b7000d3e8f091dca28ca04d02bceb60b.ppt
- Количество слайдов: 22
 Use of Solvent Iodide Ions as an Effective In-house Tool for Crystallographic Phasing Michael R. Sawaya & Duilio Cascio January 17, 2002
Use of Solvent Iodide Ions as an Effective In-house Tool for Crystallographic Phasing Michael R. Sawaya & Duilio Cascio January 17, 2002
 Phasing by solvent halide ions promises to be a revolutionary new way to phase protein structures Acta Cryst (2000). D 56, 232 -237 ØQuick soak in halide (30 seconds) ØEasy to perform ØApplicable to any protein ØNon-toxic, no heavy atom waste ØHigh quality phasing
Phasing by solvent halide ions promises to be a revolutionary new way to phase protein structures Acta Cryst (2000). D 56, 232 -237 ØQuick soak in halide (30 seconds) ØEasy to perform ØApplicable to any protein ØNon-toxic, no heavy atom waste ØHigh quality phasing
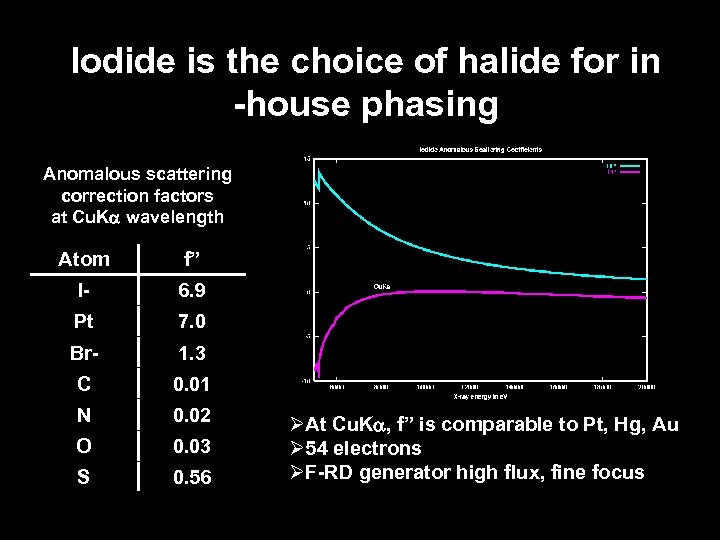 Iodide is the choice of halide for in -house phasing Anomalous scattering correction factors at Cu. Ka wavelength Atom f” I- 6. 9 Pt 7. 0 Br- 1. 3 C 0. 01 N 0. 02 O 0. 03 S 0. 56 ØAt Cu. Ka, f” is comparable to Pt, Hg, Au Ø 54 electrons ØF-RD generator high flux, fine focus
Iodide is the choice of halide for in -house phasing Anomalous scattering correction factors at Cu. Ka wavelength Atom f” I- 6. 9 Pt 7. 0 Br- 1. 3 C 0. 01 N 0. 02 O 0. 03 S 0. 56 ØAt Cu. Ka, f” is comparable to Pt, Hg, Au Ø 54 electrons ØF-RD generator high flux, fine focus
 How successful is phasing by iodide at UCLA? Original study performed with only 4 proteins using a synchrotron source. Will this method prove to be generally applicable in a real academic research lab with real proteins with real problems? What are the limitations? (e. g. resolution limits, lack of isomorphism, data quality, soaking conditions, number of iodide sites required for good phases) Will all proteins bind a sufficient number of iodides to generate good phases? Dauter et al. , suggest that the number of iodides bound is simply proportional to the surface area of the protein. Is this always true?
How successful is phasing by iodide at UCLA? Original study performed with only 4 proteins using a synchrotron source. Will this method prove to be generally applicable in a real academic research lab with real proteins with real problems? What are the limitations? (e. g. resolution limits, lack of isomorphism, data quality, soaking conditions, number of iodide sites required for good phases) Will all proteins bind a sufficient number of iodides to generate good phases? Dauter et al. , suggest that the number of iodides bound is simply proportional to the surface area of the protein. Is this always true?
