and USP <1034> Analysis of Biological Assays u <1033> Biological Assay Validation added to the suite “Roadmap” chapter (to include glossary) 66 66
 Form Consortiums to Develop White/Concept Papers u A-Mab: a Case Study in Bioprocess Development u A-Vax: Applying Quality by Design to Vaccines 67
Form Consortiums to Develop White/Concept Papers u A-Mab: a Case Study in Bioprocess Development u A-Vax: Applying Quality by Design to Vaccines 67
 Conduct Innovative Statistical Research on Regulatory Issues u Solutions based on published methods are more likely accepted by regulatory agencies 68
Conduct Innovative Statistical Research on Regulatory Issues u Solutions based on published methods are more likely accepted by regulatory agencies 68
 Take a Good Statistical Lead in Resolving Regulatory Issues 69
Take a Good Statistical Lead in Resolving Regulatory Issues 69
 Regularly Communicate with Regulatory Authorities 70
Regularly Communicate with Regulatory Authorities 70
 Conduct Joint Training 71
Conduct Joint Training 71
 Q&A 72
Q&A 72


