4fb80cf7e63102ec107e9b5823170edc.ppt
- Количество слайдов: 36

Using DBS for viral diagnosis in ressources limited setting Senegalese experiences
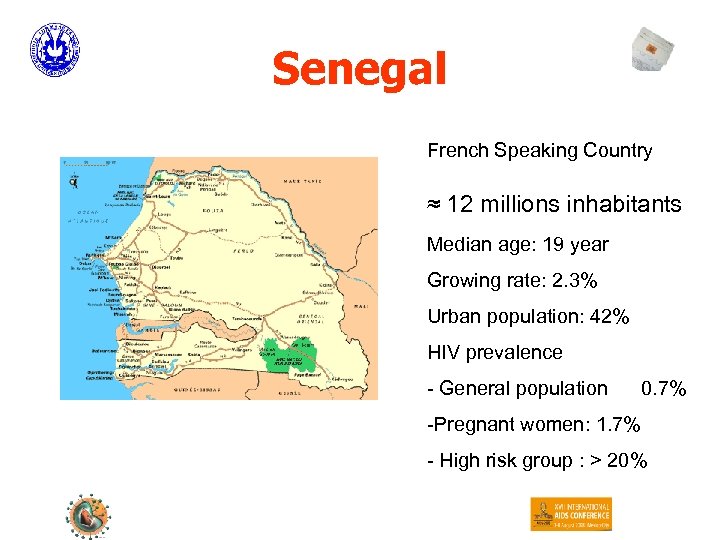
Senegal French Speaking Country ≈ 12 millions inhabitants Median age: 19 year Growing rate: 2. 3% Urban population: 42% HIV prevalence - General population 0. 7% -Pregnant women: 1. 7% - High risk group : > 20%
Viral diagnosis • Early infants diagnosis – Comparison between DNA PCR Roche 1. 5 versus Nuclisens RNA Easy. Q 1. 2 – Determination of viral load in routine on DBS using Nuclisens Easy. Q: an adapted solution for remote areas

Early Infants Diagnostics (EID)

Introduction Diagnosing HIV infection in infants is difficult. maternal HIV antibodies cross the placenta resulting in serologic tests of the baby that are positive but baby may not be infected. Therefore the usual testing methods applied in the baby are misleading and can not determine the real infection status of the baby before 18 months of age, the period needed for a baby to clear all mother’s antibodies in the blood. Early definitive HIV diagnosis of the baby requires virologic testing.

Importance of virological testing – If baby is infected but not tested early and treated it becomes life threatening (around 40% may die before the usual, standard testing is possible). – If the virological testing is performed then HIV-infected infants can get early access to the treatment and care. – Virological testing helps monitor the effectiveness of the PMTCT intervention.
Virological testing • Polymerase Chain Reaction - PCR • which is the diagnostic standard in resource-rich settings • but has been too complex and expensive for widespread use in most countries with high HIV prevalence. • Is the recommended method for Early Infant Diagnosis (EID) by the WHO and American Academy of Pediatrics, and has excellent sensitivity and specificity. – Whole blood in tubes or on DBS
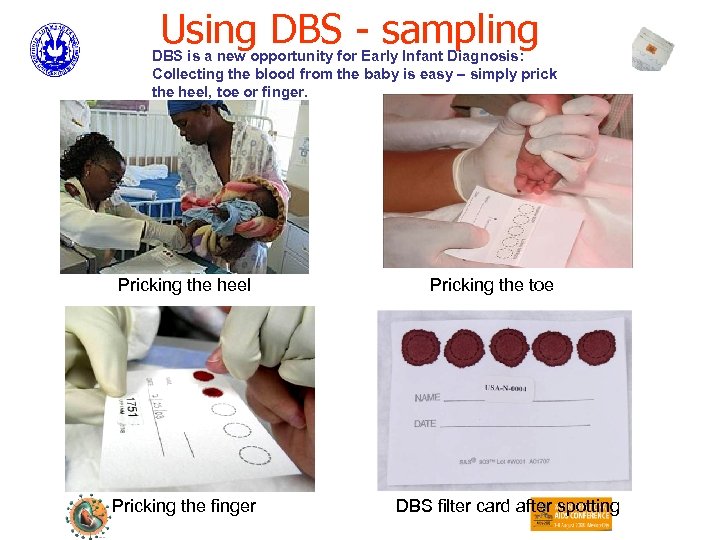
Using DBS - sampling DBS is a new opportunity for Early Infant Diagnosis: Collecting the blood from the baby is easy – simply prick the heel, toe or finger. Pricking the heel Pricking the finger Pricking the toe DBS filter card after spotting
Other alternatives – Realtime Detection • New approach for performing PCR testing. – It is an equipment that simplifies the steps of extraction, amplification and detection that were described above, resulting in shortening the time required to perform these processes. • It has more automated functions. • Currently sites in developing countries have Real-time PCR which is used mainly for monitoring patients measuring the HIV RNA level.
