7d4e4196ebac67e0d5cb5619105ef8d3.ppt
- Количество слайдов: 41
 Using mouse genetics to understand human disease Mark Daly Whitehead/Pfizer Computational Biology Fellow
Using mouse genetics to understand human disease Mark Daly Whitehead/Pfizer Computational Biology Fellow
 What we do • Genetics: the study of the inheritance of biological phenotype – Mendel recognized discrete units of inheritance – Theories rediscovered and disputed ca. 1900 – Experiments on mouse coat color proved Mendel correct and generalizable to mammals – We now recognize this inheritance as being carried by variation in DNA
What we do • Genetics: the study of the inheritance of biological phenotype – Mendel recognized discrete units of inheritance – Theories rediscovered and disputed ca. 1900 – Experiments on mouse coat color proved Mendel correct and generalizable to mammals – We now recognize this inheritance as being carried by variation in DNA
 Why mice? • Mammals, much better biological model • Easy to breed, feed, and house • Can acclimatize to human touch • Most important: we can experiment in many ways not possible in humans What do they want with me?
Why mice? • Mammals, much better biological model • Easy to breed, feed, and house • Can acclimatize to human touch • Most important: we can experiment in many ways not possible in humans What do they want with me?
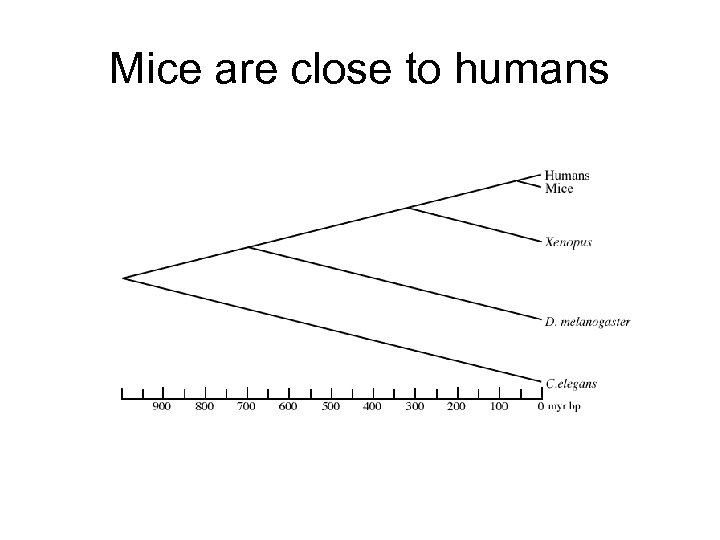 Mice are close to humans
Mice are close to humans
 Kerstin Lindblad-Toh Whitehead/MIT Center for Genome Research
Kerstin Lindblad-Toh Whitehead/MIT Center for Genome Research
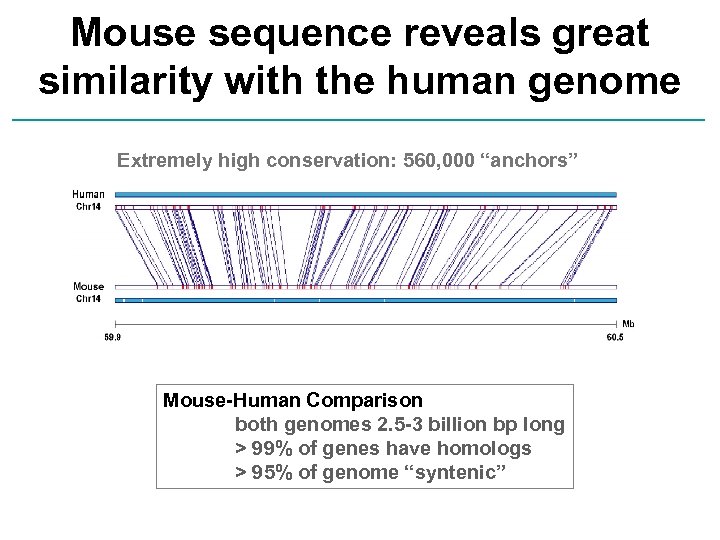 Mouse sequence reveals great similarity with the human genome Extremely high conservation: 560, 000 “anchors” Mouse-Human Comparison both genomes 2. 5 -3 billion bp long > 99% of genes have homologs > 95% of genome “syntenic”
Mouse sequence reveals great similarity with the human genome Extremely high conservation: 560, 000 “anchors” Mouse-Human Comparison both genomes 2. 5 -3 billion bp long > 99% of genes have homologs > 95% of genome “syntenic”
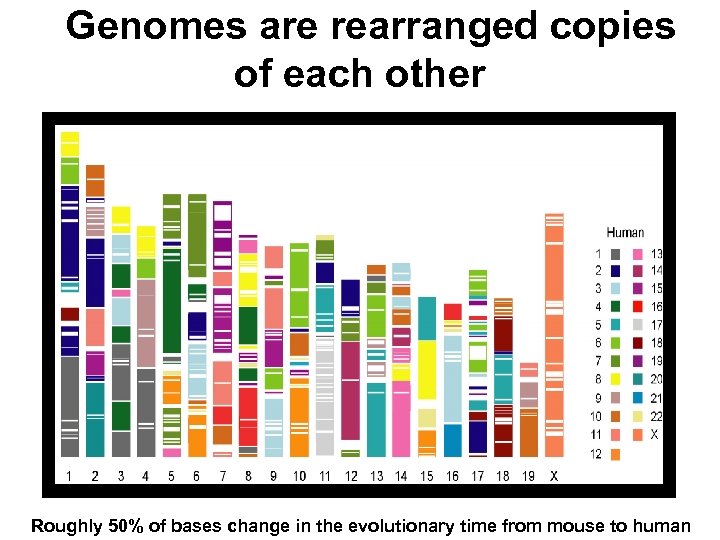 Genomes are rearranged copies of each other Roughly 50% of bases change in the evolutionary time from mouse to human
Genomes are rearranged copies of each other Roughly 50% of bases change in the evolutionary time from mouse to human
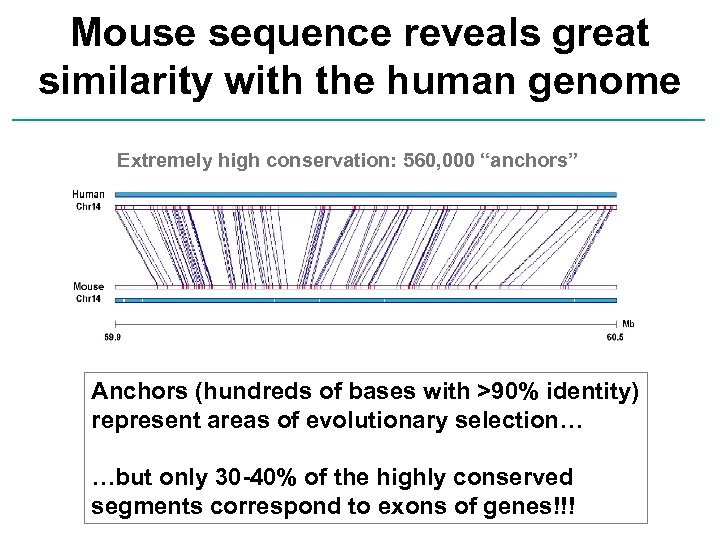 Mouse sequence reveals great similarity with the human genome Extremely high conservation: 560, 000 “anchors” Anchors (hundreds of bases with >90% identity) represent areas of evolutionary selection… …but only 30 -40% of the highly conserved segments correspond to exons of genes!!!