 Students, Post docs, and Staff from MBI generously donated their crystals to test the effectiveness of iodide soaks in phasing protein structure protein Rv 1926 c Celia Goulding Rv 3697 c Celia Goulding Rv 2878 c Celia Goulding Dsb. D-N-term Celia Goulding P 51 Chongwoo Kim Sm. AP Cameron Mura Nar. Lc complex Ann Maris RNase ds-trimer Thank You for the crystals! contributor Yanshun Liu Protein isoaspartyl methyl transferase Scott Griffith Daniel Boutz Myoglobin Maria Grzeskowiak Proteinase K Helty Adisetiyo Thaumatin Helty Adisetiyo Xylanase Helty Adisetiyo lysozyme Students of M 230 B (2001)
Students, Post docs, and Staff from MBI generously donated their crystals to test the effectiveness of iodide soaks in phasing protein structure protein Rv 1926 c Celia Goulding Rv 3697 c Celia Goulding Rv 2878 c Celia Goulding Dsb. D-N-term Celia Goulding P 51 Chongwoo Kim Sm. AP Cameron Mura Nar. Lc complex Ann Maris RNase ds-trimer Thank You for the crystals! contributor Yanshun Liu Protein isoaspartyl methyl transferase Scott Griffith Daniel Boutz Myoglobin Maria Grzeskowiak Proteinase K Helty Adisetiyo Thaumatin Helty Adisetiyo Xylanase Helty Adisetiyo lysozyme Students of M 230 B (2001)
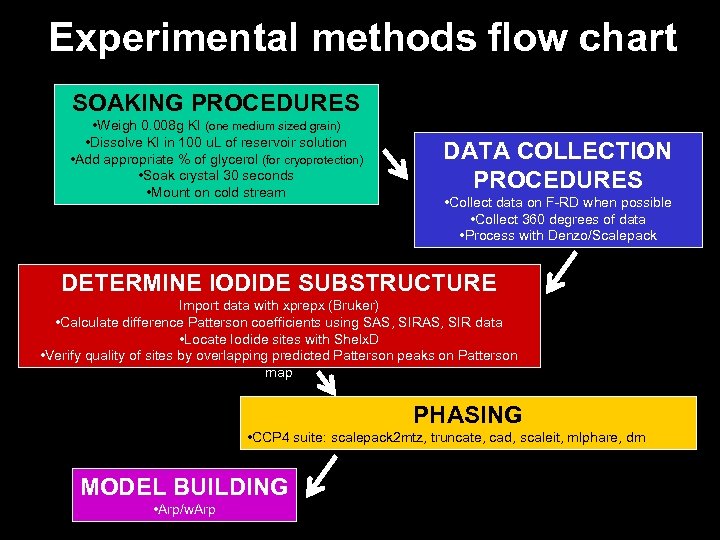 Experimental methods flow chart SOAKING PROCEDURES • Weigh 0. 008 g KI (one medium sized grain) • Dissolve KI in 100 u. L of reservoir solution • Add appropriate % of glycerol (for cryoprotection) • Soak crystal 30 seconds • Mount on cold stream DATA COLLECTION PROCEDURES • Collect data on F-RD when possible • Collect 360 degrees of data • Process with Denzo/Scalepack DETERMINE IODIDE SUBSTRUCTURE Import data with xprepx (Bruker) • Calculate difference Patterson coefficients using SAS, SIR data • Locate Iodide sites with Shelx. D • Verify quality of sites by overlapping predicted Patterson peaks on Patterson map PHASING • CCP 4 suite: scalepack 2 mtz, truncate, cad, scaleit, mlphare, dm MODEL BUILDING • Arp/w. Arp
