29c3c3bb0c74f7be5c882c70fa8bf8ea.ppt
- Количество слайдов: 26
 Using EMR Data for Population Registries Diana Gumas, JHMCIS Senior Director for Research Systems, EPR and EPR 2020/Amalga David Thiemann, Center for Clinical Data Analysis 1
Using EMR Data for Population Registries Diana Gumas, JHMCIS Senior Director for Research Systems, EPR and EPR 2020/Amalga David Thiemann, Center for Clinical Data Analysis 1
 Potential Data Uses • Sample Size Estimates (aggregate data without IRB approval) – Feasibility, grant applications, statistical planning • Identifying patients for enrollment/recruitment – By diagnosis, pathology, stage, labs, meds • Identifying/creating matched study controls • Obtaining current demographics (name, address) for mail solicitation – From research list or by clinic, provider, clinical criteria • Obtaining ongoing clinical + administrative data on a registry panel – Labs, visits, procedures, immunizations, CPT/ICD 9 codes, resource use 2
Potential Data Uses • Sample Size Estimates (aggregate data without IRB approval) – Feasibility, grant applications, statistical planning • Identifying patients for enrollment/recruitment – By diagnosis, pathology, stage, labs, meds • Identifying/creating matched study controls • Obtaining current demographics (name, address) for mail solicitation – From research list or by clinic, provider, clinical criteria • Obtaining ongoing clinical + administrative data on a registry panel – Labs, visits, procedures, immunizations, CPT/ICD 9 codes, resource use 2
 Possible research data sources • • • EPR (JHH & JHBMC) Sunrise Clinical Manager (JHH – inpatient) Meditech (Bayview) Casemix Datamart GE Centricity (JHCP) EPR 2020 Departmental Systems (ED, OR, Anesthesia) Clinical Research Management System (CRMS) IDX (professional fees) Death Registry 3
Possible research data sources • • • EPR (JHH & JHBMC) Sunrise Clinical Manager (JHH – inpatient) Meditech (Bayview) Casemix Datamart GE Centricity (JHCP) EPR 2020 Departmental Systems (ED, OR, Anesthesia) Clinical Research Management System (CRMS) IDX (professional fees) Death Registry 3
 Methods for Data Access • Historical: Researcher Negotiates Access With Clinical System /DBA – • Logistic nightmare, technical challenge Clinical Research Management System (CRMS) – • Study cohort with real-time links to enterprise data Center for Clinical Data Analysis – Monthly/quarterly data extracts from designated systems 4
Methods for Data Access • Historical: Researcher Negotiates Access With Clinical System /DBA – • Logistic nightmare, technical challenge Clinical Research Management System (CRMS) – • Study cohort with real-time links to enterprise data Center for Clinical Data Analysis – Monthly/quarterly data extracts from designated systems 4
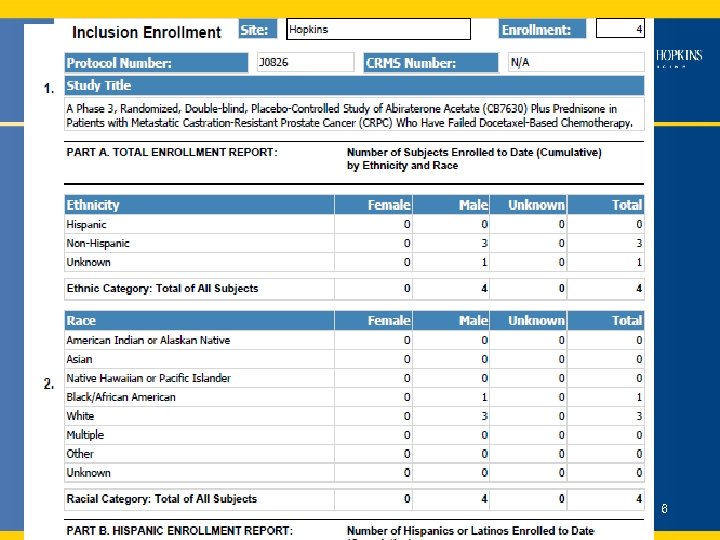
 Clinical Research Management System (CRMS) • • • 1, 054 Users 1079 Active Studies 25, 430 Participants Data Available in CRMS – e. IRB – EPR (patient demographics) – Study participants / accruals – Electronic Case Report Forms - in next 2 -3 months 5