Mouse sequence reveals great similarity with the human genome Extremely high conservation: 560, 000 “anchors” Anchors (hundreds of bases with >90% identity) represent areas of evolutionary selection… …but only 30 -40% of the highly conserved segments correspond to exons of genes!!!
 What we can do • Directed matings • Inbred lines and crosses • • Knockouts Transgenics Mutagenesis Nuclear transfer • Control exposure to pathogens, drugs, diet, etc. YIKES!!!
What we can do • Directed matings • Inbred lines and crosses • • Knockouts Transgenics Mutagenesis Nuclear transfer • Control exposure to pathogens, drugs, diet, etc. YIKES!!!
 Example: diabetes related mice available from The Jackson Labs • • Type I diabetes (3) Type II diabetes (3) Hyperglycemic (27) Hyperinsulinemic (25) Hypoglycemic (1) Hypoinsulinemic (5) Insulin resistant (30) • Impaired insulin processing (7) • Impaired wound healing (13)
Example: diabetes related mice available from The Jackson Labs • • Type I diabetes (3) Type II diabetes (3) Hyperglycemic (27) Hyperinsulinemic (25) Hypoglycemic (1) Hypoinsulinemic (5) Insulin resistant (30) • Impaired insulin processing (7) • Impaired wound healing (13)
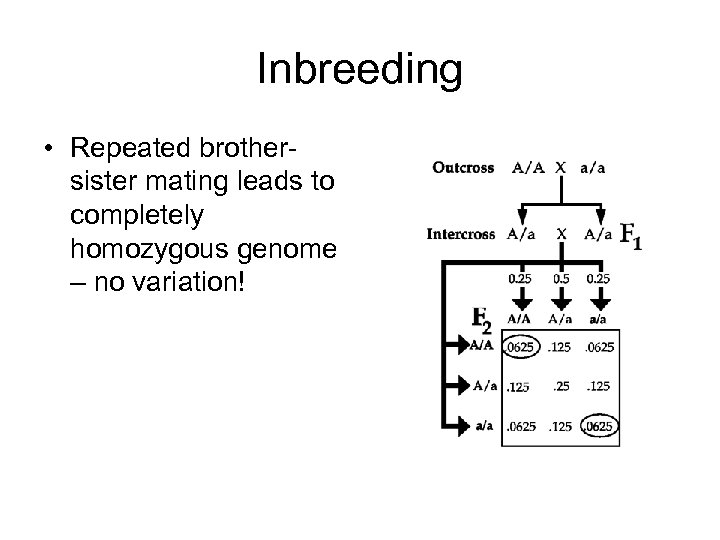 Inbreeding • Repeated brothersister mating leads to completely homozygous genome – no variation!
Inbreeding • Repeated brothersister mating leads to completely homozygous genome – no variation!
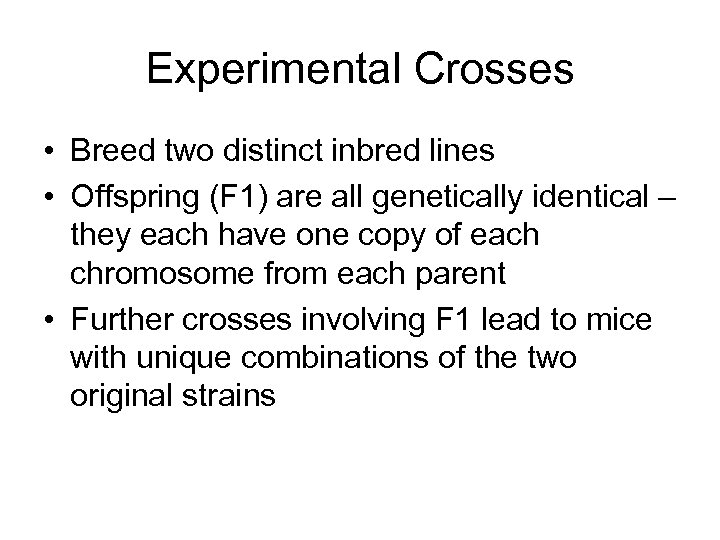 Experimental Crosses • Breed two distinct inbred lines • Offspring (F 1) are all genetically identical – they each have one copy of each chromosome from each parent • Further crosses involving F 1 lead to mice with unique combinations of the two original strains
