3f56ec3df810fa2d79a1d1a021a7b2f3.ppt
- Количество слайдов: 19

Upper GI Research and Clinical Trials Update Bridget Workman Research Manager NECRN N 21 ST November 2012

The Past History Lesson NCRN Funded by DH fully Local cancer Research networks integrated with service networks (2001) “To benefit patients by improving the coordination , integration , quality , inclusiveness and speed of cancer research” “To double recruitment into trials”

Recruitment NECRN N 2001 -2012

Recruitment NECRN S 2001 -2012
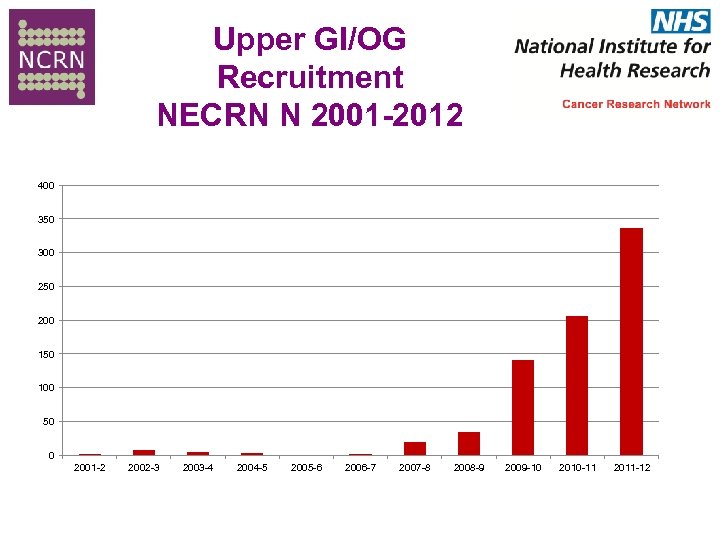
Upper GI/OG Recruitment NECRN N 2001 -2012 400 350 300 250 200 150 100 50 0 2001 -2 2002 -3 2003 -4 2004 -5 2005 -6 2006 -7 2007 -8 2008 -9 2009 -10 2010 -11 2011 -12