Experimental methods flow chart SOAKING PROCEDURES • Weigh 0. 008 g KI (one medium sized grain) • Dissolve KI in 100 u. L of reservoir solution • Add appropriate % of glycerol (for cryoprotection) • Soak crystal 30 seconds • Mount on cold stream DATA COLLECTION PROCEDURES • Collect data on F-RD when possible • Collect 360 degrees of data • Process with Denzo/Scalepack DETERMINE IODIDE SUBSTRUCTURE Import data with xprepx (Bruker) • Calculate difference Patterson coefficients using SAS, SIR data • Locate Iodide sites with Shelx. D • Verify quality of sites by overlapping predicted Patterson peaks on Patterson map PHASING • CCP 4 suite: scalepack 2 mtz, truncate, cad, scaleit, mlphare, dm MODEL BUILDING • Arp/w. Arp
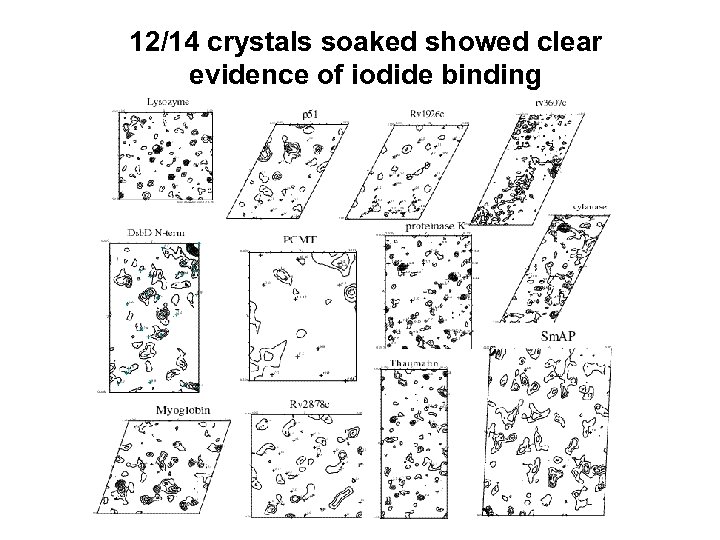 12/14 crystals soaked showed clear evidence of iodide binding
12/14 crystals soaked showed clear evidence of iodide binding
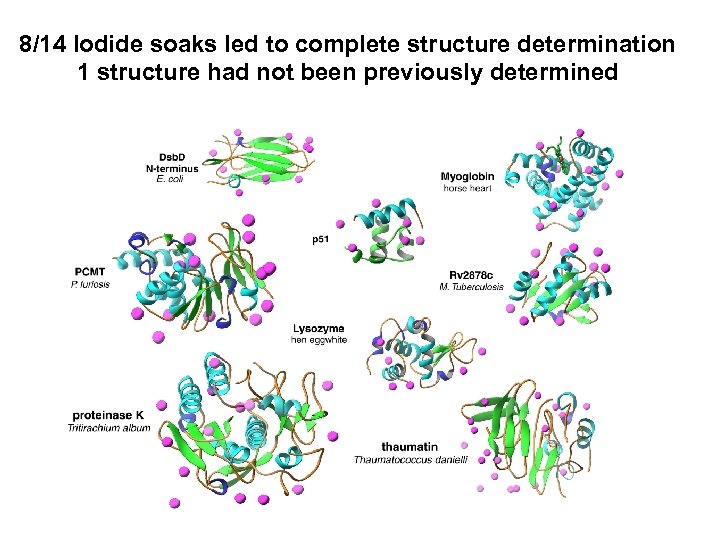 8/14 Iodide soaks led to complete structure determination 1 structure had not been previously determined
8/14 Iodide soaks led to complete structure determination 1 structure had not been previously determined
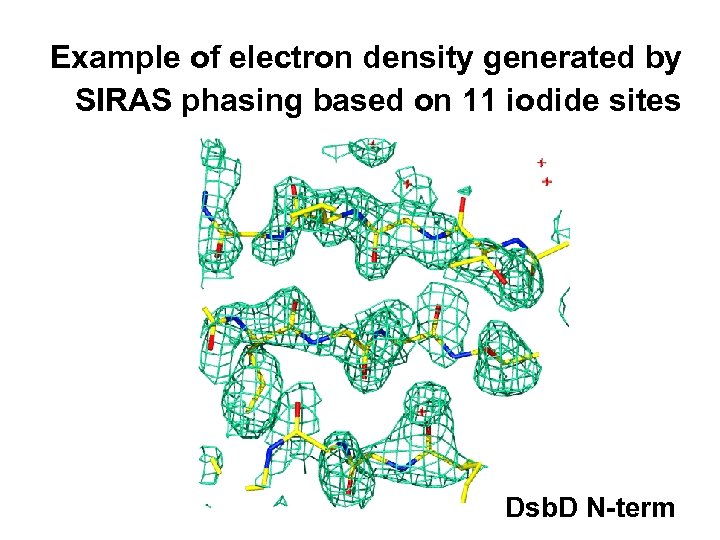 Example of electron density generated by SIRAS phasing based on 11 iodide sites Dsb. D N-term
Example of electron density generated by SIRAS phasing based on 11 iodide sites Dsb. D N-term
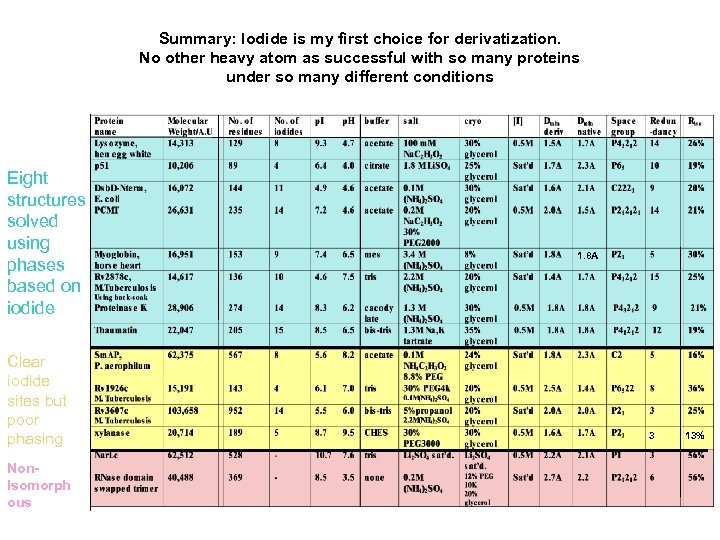 Summary: Iodide is my first choice for derivatization. No other heavy atom as successful with so many proteins under so many different conditions Eight structures solved using phases based on iodide Clear iodide sites but poor phasing Non. Isomorph ous 1. 8 A 3 13%