Experimental Crosses • Breed two distinct inbred lines • Offspring (F 1) are all genetically identical – they each have one copy of each chromosome from each parent • Further crosses involving F 1 lead to mice with unique combinations of the two original strains
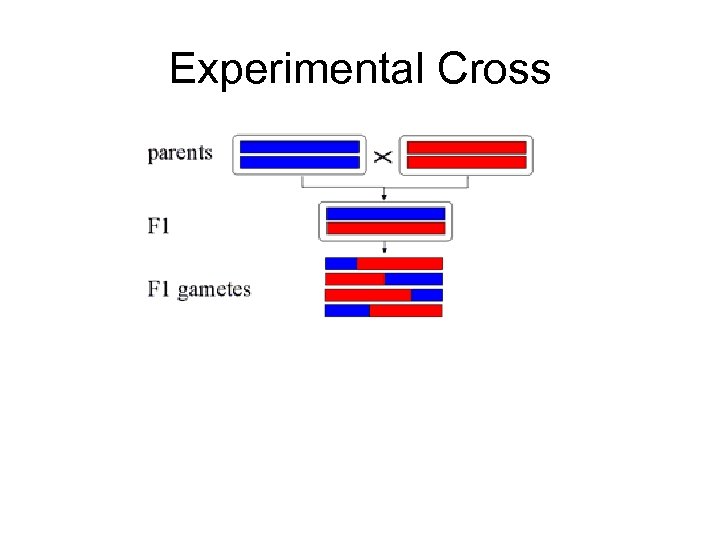 Experimental Cross
Experimental Cross
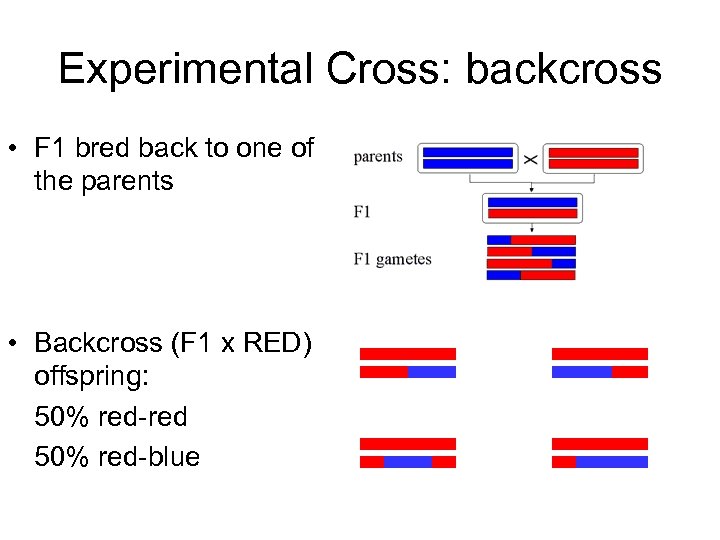 Experimental Cross: backcross • F 1 bred back to one of the parents • Backcross (F 1 x RED) offspring: 50% red-red 50% red-blue
Experimental Cross: backcross • F 1 bred back to one of the parents • Backcross (F 1 x RED) offspring: 50% red-red 50% red-blue
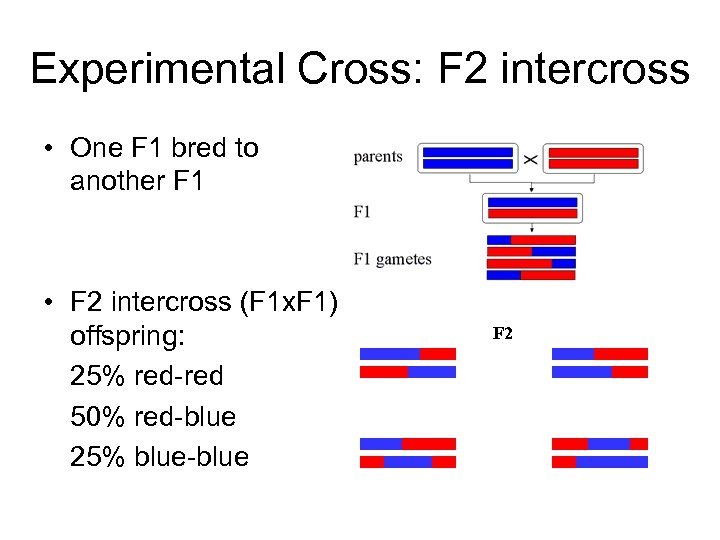 Experimental Cross: F 2 intercross • One F 1 bred to another F 1 • F 2 intercross (F 1 x. F 1) offspring: 25% red-red 50% red-blue 25% blue-blue F 2
Experimental Cross: F 2 intercross • One F 1 bred to another F 1 • F 2 intercross (F 1 x. F 1) offspring: 25% red-red 50% red-blue 25% blue-blue F 2
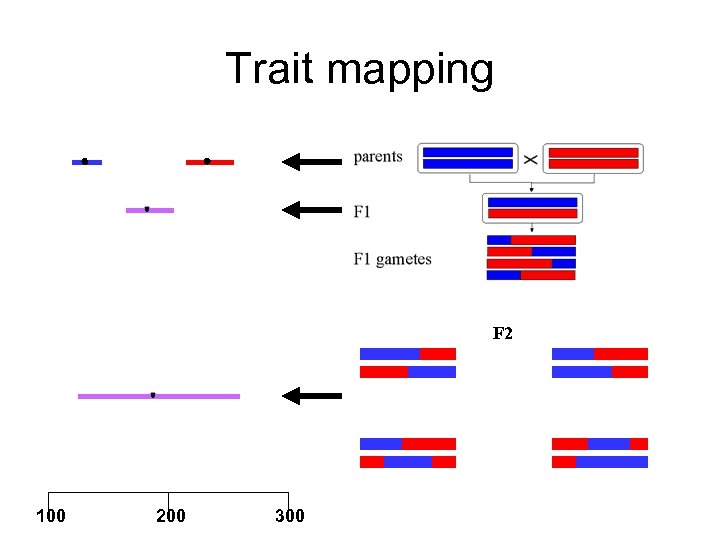 Trait mapping F 2 100 200 300
Trait mapping F 2 100 200 300
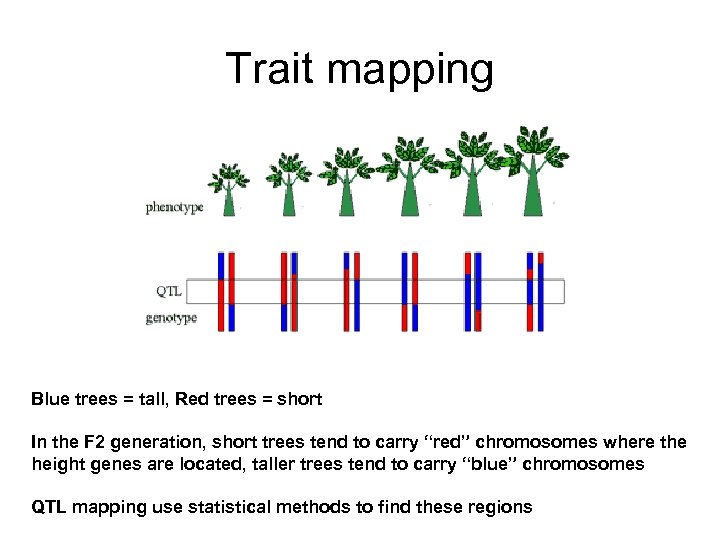 Trait mapping Blue trees = tall, Red trees = short In the F 2 generation, short trees tend to carry “red” chromosomes where the height genes are located, taller trees tend to carry “blue” chromosomes QTL mapping use statistical methods to find these regions
Trait mapping Blue trees = tall, Red trees = short In the F 2 generation, short trees tend to carry “red” chromosomes where the height genes are located, taller trees tend to carry “blue” chromosomes QTL mapping use statistical methods to find these regions
 How do we distinguish chromosomes from different strains? • Polymorphic DNA markers such as Single Nucleotide Polymorphisms (SNPs) can be used to distinguish the parental origin of offspring chromosomes ATTCGACGTATTGGCACTTACAGG ATTCGATGTATTGGCACTTACAGG SNP