Clinical Research Management System (CRMS) • • • 1, 054 Users 1079 Active Studies 25, 430 Participants Data Available in CRMS – e. IRB – EPR (patient demographics) – Study participants / accruals – Electronic Case Report Forms - in next 2 -3 months 5
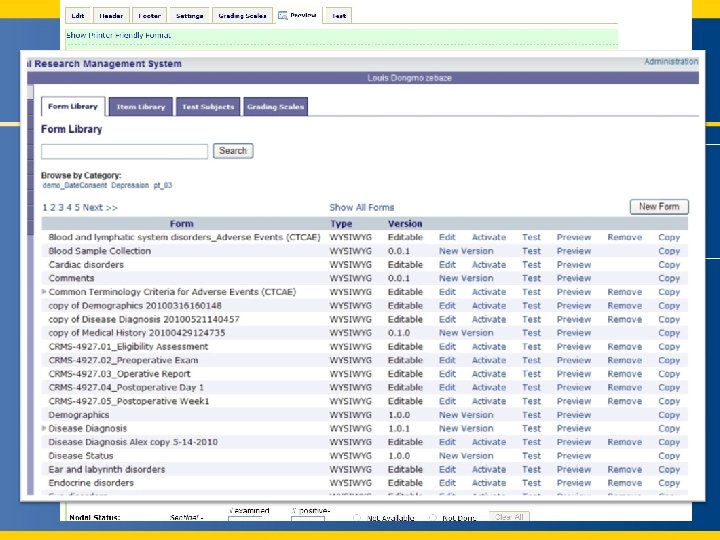
 Clinical Research Management System (CRMS) Ways to extract data – Canned Reports (click for examples) – Ad-hoc querying using SQL – Possible with CCDA support - automated study-specific data extracts 6
Clinical Research Management System (CRMS) Ways to extract data – Canned Reports (click for examples) – Ad-hoc querying using SQL – Possible with CCDA support - automated study-specific data extracts 6
 EPR 2020 Data for Researchers 4. 2 M Patients, 23. 4 M Visits 12. 3 M Documents, 6. 8 M Radiology Reports 25. 6 M Lab Results 1. 5 M Problems, 2. 2 M Medications, 140 K Allergies Planned • Bayview & JHCP data • ICD 9 diagnosis codes and CPT charges (IDX) Future • Death Registry • Blood Product Data for Transfusions • Eclipsys SCM Order data • HMED (ED), ORMIS, e. ADR/Medivision From EPR Today 7
EPR 2020 Data for Researchers 4. 2 M Patients, 23. 4 M Visits 12. 3 M Documents, 6. 8 M Radiology Reports 25. 6 M Lab Results 1. 5 M Problems, 2. 2 M Medications, 140 K Allergies Planned • Bayview & JHCP data • ICD 9 diagnosis codes and CPT charges (IDX) Future • Death Registry • Blood Product Data for Transfusions • Eclipsys SCM Order data • HMED (ED), ORMIS, e. ADR/Medivision From EPR Today 7
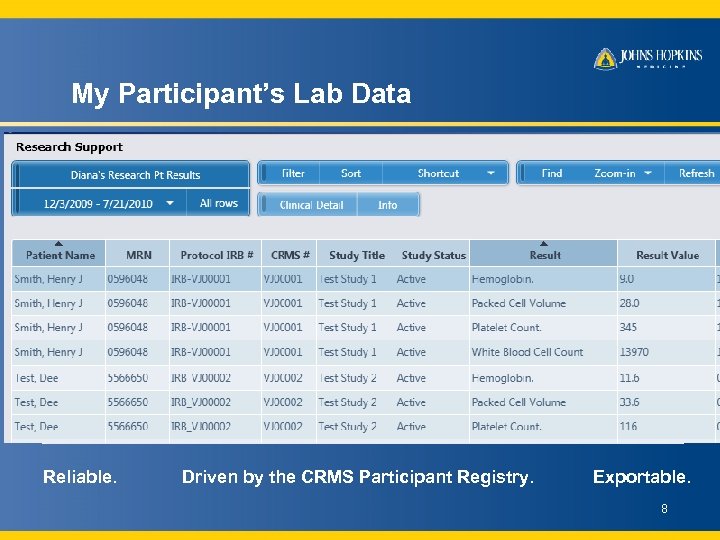 My Participant’s Lab Data Reliable. Driven by the CRMS Participant Registry. Exportable. 8
My Participant’s Lab Data Reliable. Driven by the CRMS Participant Registry. Exportable. 8
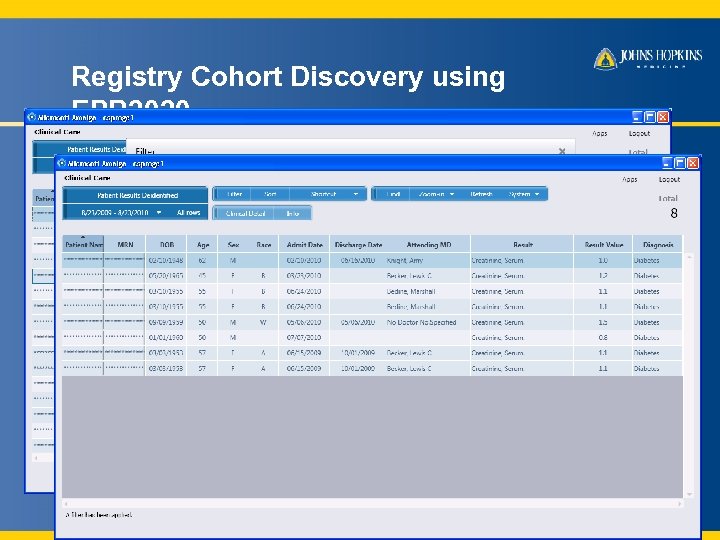 Registry Cohort Discovery using EPR 2020 A JHM investigator wants to find and enroll diabetic patients aged 45 -65 years with hemoglobin A 1 C between 7 and 9% serum creatinine < 2 mg/dl 9
Registry Cohort Discovery using EPR 2020 A JHM investigator wants to find and enroll diabetic patients aged 45 -65 years with hemoglobin A 1 C between 7 and 9% serum creatinine < 2 mg/dl 9
 Center for Clinical Data Analysis (CCDA) Provides periodic (monthly/quarterly) bulk data extracts (delimited/flat files, . xls): • Preliminary, anonymous data for feasibility, grant applications and statistical sample-size estimates • IRB-approved case-finding--for study enrollment (mailings, phone solicitation), chart review, and cohort/case-control studies • Research data extracts - monthly/quarterly integrated extracts from EPR, POE, ORMIS, lab/PDS, billing systems, vaccination/transfusion/culture data, etc. 10
Center for Clinical Data Analysis (CCDA) Provides periodic (monthly/quarterly) bulk data extracts (delimited/flat files, . xls): • Preliminary, anonymous data for feasibility, grant applications and statistical sample-size estimates • IRB-approved case-finding--for study enrollment (mailings, phone solicitation), chart review, and cohort/case-control studies • Research data extracts - monthly/quarterly integrated extracts from EPR, POE, ORMIS, lab/PDS, billing systems, vaccination/transfusion/culture data, etc. 10