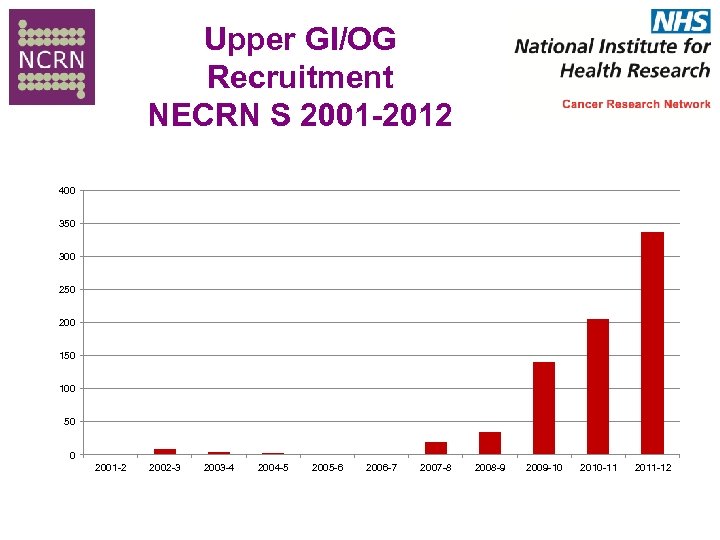
Upper GI/OG Recruitment NECRN S 2001 -2012 400 350 300 250 200 150 100 50 0 2001 -2 2002 -3 2003 -4 2004 -5 2005 -6 2006 -7 2007 -8 2008 -9 2009 -10 2010 -11 2011 -12
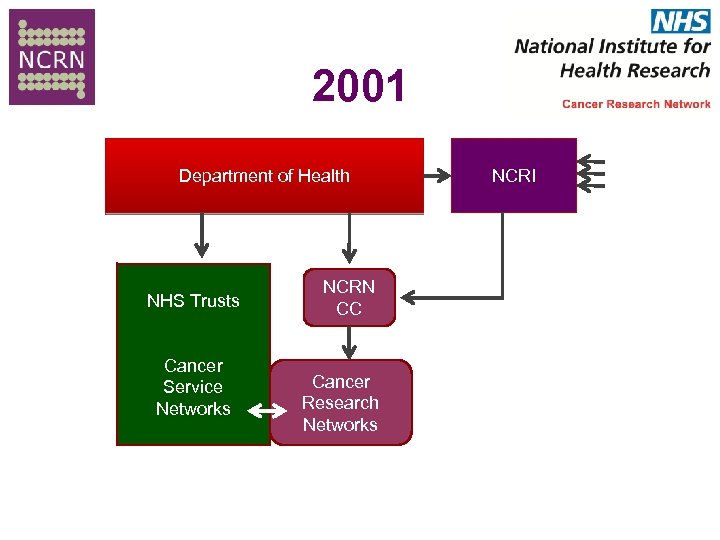
2001 Department of Health NHS Trusts Cancer Service Networks NCRN CC Cancer Research Networks NCRI
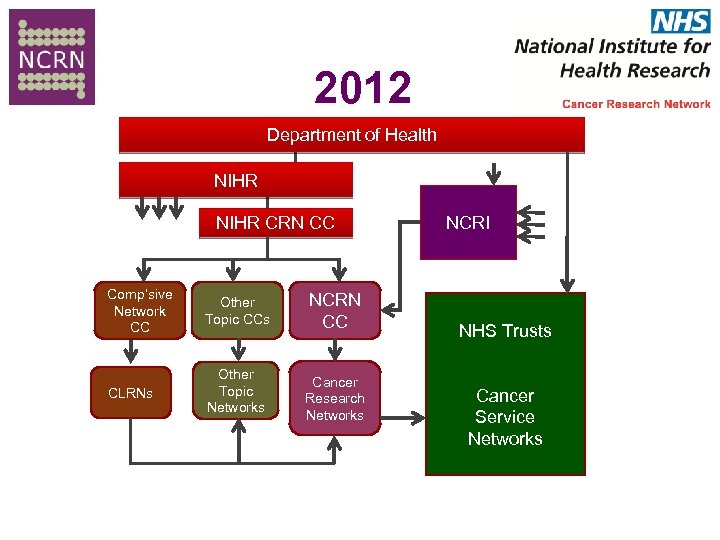
2012 Department of Health NIHR CRN CC Comp’sive Network CC Other Topic CCs CLRNs Other Topic Networks NCRN CC Cancer Research Networks NCRI NHS Trusts Cancer Service Networks