How do we distinguish chromosomes from different strains? • Polymorphic DNA markers such as Single Nucleotide Polymorphisms (SNPs) can be used to distinguish the parental origin of offspring chromosomes ATTCGACGTATTGGCACTTACAGG ATTCGATGTATTGGCACTTACAGG SNP
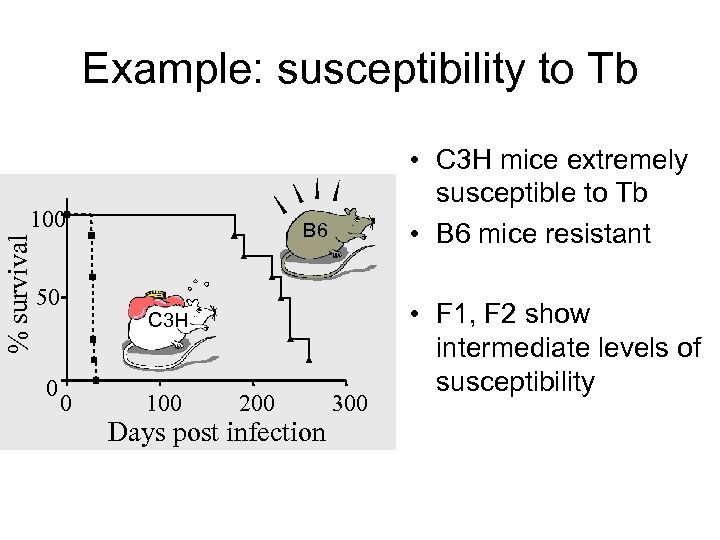 Example: susceptibility to Tb % survival 100 50 0 • C 3 H mice extremely susceptible to Tb • B 6 mice resistant B 6 C 3 H 0 100 200 Days post infection 300 • F 1, F 2 show intermediate levels of susceptibility
Example: susceptibility to Tb % survival 100 50 0 • C 3 H mice extremely susceptible to Tb • B 6 mice resistant B 6 C 3 H 0 100 200 Days post infection 300 • F 1, F 2 show intermediate levels of susceptibility
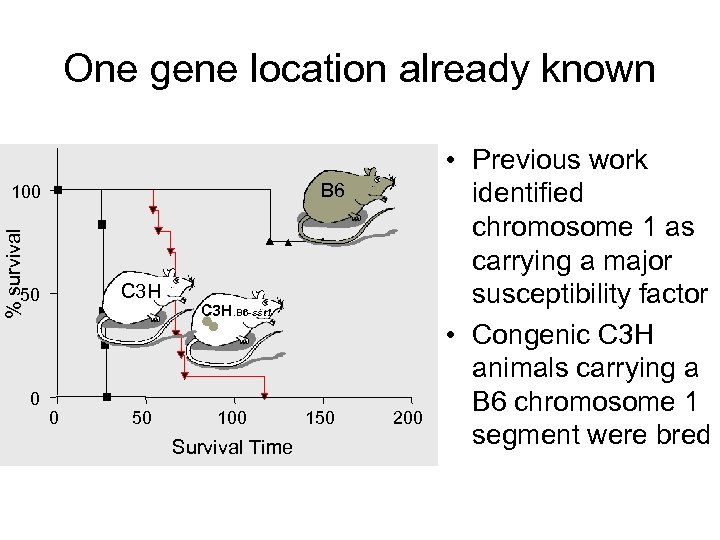 One gene location already known B 6 % survival 100 C 3 H 50 0 0 50 C 3 H. B 6 -sst 1 100 Survival Time 150 200 • Previous work identified chromosome 1 as carrying a major susceptibility factor • Congenic C 3 H animals carrying a B 6 chromosome 1 segment were bred
One gene location already known B 6 % survival 100 C 3 H 50 0 0 50 C 3 H. B 6 -sst 1 100 Survival Time 150 200 • Previous work identified chromosome 1 as carrying a major susceptibility factor • Congenic C 3 H animals carrying a B 6 chromosome 1 segment were bred
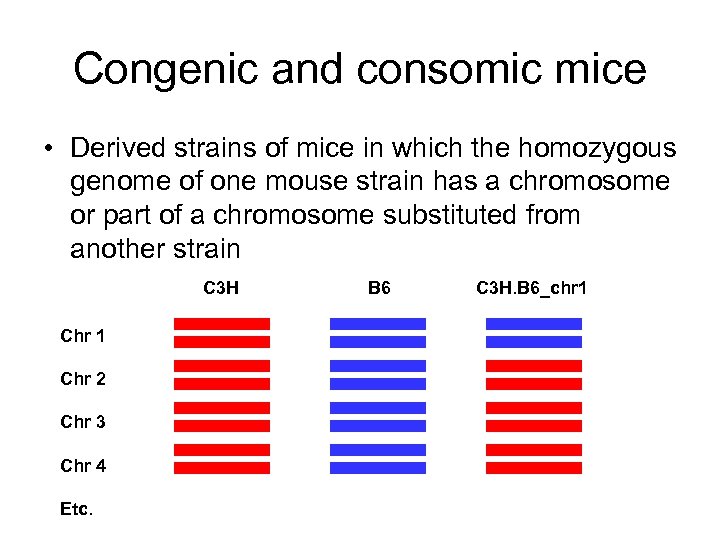 Congenic and consomic mice • Derived strains of mice in which the homozygous genome of one mouse strain has a chromosome or part of a chromosome substituted from another strain C 3 H Chr 1 Chr 2 Chr 3 Chr 4 Etc. B 6 C 3 H. B 6_chr 1