Objective – Evaluate performances for HIV-1 proviral detection using Roche DNA Amplicor 1. 5 versus RNA detection using Nucli. Sens Easy Q® – Compare efficiency and turn around time.
Methodology Study Population : – 123 Samples → peripheral and regional sites included in PMTCT, or infants with clinical symptoms mainly malnutrition. • Types of samples : – 43 EDTA Tubes : 5 m. L transported within 6 h in the lab – 80 Paper filter ou DBS
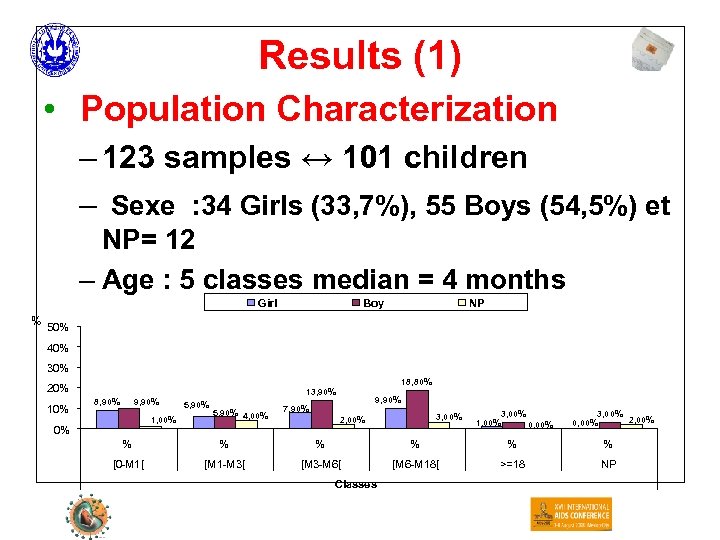
Results (1) • Population Characterization – 123 samples ↔ 101 children – Sexe : 34 Girls (33, 7%), 55 Boys (54, 5%) et NP= 12 – Age : 5 classes median = 4 months Girl Boy NP % 50% 40% 30% 18, 80% 20% 10% 8, 90% 9, 90% 1, 00% 0% 13, 90% 5, 90% 4, 00% 9, 90% 7, 90% 3, 00% 2, 00% 3, 00% 1, 00% 0, 00% 3, 00% 2, 00% 0, 00% % % % [0 -M 1[ [M 1 -M 3[ [M 3 -M 6[ [M 6 -M 18[ >=18 NP Classes
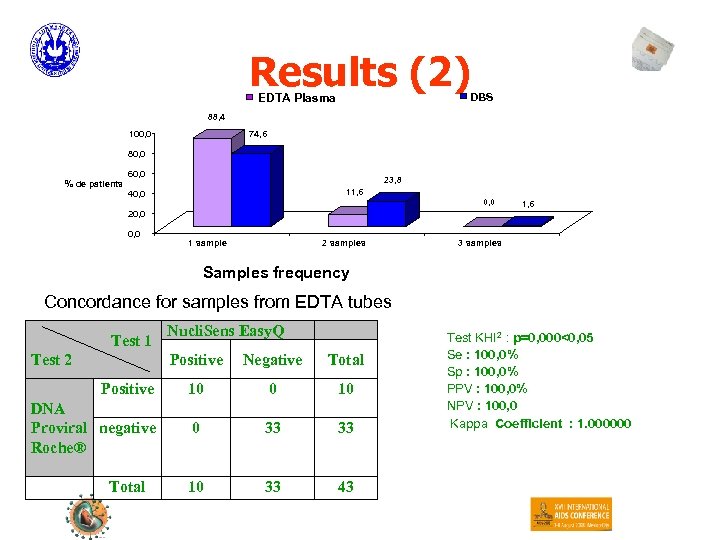
Results (2) DBS EDTA Plasma 88, 4 100, 0 74, 6 80, 0 % de patients 60, 0 23, 8 11, 6 40, 0 1, 6 20, 0 1 sample 2 samples 3 samples Samples frequency Concordance for samples from EDTA tubes Test 1 Nucli. Sens Easy. Q Positive Negative Total Positive 10 0 10 DNA Proviral negative Roche® 0 33 33 10 33 43 Test 2 Total Test KHI 2 : p=0, 000<0, 05 Se : 100, 0% Sp : 100, 0% PPV : 100, 0% NPV : 100, 0 Kappa Coefficient : 1. 000000
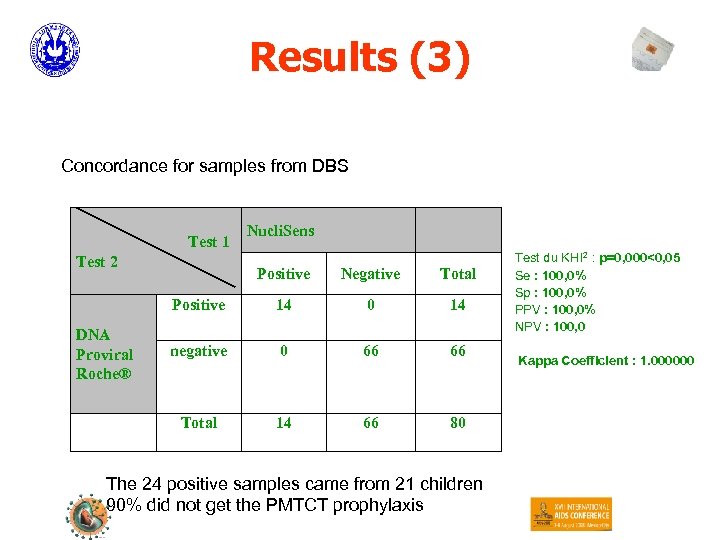