The Present NCRN Strategy – 4 key areas NIHR – High level Objectives

1. Impact • Highlighting the need to better demonstrate the advantages to patients and public of delivering cancer trials alongside NHS cancer services. • Demonstrate and measure the impact of individual studies

2. Industry • Highlights continued need to attract and retain pharmaceutical industry investment. Requires expansion of industry trial portfolio and targets have been set for this. • Minimum of 50 industry studies by 2011 • Minimum of 75 industry studies by 2014 • Requirement to deliver industry studies to time and target.

3. Network Performance • Continued success of Cancer Research Networks will be assured by proactive performance management process • Increased research participation links to NHS Operating Framework, NCRI Strategic Plan and Improving Outcomes a strategy for Cancer (2011) to improve patient outcomes and enhancing delivery.

4. Portfolio Balance & Delivery • Ensure NIHR portfolio reflects contemporary developments in stratified medicine as well as NCRI priorities in surgery, radiotherapy and nondrug trials. • Extend portfolio to studies designed to prevent and diagnose cancer at earlier stage • Studies opening and recruiting to agreed targets and timelines • Continue to strengthen international links EORTC (Europe) NCI ( US)
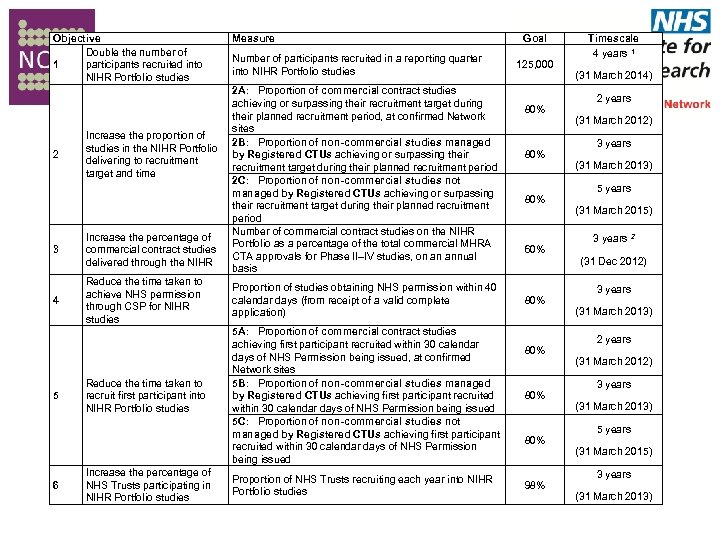
Objective Double the number of 1 participants recruited into NIHR Portfolio studies 2 Increase the proportion of studies in the NIHR Portfolio delivering to recruitment target and time Measure Number of participants recruited in a reporting quarter into NIHR Portfolio studies 2 A: Proportion of commercial contract studies achieving or surpassing their recruitment target during their planned recruitment period, at confirmed Network sites 2 B: Proportion of non-commercial studies managed by Registered CTUs achieving or surpassing their recruitment target during their planned recruitment period 2 C: Proportion of non-commercial studies not managed by Registered CTUs achieving or surpassing their recruitment target during their planned recruitment period Number of commercial contract studies on the NIHR Portfolio as a percentage of the total commercial MHRA CTA approvals for Phase II–IV studies, on an annual basis 3 Increase the percentage of commercial contract studies delivered through the NIHR 4 Reduce the time taken to achieve NHS permission through CSP for NIHR studies Proportion of studies obtaining NHS permission within 40 calendar days (from receipt of a valid complete application) Reduce the time taken to recruit first participant into NIHR Portfolio studies 5 A: Proportion of commercial contract studies achieving first participant recruited within 30 calendar days of NHS Permission being issued, at confirmed Network sites 5 B: Proportion of non-commercial studies managed by Registered CTUs achieving first participant recruited within 30 calendar days of NHS Permission being issued 5 C: Proportion of non-commercial studies not managed by Registered CTUs achieving first participant recruited within 30 calendar days of NHS Permission being issued 5 6 Increase the percentage of NHS Trusts participating in NIHR Portfolio studies Proportion of NHS Trusts recruiting each year into NIHR Portfolio studies Goal 125, 000 80% 80% 60% 80% 80% 98% Timescale 4 years 1 (31 March 2014) 2 years (31 March 2012) 3 years (31 March 2013) 5 years (31 March 2015) 3 years 2 (31 Dec 2012) 3 years (31 March 2013) 2 years (31 March 2012) 3 years (31 March 2013) 5 years (31 March 2015) 3 years (31 March 2013)

2012 -2013
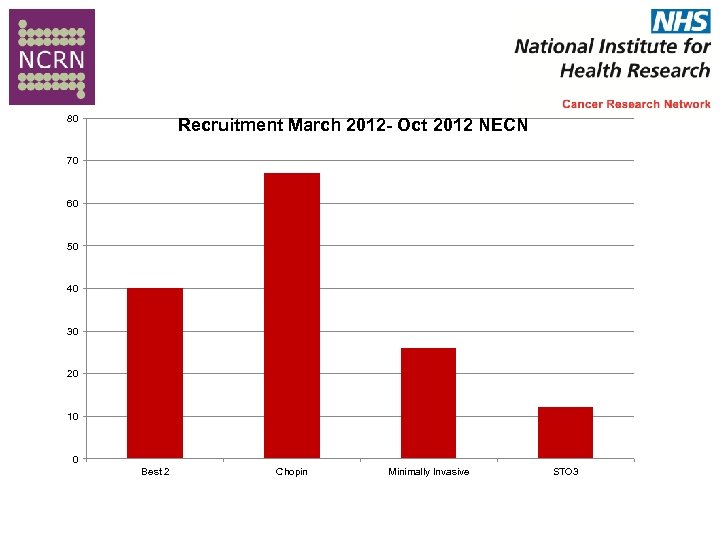
80 Recruitment March 2012 - Oct 2012 NECN 70 60 50 40 30 20 10 0 Best 2 Chopin Minimally Invasive STO 3

The Future Governance Review for Research • New Structure Research 2014 – 14 -18 Geographically based Clinical Research Networks – Greater integration across different Topics Specialties – Single Host Organisation • So now in period of Transition • Other considerations – Clinical Service Network – Academic Science Networks •

What does this mean ? • Business as Usual • Balanced OG portfolio – Opening to time and target – Recruiting to Time and target – Including Industry Trials • Equity of Access - referral pathways – Handbooks and Portfolio Maps for NSSGS’s and MDT’s

Thank You
3f56ec3df810fa2d79a1d1a021a7b2f3.ppt