Congenic and consomic mice • Derived strains of mice in which the homozygous genome of one mouse strain has a chromosome or part of a chromosome substituted from another strain C 3 H Chr 1 Chr 2 Chr 3 Chr 4 Etc. B 6 C 3 H. B 6_chr 1
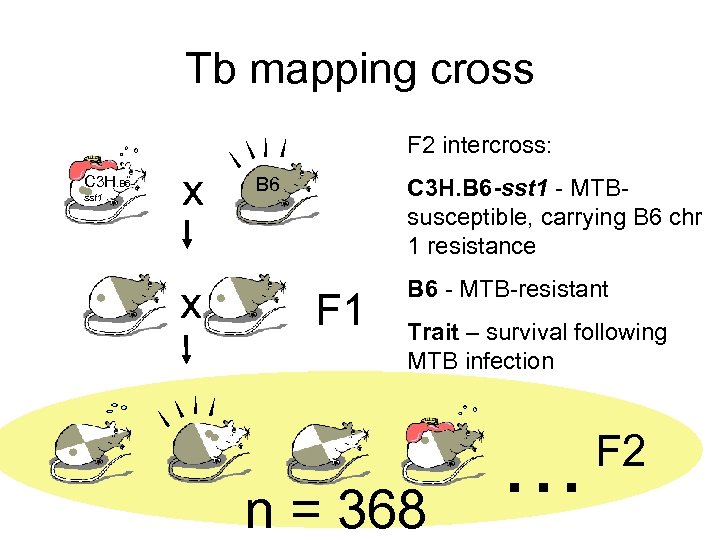 Tb mapping cross F 2 intercross: C 3 H. B 6 sst 1 x x B 6 C 3 H. B 6 -sst 1 - MTBsusceptible, carrying B 6 chr 1 resistance F 1 B 6 - MTB-resistant Trait – survival following MTB infection n = 368 … F 2
Tb mapping cross F 2 intercross: C 3 H. B 6 sst 1 x x B 6 C 3 H. B 6 -sst 1 - MTBsusceptible, carrying B 6 chr 1 resistance F 1 B 6 - MTB-resistant Trait – survival following MTB infection n = 368 … F 2
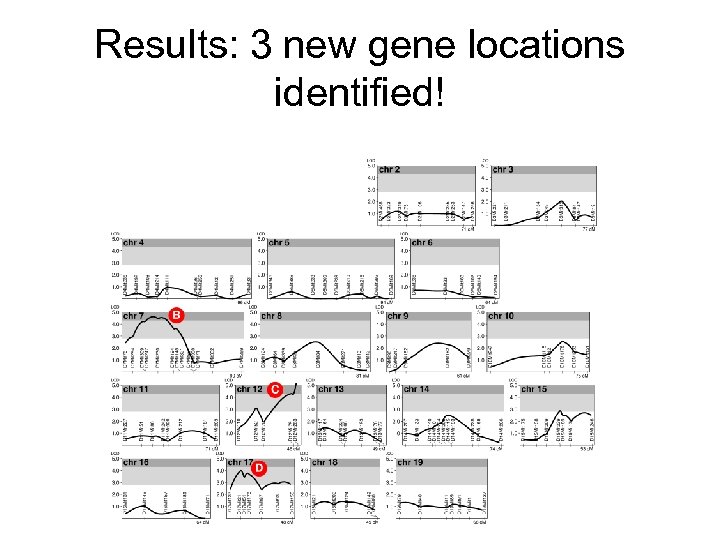 Results: 3 new gene locations identified!
Results: 3 new gene locations identified!
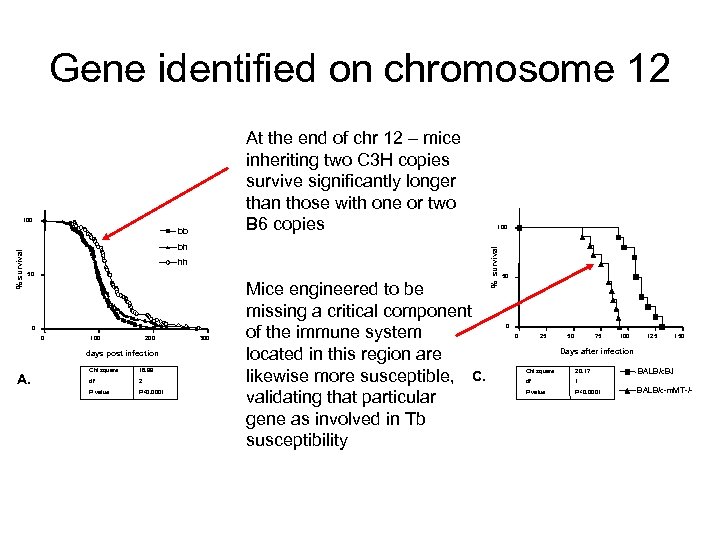 Gene identified on chromosome 12 100 bh % survival bb hh 50 Mice engineered to be missing a critical component of the immune system Days after infection located in this region are C 57 Bl/6 J B. likewise more susceptible, C. B 6 -Igh 6 validating that particular gene as involved in Tb susceptibility 0 0 0 100 200 days post infection A. 50 Chi square 18. 99 df 2 P value P<0. 0001 300 0 25 50 75 100 125 150 100 % survival At the end of chr 12 – mice inheriting two C 3 H copies survive significantly longer than those with one or two B 6 -IL 12 -/B 6 copies 50 0 0 25 50 75 100 125 150 Days after infection Chi square 30. 02 Chi square 20. 17 df 2 df P value P<0. 0001 BALB/c. BJ 1 BALB/c-m. MT-/-
Gene identified on chromosome 12 100 bh % survival bb hh 50 Mice engineered to be missing a critical component of the immune system Days after infection located in this region are C 57 Bl/6 J B. likewise more susceptible, C. B 6 -Igh 6 validating that particular gene as involved in Tb susceptibility 0 0 0 100 200 days post infection A. 50 Chi square 18. 