Summary: Iodide is my first choice for derivatization. No other heavy atom as successful with so many proteins under so many different conditions Eight structures solved using phases based on iodide Clear iodide sites but poor phasing Non. Isomorph ous 1. 8 A 3 13%
 Tips for successful phasing with iodide ØPoor peak heights in difference Patterson map? High redundancy of intensity measurements is crucial to locating heavy atom sites and phasing. Collect 360 degrees of data. Not just iodide, but any derivative would benefit. ØIodide soak is non isomorphous with native? Nonisomorphorism can be reduced by a quick back-soak in cryoconditions lacking iodide. (eg. Rv 2878 c) ØIodide sites not convincing? Shelx. D often succeeds at finding iodide sites based on anomalous differences alone. But, If the solution is not clear, try using isomorphous differences (SIR) or a combination of isomorphous and anomalous differences (SIRAS) output by xprepx.
Tips for successful phasing with iodide ØPoor peak heights in difference Patterson map? High redundancy of intensity measurements is crucial to locating heavy atom sites and phasing. Collect 360 degrees of data. Not just iodide, but any derivative would benefit. ØIodide soak is non isomorphous with native? Nonisomorphorism can be reduced by a quick back-soak in cryoconditions lacking iodide. (eg. Rv 2878 c) ØIodide sites not convincing? Shelx. D often succeeds at finding iodide sites based on anomalous differences alone. But, If the solution is not clear, try using isomorphous differences (SIR) or a combination of isomorphous and anomalous differences (SIRAS) output by xprepx.