Results (3) Concordance for samples from DBS Test 1 Test 2 Nucli. Sens Negative Total Positive 14 0 14 negative 0 66 66 Total DNA Proviral Roche® Positive 14 66 80 The 24 positive samples came from 21 children 90% did not get the PMTCT prophylaxis Test du KHI 2 : p=0, 000<0, 05 Se : 100, 0% Sp : 100, 0% PPV : 100, 0% NPV : 100, 0 Kappa Coefficient : 1. 000000
Discussion • Using RNA detection as tool of EID – ROUET et al. (2001) Côte d’Ivoire : quantitation of HIV RNA among breastfeeded children • Comparison of RNA/DNA techniques – on EDTA tube • Young et al. (2000) : 100% specificity and comparing PCR DNA/RNA – On DBS and tube • COMEAU et al. (1996): sensitivity 96% comparing an house DNA PCR ADN on tube and DBS samples. • JACOB et al. (2008): sensitivity 95% comparing an house DNA PCR ADN on tube and DBS samples. • Turnaround time – Nucli. Sens: simple, easy to perform 3 -4 h – Amplicor ADN VIH-1 (ROCHE): turnaround longer 6 -8 h
Conclusions • DBS – Useful for early infants diagnosis • DNA PCR • RNA detection – Equivalent performances • Nuclisens Easy. Q vs Amplicor DNA 1. 5

Determination of viral load in routine

Background • Laboratory tests are essential for – monitoring the efficacy of antivirals – the optimal management of antiretroviral treatment. • LTCD 4+ count • Viral load • The measurement of HIV RNA levels in plasma – Several methodologies are available • They require adequate facilities and trained personnel, which are available in a limited number of laboratories in developing countries.