 How CCDA works • Email CCDA@jhmi. edu, cc: dthiema 1@jhmi. edu; phone 410 -955 -65558 (Thiemann) • For IRB-approved research: – Provide full protocol + IRB approval – Meet to discuss query methods, format – Iterate, then schedule prod (email extracts, Jshare) – Cost: $100/hour • For non-IRB projects (exploratory analyses, QI) – Same process, cost subsidized by ICTR/JHM – Do NOT implicitly morph QI into IRB 11
How CCDA works • Email CCDA@jhmi. edu, cc: dthiema 1@jhmi. edu; phone 410 -955 -65558 (Thiemann) • For IRB-approved research: – Provide full protocol + IRB approval – Meet to discuss query methods, format – Iterate, then schedule prod (email extracts, Jshare) – Cost: $100/hour • For non-IRB projects (exploratory analyses, QI) – Same process, cost subsidized by ICTR/JHM – Do NOT implicitly morph QI into IRB 11
 The Basics: Getting Clinical Data Into a Registry Database • Real work, not ad hoc/bootstrap • Need $$$ and FTE(s) • Smart analyst(s) who know database technology and understand (or can learn) nuances of the sources and content domain • Hands-on PI management/guidance • Statistical liason early, before database schema and ETL methods are set in stone 12
The Basics: Getting Clinical Data Into a Registry Database • Real work, not ad hoc/bootstrap • Need $$$ and FTE(s) • Smart analyst(s) who know database technology and understand (or can learn) nuances of the sources and content domain • Hands-on PI management/guidance • Statistical liason early, before database schema and ETL methods are set in stone 12
 The Extract-Transform-Load process: Getting Clinical Data into Research DB • Raw clinical/administrative data is useless for research • Build an intermediate (staging) database – Don’t do data management in SAS/Stata/Excel • Data dictionary—derivation for each field • Templated, tested, documented cleanup scripts/routines. • Intermediate tables: Log each step/modification – – – For each batch, be able to re-create data transform from scratch Version control, change control and documentation are vital Build data versioning into the database 13
The Extract-Transform-Load process: Getting Clinical Data into Research DB • Raw clinical/administrative data is useless for research • Build an intermediate (staging) database – Don’t do data management in SAS/Stata/Excel • Data dictionary—derivation for each field • Templated, tested, documented cleanup scripts/routines. • Intermediate tables: Log each step/modification – – – For each batch, be able to re-create data transform from scratch Version control, change control and documentation are vital Build data versioning into the database 13
 Transforming Data • Raw data typically string (char/text) fields • Unanalyzable characters (* < >, comments) still have meaning – • ~3% of pts have multiple/non-preferred MRNs – • Need 1 -to-many link table Assays/reference ranges/coding changes – – • Put non-numeric data in separate field. Avoid numerical recoding (999) Avoid using raw codes (CPT/ICD) in research db Map clinical codes to research terms Defer analytic assumptions. When recoding data, anticipate problems. Keep options open. 14