99 df 2 P value P<0. 0001 300 0 25 50 75 100 125 150 100 % survival At the end of chr 12 – mice inheriting two C 3 H copies survive significantly longer than those with one or two B 6 -IL 12 -/B 6 copies 50 0 0 25 50 75 100 125 150 Days after infection Chi square 30. 02 Chi square 20. 17 df 2 df P value P<0. 0001 BALB/c. BJ 1 BALB/c-m. MT-/-

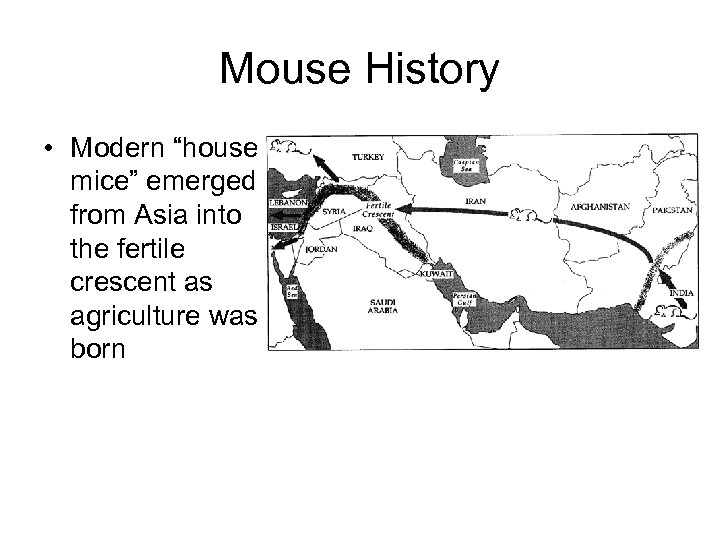 Mouse History • Modern “house mice” emerged from Asia into the fertile crescent as agriculture was born
Mouse History • Modern “house mice” emerged from Asia into the fertile crescent as agriculture was born
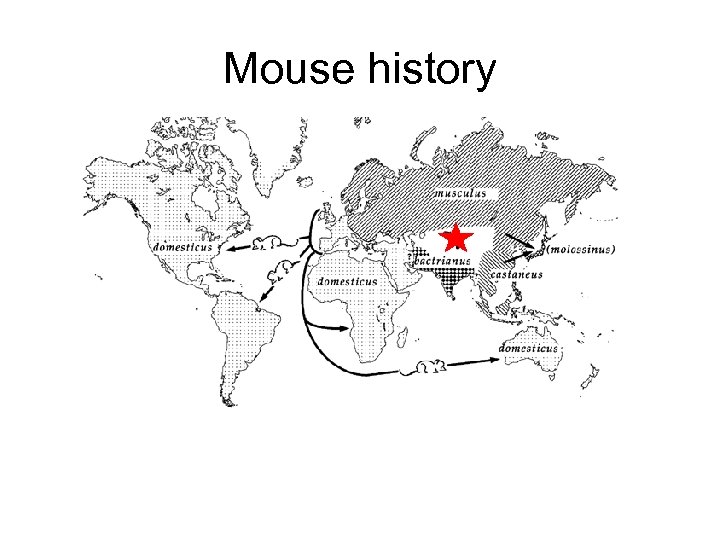 Mouse history
Mouse history
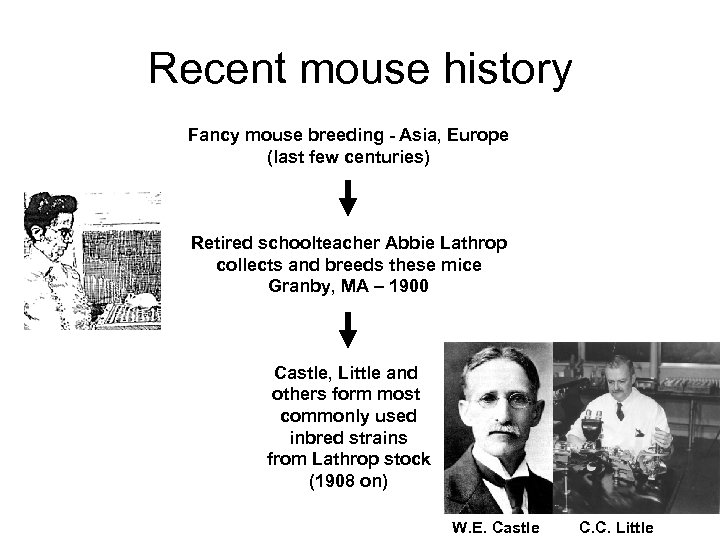 Recent mouse history Fancy mouse breeding - Asia, Europe (last few centuries) Retired schoolteacher Abbie Lathrop collects and breeds these mice Granby, MA – 1900 Castle, Little and others form most commonly used inbred strains from Lathrop stock (1908 on) W. E. Castle C. C. Little
Recent mouse history Fancy mouse breeding - Asia, Europe (last few centuries) Retired schoolteacher Abbie Lathrop collects and breeds these mice Granby, MA – 1900 Castle, Little and others form most commonly used inbred strains from Lathrop stock (1908 on) W. E. Castle C. C. Little
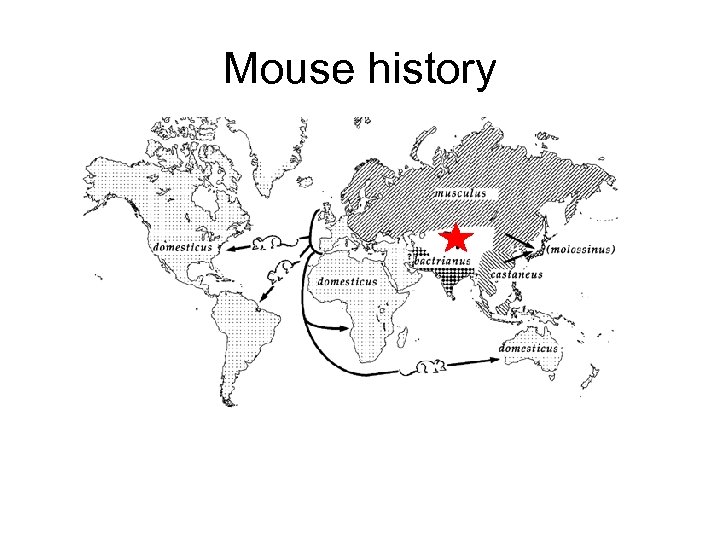 Mouse history
Mouse history
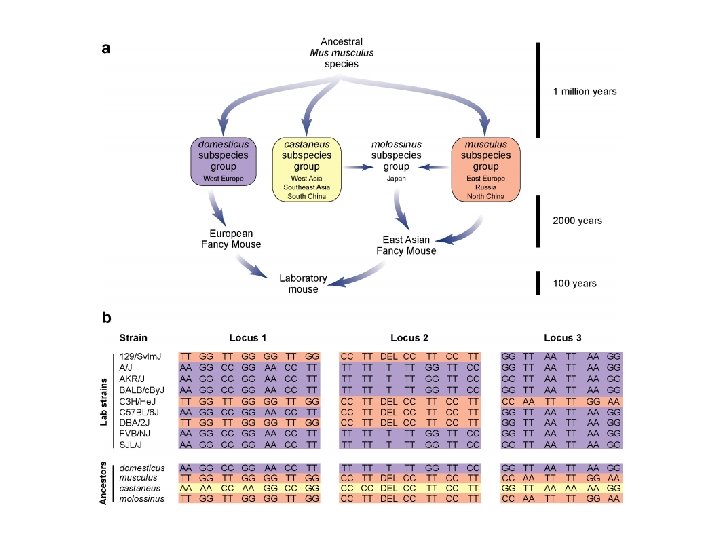
 Mouse history • Asian musculus and European domesticus mice dominate the world but have evolved separately over ~ 1 Million years • Mixing in Abbie Lathrop’s schoolhouse created all our commonly used mice from these two distinct founder groups