Background (cont’d) • Antiretroviral drugs are increasingly available in resourcelimited countries, – need to develop more practical and reliable methods that are adapted to field conditions for the collection, transportation and accurate measurement of HIV-1 viral load. • Dried blood spots have the potential to further reduce the expense of virological testing by simplifying sample collection, storage and shipment for VL determination. – Proven to be effective for: • the detection of HIV-1 DNA (Cassol et al, 1991), • quantification of HIV-1 RNA (Alvarez-Muñoz et al. , 2005; Brambilla et al. , 2003; Cassol et al. , 1997; Fiscus et al. , 1998; Mwaba et al. , 2003 a; Uttayamulkul et al. , 2005) ), • monitoring of the emergence of drug resistance mutations (Plantier and al 2005)

Objective • The present work aimed at : – evaluating the value of dried blood spots for transport and storage of blood specimens collected under field conditions and their use for real-time determination of HIV-1 viral load with Nucli. SENS Easy. Q. – RNA levels obtained with dried blood spots after storage at 37°C for 8 and 15 days were compared with those obtained with plasma from a set of 41 clinical samples collected in suburban dispensaries. – Applying in 7 settings throughout the country
Deliverables • Determine concordance between – DBS, – blood – plasma viral load results • • • Investigate stability of DBS Investigate reproducibility Applications on the remote areas
Methodology • Optimisation, Reproducibility, Field Application – Clinical, HIV-1 positive patients – HIV-1 control material (Pelyspy = virions!) – DBS • 5 spots (50 µl blood) are applied to individual circles of Guthrie cards (S&S 903 Specimen Collection Paper (Schleicher & Schuell)) by using a micropipettor and plugged tips. – cards were stored in zip bags with desiccant at 37 °C and used for isolation of nucleic acids on day 8 (t = 8) and day 15 (t = 15).
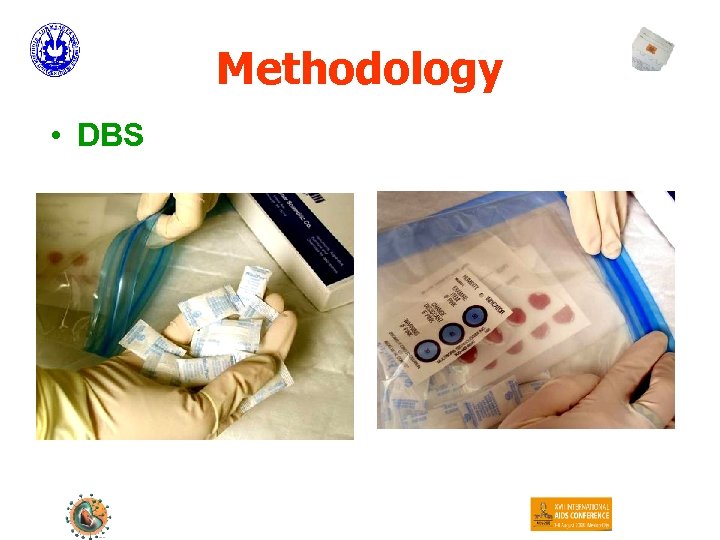
Methodology • DBS
Methodology Study outline • Concordance, Stability and Reproducibility (1, 8 and 15 days) – Between the HIV-1 viral load results of DBSs and plasma. • • 20 HIV-1 -positive patients’ samples 3 controls whole blood, plasma, and DBS Concordance (8 and 15 days) – 41 fields samples Analyses between the methods, the Bland & Altman approach was used, • Field Application
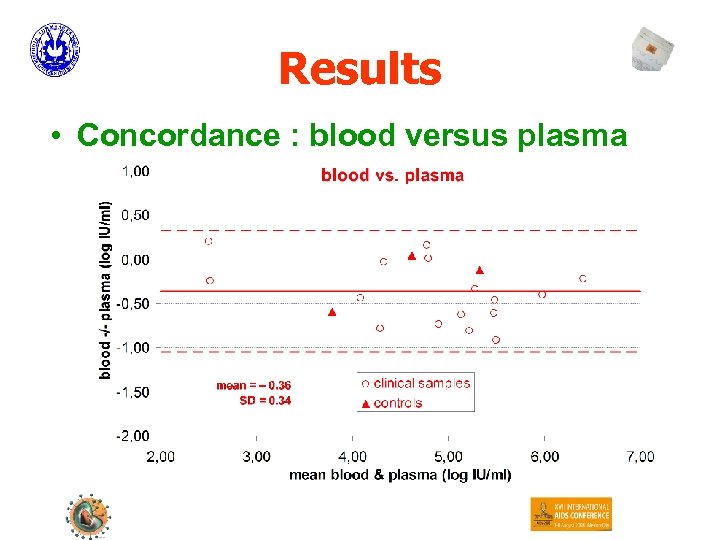
Results • Concordance : blood versus plasma
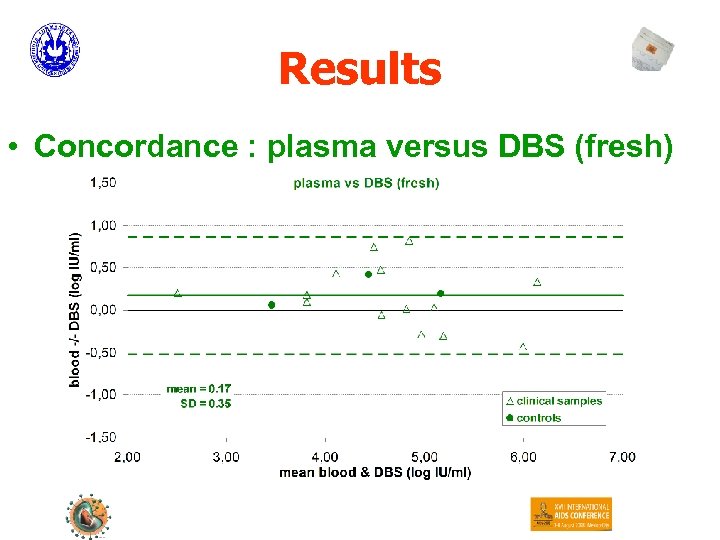
Results • Concordance : plasma versus DBS (fresh)
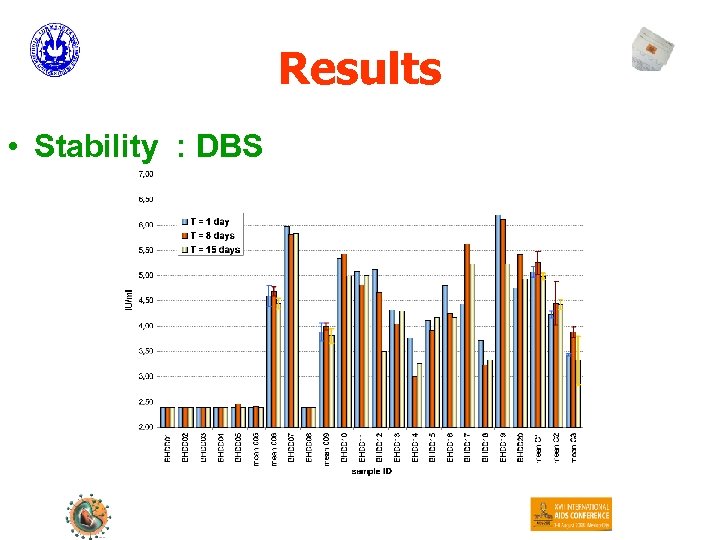
Results • Stability : DBS
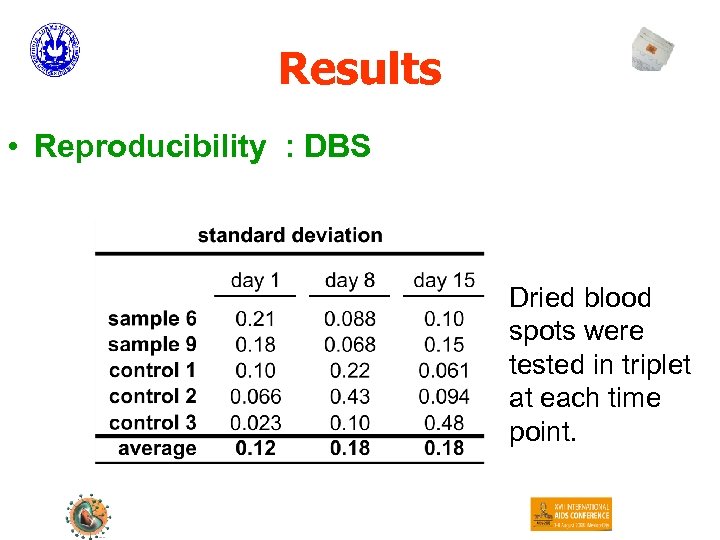
Results • Reproducibility : DBS Dried blood spots were tested in triplet at each time point.