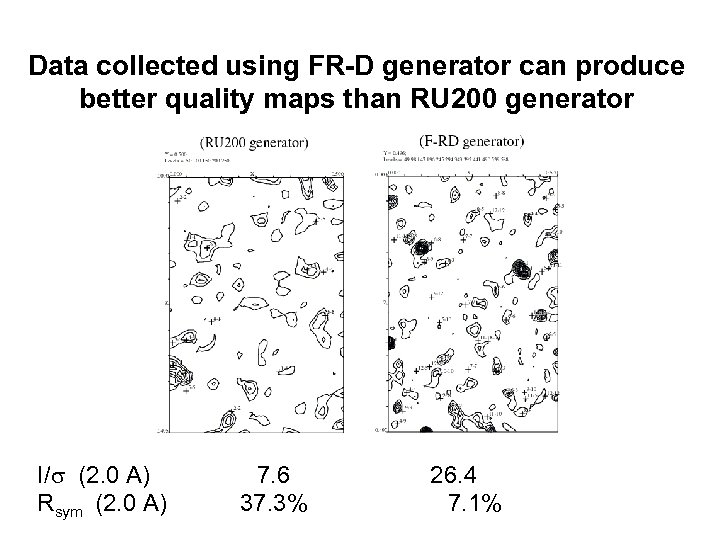 Data collected using FR-D generator can produce better quality maps than RU 200 generator I/s (2. 0 A) Rsym (2. 0 A) 7. 6 37. 3% 26. 4 7. 1%
Data collected using FR-D generator can produce better quality maps than RU 200 generator I/s (2. 0 A) Rsym (2. 0 A) 7. 6 37. 3% 26. 4 7. 1%
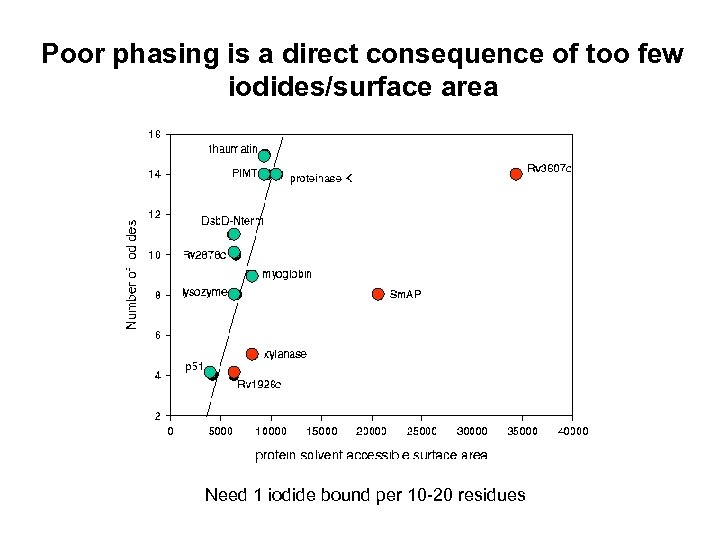 Poor phasing is a direct consequence of too few iodides/surface area Need 1 iodide bound per 10 -20 residues
Poor phasing is a direct consequence of too few iodides/surface area Need 1 iodide bound per 10 -20 residues
 Why do some proteins bind disproportionately fewer iodides/surface area? Two possibilities 1) Soaking conditions (e. g. p. H, salt, buffer) disfavor or compete with iodide binding. If true then we could search for conditions that favor iodide binding. 2) or 2) Residue composition of the protein surface disfavors iodide binding. Make predictions about iodide binding based on amino acid composition.
Why do some proteins bind disproportionately fewer iodides/surface area? Two possibilities 1) Soaking conditions (e. g. p. H, salt, buffer) disfavor or compete with iodide binding. If true then we could search for conditions that favor iodide binding. 2) or 2) Residue composition of the protein surface disfavors iodide binding. Make predictions about iodide binding based on amino acid composition.
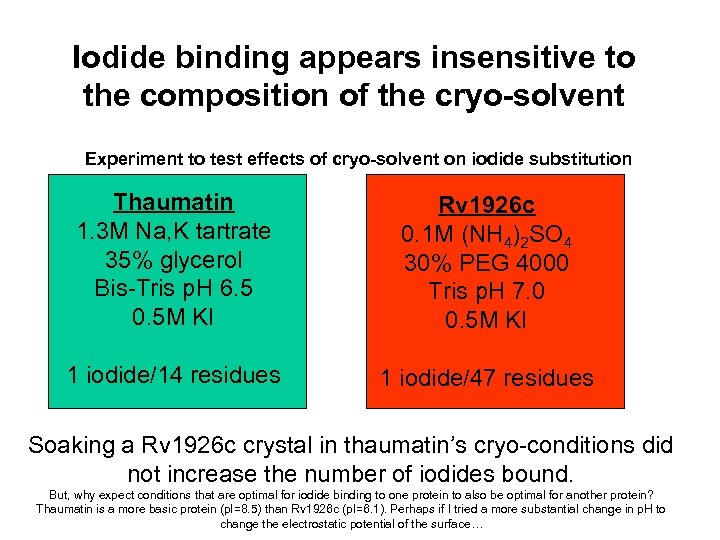 Iodide binding appears insensitive to the composition of the cryo-solvent Experiment to test effects of cryo-solvent on iodide substitution Thaumatin 1. 3 M Na, K tartrate 35% glycerol Bis-Tris p. H 6. 5 0. 5 M KI Rv 1926 c 0. 1 M (NH 4)2 SO 4 30% PEG 4000 Tris p. H 7. 0 0. 