Transforming Data • Raw data typically string (char/text) fields • Unanalyzable characters (* < >, comments) still have meaning – • ~3% of pts have multiple/non-preferred MRNs – • Need 1 -to-many link table Assays/reference ranges/coding changes – – • Put non-numeric data in separate field. Avoid numerical recoding (999) Avoid using raw codes (CPT/ICD) in research db Map clinical codes to research terms Defer analytic assumptions. When recoding data, anticipate problems. Keep options open. 14
 More Data Transform Challenges • NEVER trust raw data. Learn business logic of source system. – – CPTs morph annually, internal complexity/redundancy Lab assays/reference/terms change Parsing is inherently unreliable Administrative names/groups change (clinic #s, departments). • Duplicate-value problems (labs, orders) • System-attribution source/datetime (POE, lab) • Always run an aggregate (“group by” ) query to identify alternative names (eg lab name) and values (number, result) before transform. Otherwise you’ll miss something 15
More Data Transform Challenges • NEVER trust raw data. Learn business logic of source system. – – CPTs morph annually, internal complexity/redundancy Lab assays/reference/terms change Parsing is inherently unreliable Administrative names/groups change (clinic #s, departments). • Duplicate-value problems (labs, orders) • System-attribution source/datetime (POE, lab) • Always run an aggregate (“group by” ) query to identify alternative names (eg lab name) and values (number, result) before transform. Otherwise you’ll miss something 15
 Understanding Business Logic • Trust but verify: Test coding accuracy – – – • Run min/max queries, aggregates, outer joins – • Providers may habitually use imprecise/inaccurate diagnosis codes (especially in profee data) ICD 9 procedure indications often a billing fiction Trained coders may make systematic errors Different content domains may have different standards (inpt vs outpt coders) Don’t infer/assume dependencies unless enforced by source system. Confirm date ranges, data ranges, relative proportions by year Don’t assume that null rows actually are empty. Maybe the query missed something 16
Understanding Business Logic • Trust but verify: Test coding accuracy – – – • Run min/max queries, aggregates, outer joins – • Providers may habitually use imprecise/inaccurate diagnosis codes (especially in profee data) ICD 9 procedure indications often a billing fiction Trained coders may make systematic errors Different content domains may have different standards (inpt vs outpt coders) Don’t infer/assume dependencies unless enforced by source system. Confirm date ranges, data ranges, relative proportions by year Don’t assume that null rows actually are empty. Maybe the query missed something 16
 JHM Clinical Data Landscape: Past, Present and Future Past : Babble of unintegrated systems • EPR (antiquated technology, VSAM files, DB 2) contains text, not queryable, analyzable data Present: EPR 2020 (aka Amalga) –integrated data!! • Has everything in EPR, plus JHCP, plus gradually adding data from clinical/departmental/administative systems (IDX CPTs, transfusion medicine, ORMIS, HMED, e. ADR, death registry, ad infinitum) Future: ? Epic, ? JHM Data Warehouse • Epic: One system replacing all major JHM systems • JHH timeline: 4+ years 17