Mouse history • Asian musculus and European domesticus mice dominate the world but have evolved separately over ~ 1 Million years • Mixing in Abbie Lathrop’s schoolhouse created all our commonly used mice from these two distinct founder groups
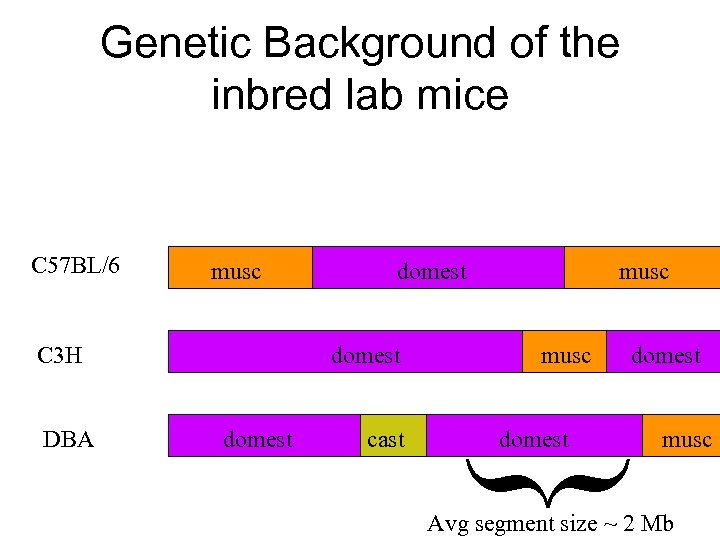 Genetic Background of the inbred lab mice musc C 3 H DBA domest cast musc domest { C 57 BL/6 domest musc Avg segment size ~ 2 Mb
Genetic Background of the inbred lab mice musc C 3 H DBA domest cast musc domest { C 57 BL/6 domest musc Avg segment size ~ 2 Mb
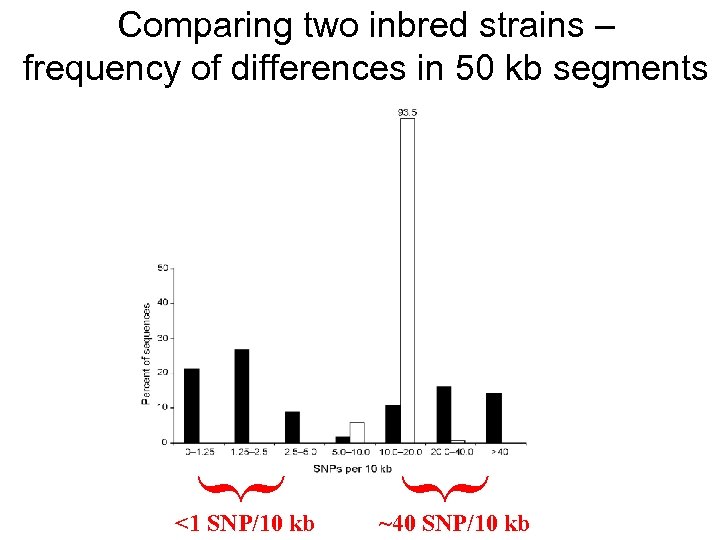 <1 SNP/10 kb { { Comparing two inbred strains – frequency of differences in 50 kb segments ~40 SNP/10 kb
<1 SNP/10 kb { { Comparing two inbred strains – frequency of differences in 50 kb segments ~40 SNP/10 kb
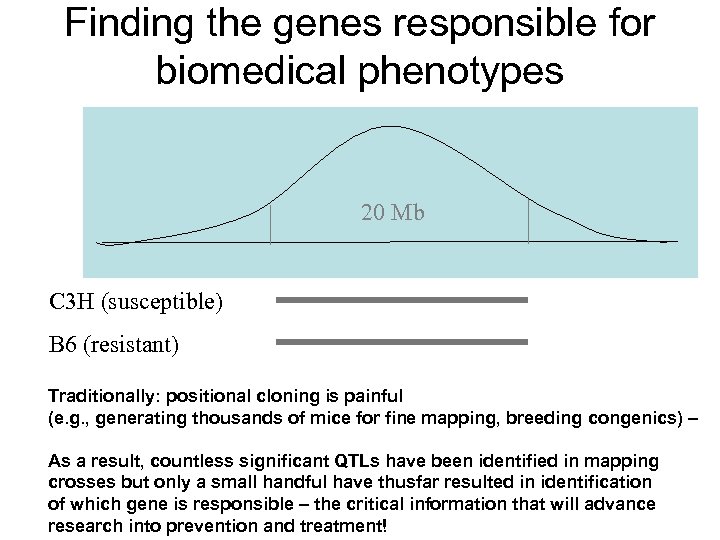 Finding the genes responsible for biomedical phenotypes 20 Mb C 3 H (susceptible) B 6 (resistant) Traditionally: positional cloning is painful (e. g. , generating thousands of mice for fine mapping, breeding congenics) – As a result, countless significant QTLs have been identified in mapping crosses but only a small handful have thusfar resulted in identification of which gene is responsible – the critical information that will advance research into prevention and treatment!
Finding the genes responsible for biomedical phenotypes 20 Mb C 3 H (susceptible) B 6 (resistant) Traditionally: positional cloning is painful (e. g. , generating thousands of mice for fine mapping, breeding congenics) – As a result, countless significant QTLs have been identified in mapping crosses but only a small handful have thusfar resulted in identification of which gene is responsible – the critical information that will advance research into prevention and treatment!