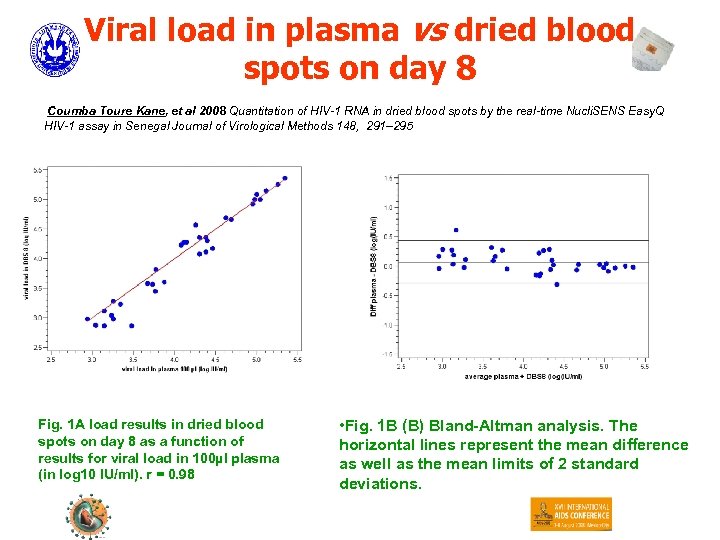
Viral load in plasma vs dried blood spots on day 8 Coumba Toure Kane, et al 2008 Quantitation of HIV-1 RNA in dried blood spots by the real-time Nucli. SENS Easy. Q HIV-1 assay in Senegal Journal of Virological Methods 148, 291– 295 Fig. 1 A load results in dried blood spots on day 8 as a function of results for viral load in 100µl plasma (in log 10 IU/ml). r = 0. 98 • Fig. 1 B (B) Bland-Altman analysis. The horizontal lines represent the mean difference as well as the mean limits of 2 standard deviations.
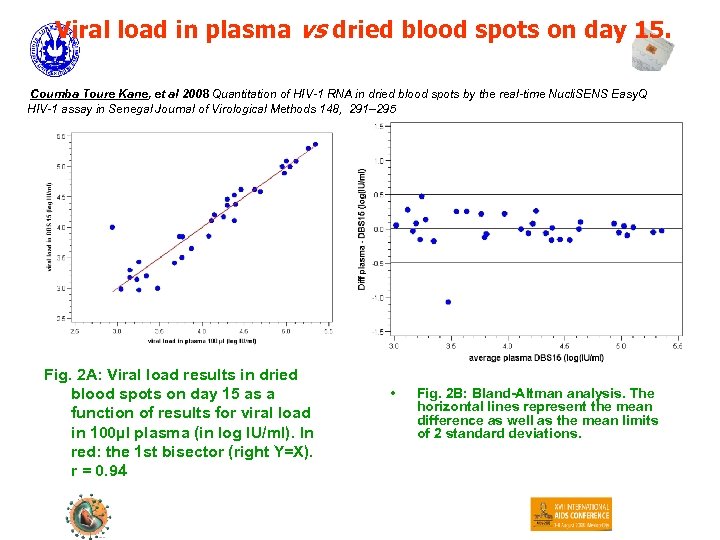
Viral load in plasma vs dried blood spots on day 15. Coumba Toure Kane, et al 2008 Quantitation of HIV-1 RNA in dried blood spots by the real-time Nucli. SENS Easy. Q HIV-1 assay in Senegal Journal of Virological Methods 148, 291– 295 Fig. 2 A: Viral load results in dried blood spots on day 15 as a function of results for viral load in 100µl plasma (in log IU/ml). In red: the 1 st bisector (right Y=X). r = 0. 94 • Fig. 2 B: Bland-Altman analysis. The horizontal lines represent the mean difference as well as the mean limits of 2 standard deviations.