JHM Clinical Data Landscape: Past, Present and Future Past : Babble of unintegrated systems • EPR (antiquated technology, VSAM files, DB 2) contains text, not queryable, analyzable data Present: EPR 2020 (aka Amalga) –integrated data!! • Has everything in EPR, plus JHCP, plus gradually adding data from clinical/departmental/administative systems (IDX CPTs, transfusion medicine, ORMIS, HMED, e. ADR, death registry, ad infinitum) Future: ? Epic, ? JHM Data Warehouse • Epic: One system replacing all major JHM systems • JHH timeline: 4+ years 17
 JHM Data Sources: Casemix Datamart • Gold standard for JHM (non-profee) administrative data, including payer/insurance data • Combines data from Keane (hospital charges), ADT (admission/discharge/transfer), HDM (ICD 9 diagnosis + procedure coding), HSCRC (regulatory submissions) • Not a true data warehouse; meager reconciliation • Best source for length of stay, resource use, ICD 9 diagnoses • Outpatient ICD 9 s limited • Has JHH + BMC + HCGH data 18
JHM Data Sources: Casemix Datamart • Gold standard for JHM (non-profee) administrative data, including payer/insurance data • Combines data from Keane (hospital charges), ADT (admission/discharge/transfer), HDM (ICD 9 diagnosis + procedure coding), HSCRC (regulatory submissions) • Not a true data warehouse; meager reconciliation • Best source for length of stay, resource use, ICD 9 diagnoses • Outpatient ICD 9 s limited • Has JHH + BMC + HCGH data 18
 JHM Data Sources: IDX (profee) • Gold standard for inpatient +outpatient CPT (profee charge) data • ICD 9 diagnosis data problematic • Limitation: No data from non-faculty providers (private physicians, etc. ) • Difficult to query. Has a data warehouse, limited access. • Early target for EPR 2020/Amalga integration. 19
JHM Data Sources: IDX (profee) • Gold standard for inpatient +outpatient CPT (profee charge) data • ICD 9 diagnosis data problematic • Limitation: No data from non-faculty providers (private physicians, etc. ) • Difficult to query. Has a data warehouse, limited access. • Early target for EPR 2020/Amalga integration. 19
 JHH Data Sources: SCM/POE • Sunrise Clinical Manager/Provider Order Entry • Replicated transactional database, difficult to query • For registry purposes POE has large attribution/process challenges: Stutter-step orders, multiple alerts, imputed times • Great source for inpatient meds, labs, physiologic monitor data • No codified ICD 9/Snomed/Rx. Norm data • No outpatient data 20
JHH Data Sources: SCM/POE • Sunrise Clinical Manager/Provider Order Entry • Replicated transactional database, difficult to query • For registry purposes POE has large attribution/process challenges: Stutter-step orders, multiple alerts, imputed times • Great source for inpatient meds, labs, physiologic monitor data • No codified ICD 9/Snomed/Rx. Norm data • No outpatient data 20