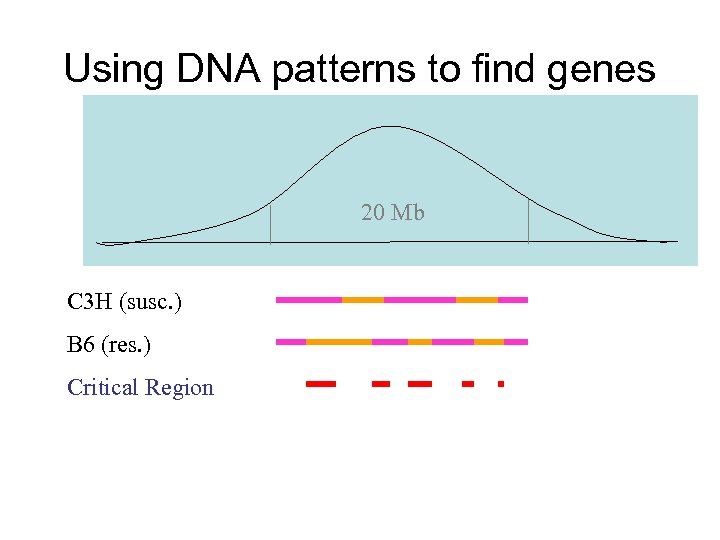 Using DNA patterns to find genes 20 Mb C 3 H (susc. ) B 6 (res. ) Critical Region
Using DNA patterns to find genes 20 Mb C 3 H (susc. ) B 6 (res. ) Critical Region
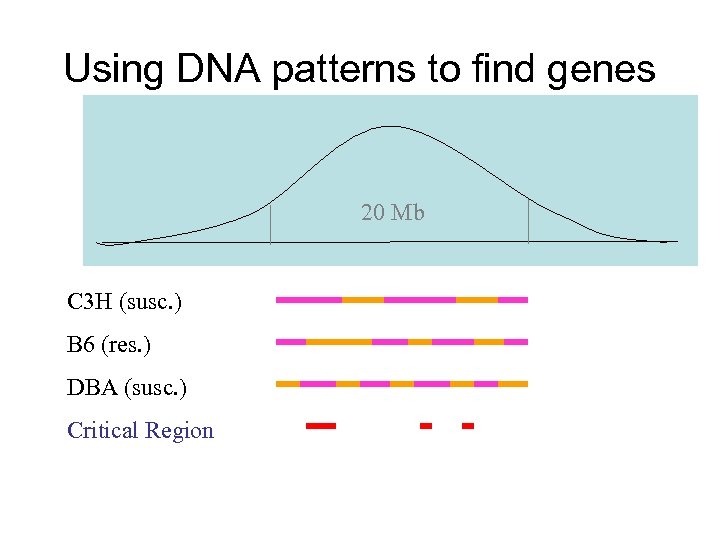 Using DNA patterns to find genes 20 Mb C 3 H (susc. ) B 6 (res. ) DBA (susc. ) Critical Region
Using DNA patterns to find genes 20 Mb C 3 H (susc. ) B 6 (res. ) DBA (susc. ) Critical Region
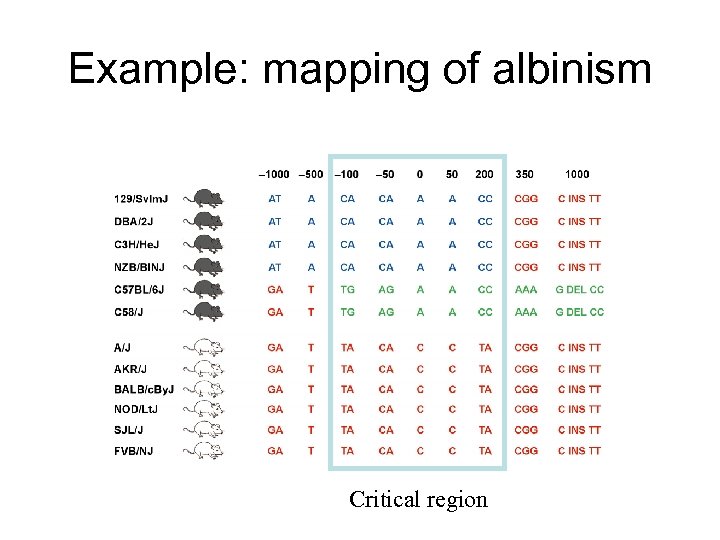 Example: mapping of albinism Critical region
Example: mapping of albinism Critical region
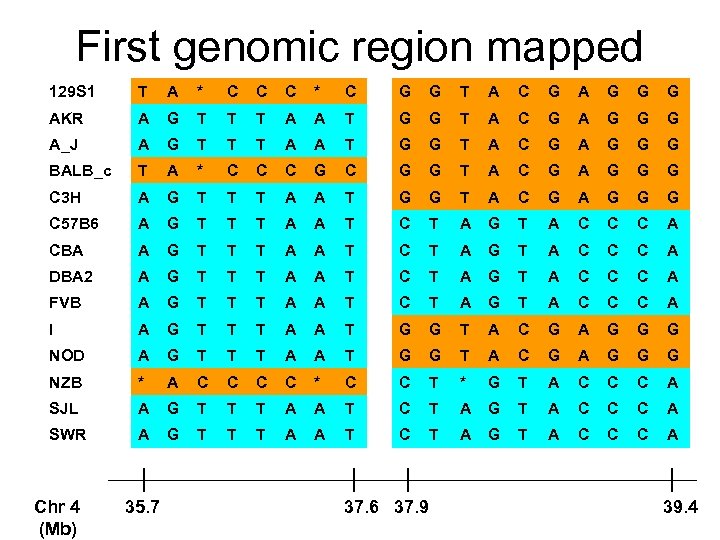 First genomic region mapped 129 S 1 T A * C C C * C G G T A C G A G G G AKR A G T T T A A T G G T A C G A G G G A_J A G T T T A A T G G T A C G A G G G BALB_c T A * C C C G G T A C G A G G G C 3 H A G T T T A A T G G T A C G A G G G C 57 B 6 A G T T T A A T C T A G T A C C C A CBA A G T T T A A T C T A G T A C C C A DBA 2 A G T T T A A T C T A G T A C C C A FVB A G T T T A A T C T A G T A C C C A I A G T T T A A T G G T A C G A G G G NOD A G T T T A A T G G T A C G A G G G NZB * A C C * C C T * G T A C C C A SJL A G T T T A A T C T A G T A C C C A SWR A G T T T A A T C T A G T A C C C A Chr 4 (Mb) 35. 7 37. 6 37. 9 39. 4
First genomic region mapped 129 S 1 T A * C C C * C G G T A C G A G G G AKR A G T T T A A T G G T A C G A G G G A_J A G T T T A A T G G T A C G A G G G BALB_c T A * C C C G G T A C G A G G G C 3 H A G T T T A A T G G T A C G A G G G C 57 B 6 A G T T T A A T C T A G T A C C C A CBA A G T T T A A T C T A G T A C C C A DBA 2 A G T T T A A T C T A G T A C C C A FVB A G T T T A A T C T A G T A C C C A I A G T T T A A T G G T A C G A G G G NOD A G T T T A A T G G T A C G A G G G NZB * A C C * C C T * G T A C C C A SJL A G T T T A A T C T A G T A C C C A SWR A G T T T A A T C T A G T A C C C A Chr 4 (Mb) 35. 7 37. 6 37. 9 39. 4
 Future Genetic Studies Mapping Expression Pathways Model Systems
Future Genetic Studies Mapping Expression Pathways Model Systems
 Thanks to (Whitehead Institute) Claire Wade Andrew Kirby (MIT Genome Center) EJ Kulbokas Mike Zody Eric Lander Kerstin Lindblad-Toh Funding: Whitehead Institute Pfizer, Inc. National Human Genome Research Institute
Thanks to (Whitehead Institute) Claire Wade Andrew Kirby (MIT Genome Center) EJ Kulbokas Mike Zody Eric Lander Kerstin Lindblad-Toh Funding: Whitehead Institute Pfizer, Inc. National Human Genome Research Institute