5 M KI 1 iodide/14 residues 1 iodide/47 residues Soaking a Rv 1926 c crystal in thaumatin’s cryo-conditions did not increase the number of iodides bound. But, why expect conditions that are optimal for iodide binding to one protein to also be optimal for another protein? Thaumatin is a more basic protein (p. I=8. 5) than Rv 1926 c (p. I=6. 1). Perhaps if I tried a more substantial change in p. H to change the electrostatic potential of the surface…
Iodide binding appears insensitive to the composition of the cryo-solvent Experiment to test effects of cryo-solvent on iodide substitution Thaumatin 1. 3 M Na, K tartrate 35% glycerol Bis-Tris p. H 6. 5 0. 5 M KI Rv 1926 c 0. 1 M (NH 4)2 SO 4 30% PEG 4000 Tris p. H 7. 0 0. 5 M KI 1 iodide/14 residues 1 iodide/47 residues Soaking a Rv 1926 c crystal in thaumatin’s cryo-conditions did not increase the number of iodides bound. But, why expect conditions that are optimal for iodide binding to one protein to also be optimal for another protein? Thaumatin is a more basic protein (p. I=8. 5) than Rv 1926 c (p. I=6. 1). Perhaps if I tried a more substantial change in p. H to change the electrostatic potential of the surface…
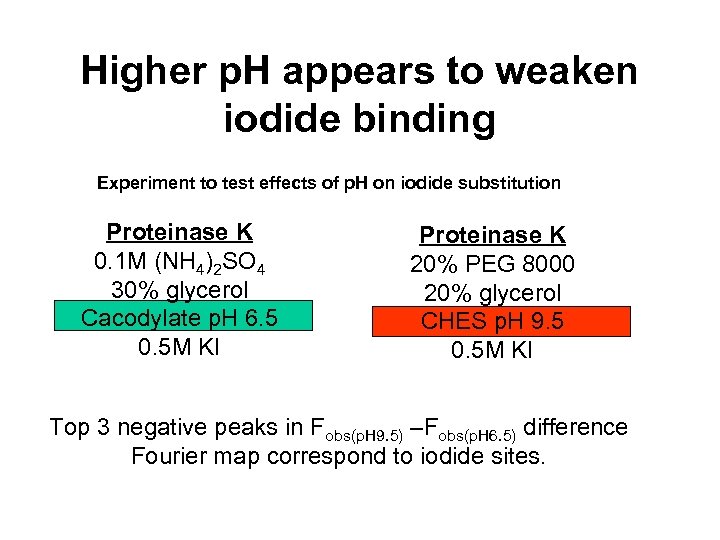 Higher p. H appears to weaken iodide binding Experiment to test effects of p. H on iodide substitution Proteinase K 0. 1 M (NH 4)2 SO 4 30% glycerol Cacodylate p. H 6. 5 0. 5 M KI Proteinase K 20% PEG 8000 20% glycerol CHES p. H 9. 5 0. 5 M KI Top 3 negative peaks in Fobs(p. H 9. 5) –Fobs(p. H 6. 5) difference Fourier map correspond to iodide sites.
Higher p. H appears to weaken iodide binding Experiment to test effects of p. H on iodide substitution Proteinase K 0. 1 M (NH 4)2 SO 4 30% glycerol Cacodylate p. H 6. 5 0. 5 M KI Proteinase K 20% PEG 8000 20% glycerol CHES p. H 9. 5 0. 5 M KI Top 3 negative peaks in Fobs(p. H 9. 5) –Fobs(p. H 6. 5) difference Fourier map correspond to iodide sites.