 JHH Data Sources: SCC/AIM • Sunrise Critical Care (aka Emtek, Eclipsys). JHH ICUs + stepdown units + oncology • AIM analytic database contains selected but comprehensive batch extract • Sunsets as ICUs switch to POE Clin. Doc • Challenging to query. Lots of denormalized fields 21
JHH Data Sources: SCC/AIM • Sunrise Critical Care (aka Emtek, Eclipsys). JHH ICUs + stepdown units + oncology • AIM analytic database contains selected but comprehensive batch extract • Sunsets as ICUs switch to POE Clin. Doc • Challenging to query. Lots of denormalized fields 21
 JHH + BMC Data Sources: PDS • PDS=Pathology Data Systems • Includes lab, transfusion medicine, anatomic pathology, cytopath, John Boitnott’s death registry • Lab data also available via EPR 2020/Amalga and POE 22
JHH + BMC Data Sources: PDS • PDS=Pathology Data Systems • Includes lab, transfusion medicine, anatomic pathology, cytopath, John Boitnott’s death registry • Lab data also available via EPR 2020/Amalga and POE 22
 BMC Data Sources: Meditech • Shrink-wrapped, comprehensive inpatient + outpatient clinical + financial system • Difficult for ad hoc research queries. • Exports data to Datamart and EPR 2020 • BMC-JHH patient linkage doable but difficult, needs caution 23
BMC Data Sources: Meditech • Shrink-wrapped, comprehensive inpatient + outpatient clinical + financial system • Difficult for ad hoc research queries. • Exports data to Datamart and EPR 2020 • BMC-JHH patient linkage doable but difficult, needs caution 23
 JHCP Data Sources: GE Centricity • All clinical + administrative data for JHCP clinics • Largely opaque to research query; JHCP sometimes collaborates directly, especially for its physician/investigators • Early target for EPR 2020/Amalga integration • Linkage challenges to BMC and JHH mrns 24
JHCP Data Sources: GE Centricity • All clinical + administrative data for JHCP clinics • Largely opaque to research query; JHCP sometimes collaborates directly, especially for its physician/investigators • Early target for EPR 2020/Amalga integration • Linkage challenges to BMC and JHH mrns 24
 JHH Departmental Data: ORMIS + e. ADR/Medivision • ORMIS: Operating Room Management Information System • Mostly transactional scheduling/tracking/administrative data, limited clinical data. • Has diagnoses, procedures, case start/stop times • e. ADR/Medivision (anesthesia) still evolving, limited research data access • Design challenges similar to legacy SCC critical-care system. 25
JHH Departmental Data: ORMIS + e. ADR/Medivision • ORMIS: Operating Room Management Information System • Mostly transactional scheduling/tracking/administrative data, limited clinical data. • Has diagnoses, procedures, case start/stop times • e. ADR/Medivision (anesthesia) still evolving, limited research data access • Design challenges similar to legacy SCC critical-care system. 25
 JHH Departmental Data: HMED (Emergency Department) • Mostly opaque to research • Replicated data hosted by Datamart 26
JHH Departmental Data: HMED (Emergency Department) • Mostly opaque to research • Replicated data hosted by Datamart 26