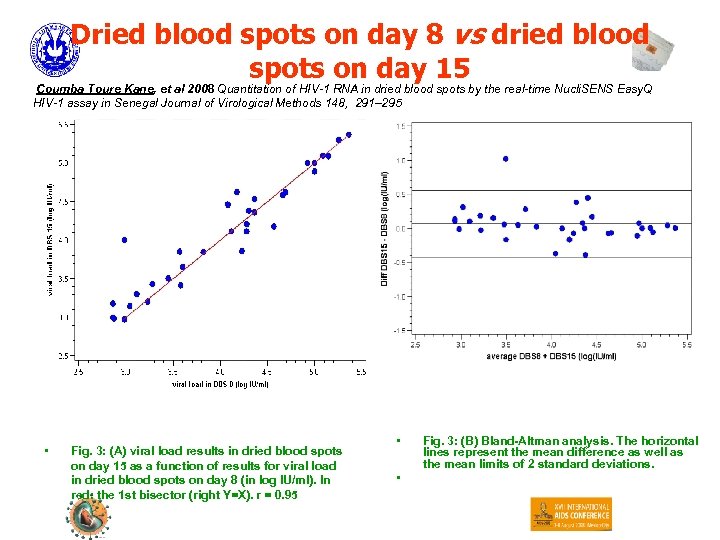
Dried blood spots on day 8 vs dried blood spots on day 15 Coumba Toure Kane, et al 2008 Quantitation of HIV-1 RNA in dried blood spots by the real-time Nucli. SENS Easy. Q HIV-1 assay in Senegal Journal of Virological Methods 148, 291– 295 • Fig. 3: (A) viral load results in dried blood spots on day 15 as a function of results for viral load in dried blood spots on day 8 (in log IU/ml). In red: the 1 st bisector (right Y=X). r = 0. 95 • • Fig. 3: (B) Bland-Altman analysis. The horizontal lines represent the mean difference as well as the mean limits of 2 standard deviations.

Field application • Senegal : Training number of sites – Early Infants Diagnostic n= 8 – Viral load n= 8 – Genotyping n = 8 • Sub Region – Bissau Guinea • Bandim Project coordinated Statens Serum Institute Copenhagen Denmark Applied sites
Field application • Senegal – 219 patients under HAART since 1 year • 74 detectable level > 4 log 10 copies/ml • 145 under detectable level – Genotyping on the extract is ongoing

Conclusions • DBS – Showed high concordance, even after two weeks incubation at 37°C. – HIV‑ 1 quantitation could be used in dried blood testing and hence offers a good alternative for centralized testing in resource-poor countries. – Need to determine the low detectable level – Could be extended on genotyping testing

Acknowledgments • Senegal team – Coumba Toure-Kane 1, Halimatou Diop Ndiaye 1, Sada Diallo 1, Ibrahima Ndiaye 2, Abdoulaye Sidibé Wade 3, Souleymane Mboup 1 – 1 Bacteriology-Virology (HALD) – 2 Infectious disease Clinic (FANN) – 3 Ministry of Health • International partners – bio. Mérieux (Lyon France, Boxtel Netherland) – BMS Foundation (STF)

Thanks for attention
4fb80cf7e63102ec107e9b5823170edc.ppt