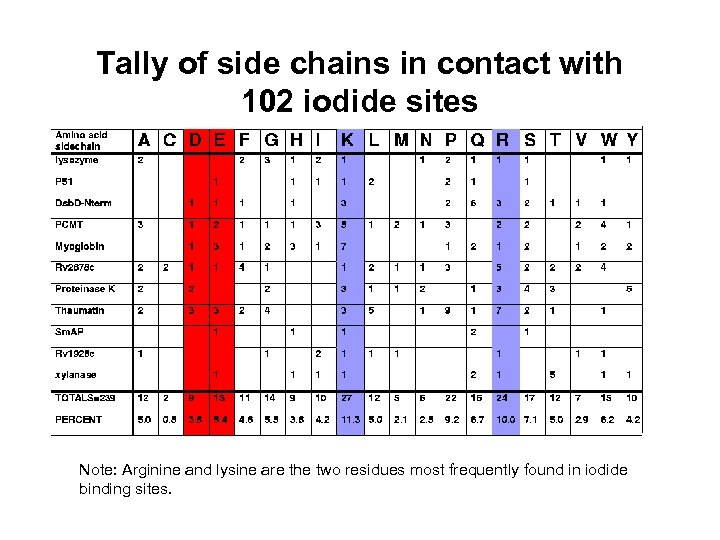 Tally of side chains in contact with 102 iodide sites Note: Arginine and lysine are the two residues most frequently found in iodide binding sites.
Tally of side chains in contact with 102 iodide sites Note: Arginine and lysine are the two residues most frequently found in iodide binding sites.
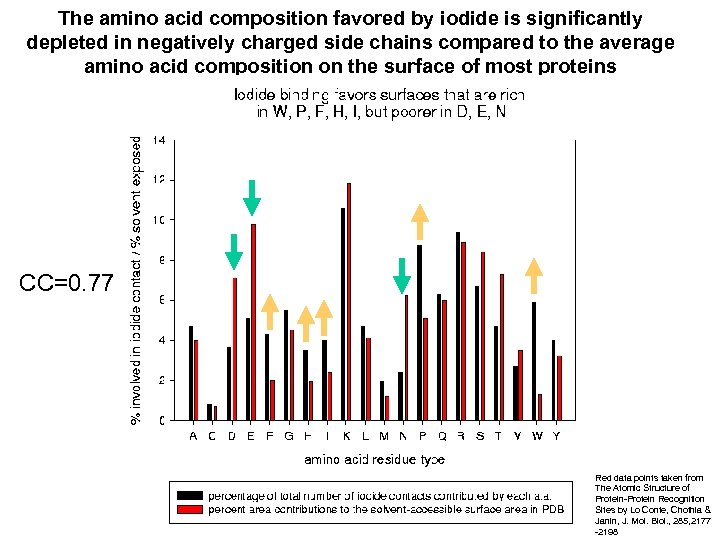 The amino acid composition favored by iodide is significantly depleted in negatively charged side chains compared to the average amino acid composition on the surface of most proteins CC=0. 77 Red data points taken from The Atomic Structure of Protein-Protein Recognition Sites by Lo Conte, Chothia & Janin, J. Mol. Biol. , 285, 2177 -2198
The amino acid composition favored by iodide is significantly depleted in negatively charged side chains compared to the average amino acid composition on the surface of most proteins CC=0. 77 Red data points taken from The Atomic Structure of Protein-Protein Recognition Sites by Lo Conte, Chothia & Janin, J. Mol. Biol. , 285, 2177 -2198
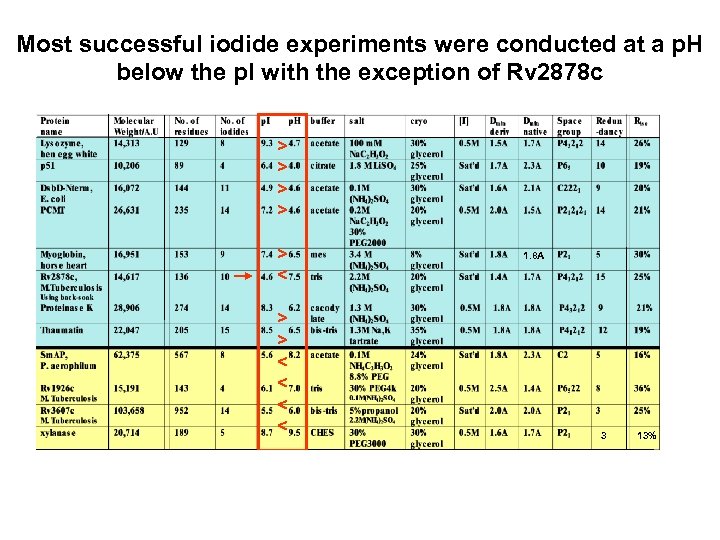 Most successful iodide experiments were conducted at a p. H below the p. I with the exception of Rv 2878 c > > > < < 1. 8 A 3 13%
Most successful iodide experiments were conducted at a p. H below the p. I with the exception of Rv 2878 c > > > < < 1. 8 A 3 13%
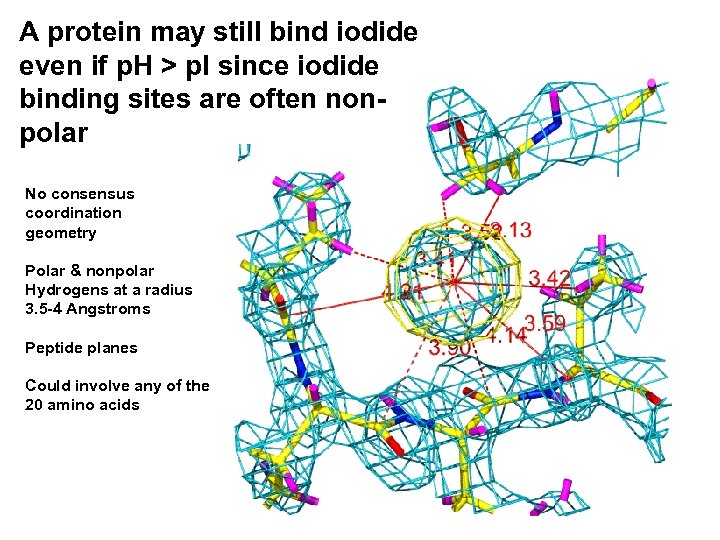 A protein may still bind iodide even if p. H > p. I since iodide binding sites are often nonpolar No consensus coordination geometry Polar & nonpolar Hydrogens at a radius 3. 5 -4 Angstroms Peptide planes Could involve any of the 20 amino acids
A protein may still bind iodide even if p. H > p. I since iodide binding sites are often nonpolar No consensus coordination geometry Polar & nonpolar Hydrogens at a radius 3. 5 -4 Angstroms Peptide planes Could involve any of the 20 amino acids
 Conclusions ØSIRAS phasing from iodide soaks in-house is effective, quick, easy, and non-toxic. 8/14 structures could be determined at UCLA ØEven in cases where there are too few iodide sites to produce a good map, iodide sites could be used in combination with other derivatives (e. g. Cs. Cl). ØHigh redundancy, high resolution, and a bright, focused x-ray source (F-RD) are important factors for success. ØSoaking at p. H < p. I improves chances of success Future: Lower the p. H of cryo-conditions of Rv 1926 c or xylanase to increase iodide binding and solve another structure.
Conclusions ØSIRAS phasing from iodide soaks in-house is effective, quick, easy, and non-toxic. 8/14 structures could be determined at UCLA ØEven in cases where there are too few iodide sites to produce a good map, iodide sites could be used in combination with other derivatives (e. g. Cs. Cl). ØHigh redundancy, high resolution, and a bright, focused x-ray source (F-RD) are important factors for success. ØSoaking at p. H < p. I improves chances of success Future: Lower the p. H of cryo-conditions of Rv 1926 c or xylanase to increase iodide binding and solve another structure.
 Acknowledgements Duilio Cascio- partner in experiments, advice, inspiration CRYSTALS Celia Goulding Chongwoo Kim Cam Mura Ann Maris Yanshun Liu Scott Griffith Daniel Boutz Maria Grzeskowiak Helty Adisetiyo STATISTICS Gary Kleiger Todd Norcross SUPPORT David Eisenberg Todd Yeates Richard Dickerson James Bowie Zbigniew Dauter-advice on back-soaking, shelx. D, xprepx. Peter Muller-xprep connections Kim Ma –X-ray maintenance
Acknowledgements Duilio Cascio- partner in experiments, advice, inspiration CRYSTALS Celia Goulding Chongwoo Kim Cam Mura Ann Maris Yanshun Liu Scott Griffith Daniel Boutz Maria Grzeskowiak Helty Adisetiyo STATISTICS Gary Kleiger Todd Norcross SUPPORT David Eisenberg Todd Yeates Richard Dickerson James Bowie Zbigniew Dauter-advice on back-soaking, shelx. D, xprepx. Peter Muller-xprep connections Kim Ma –X-ray maintenance


