648b1a919fb7f3612b7d7f19b11023d9.ppt
- Количество слайдов: 55
 Updates on Specific Technical requirements for accreditation of cytopathology laboratories (STR 2. 1) By Dato’ Dr. Halimah Yahaya 25 th November, 2017
Updates on Specific Technical requirements for accreditation of cytopathology laboratories (STR 2. 1) By Dato’ Dr. Halimah Yahaya 25 th November, 2017
 CONTENTS • 1. Introduction on Malaysian accreditation standards • 2. Development of accreditation schemes • 3. Malaysian standards documents of accreditation • 4. Updates in STR 2. 1 • 5. Benefits of accreditation
CONTENTS • 1. Introduction on Malaysian accreditation standards • 2. Development of accreditation schemes • 3. Malaysian standards documents of accreditation • 4. Updates in STR 2. 1 • 5. Benefits of accreditation
 Introduction • SOPs • Year 2000 - The idea of accreditation of pathology laboratories. • Started with ISO 17025 accreditation program • The medical testing services need to provide services based on standards in the form of laboratory accreditation • Year 2004 – NATA accreditation of MOH state hospitals and IMR using ISO 15189
Introduction • SOPs • Year 2000 - The idea of accreditation of pathology laboratories. • Started with ISO 17025 accreditation program • The medical testing services need to provide services based on standards in the form of laboratory accreditation • Year 2004 – NATA accreditation of MOH state hospitals and IMR using ISO 15189
 Development of Accreditation Scheme for medical testing 5/10/2002: Signing of MOU with COP 26/6/2004: Core group for medical testing SC 2 draft document ready 28/6/2004: Minister of Science Technology and Innovation approved the adoption of ISO 15189 30/6/2004 till 1/8/2004: SC 2 and STR documents were finalised. 4/12/2004: Launching of accreditation scheme for medical testing laboratories (MS ISO 15189)
Development of Accreditation Scheme for medical testing 5/10/2002: Signing of MOU with COP 26/6/2004: Core group for medical testing SC 2 draft document ready 28/6/2004: Minister of Science Technology and Innovation approved the adoption of ISO 15189 30/6/2004 till 1/8/2004: SC 2 and STR documents were finalised. 4/12/2004: Launching of accreditation scheme for medical testing laboratories (MS ISO 15189)
 DSM as an Accreditation Body • Complies with ISO/IEC Guide 58 • Recognised by – – Pacific Accreditation Cooperation (PAC), 1998 International Accreditation Forum (IAF), 1999 Asia Pacific Laboratory Accreditation Cooperation (APLAC) International Laboratory Accreditation Cooperation (ILAC) Scope of accreditation – Histopathology, Cytopathology, Virology, Bacteriology, Chemical pathology, Haematology, Cytogenetics, IVF lab,
DSM as an Accreditation Body • Complies with ISO/IEC Guide 58 • Recognised by – – Pacific Accreditation Cooperation (PAC), 1998 International Accreditation Forum (IAF), 1999 Asia Pacific Laboratory Accreditation Cooperation (APLAC) International Laboratory Accreditation Cooperation (ILAC) Scope of accreditation – Histopathology, Cytopathology, Virology, Bacteriology, Chemical pathology, Haematology, Cytogenetics, IVF lab,
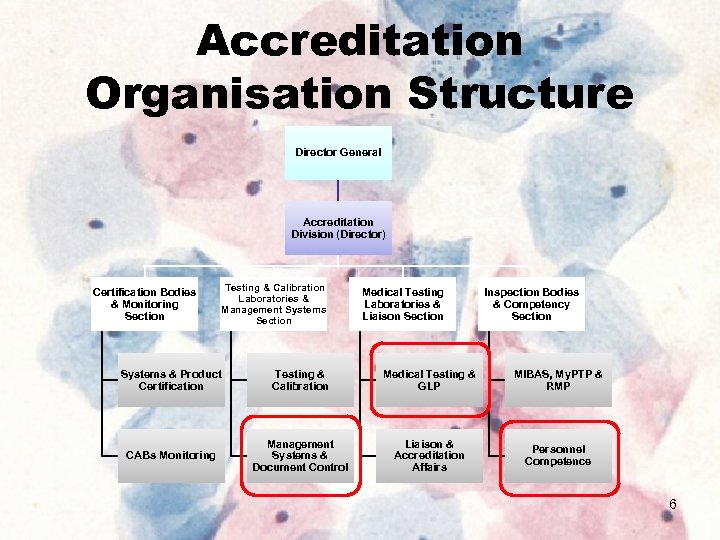 Accreditation Organisation Structure Director General Accreditation Division (Director) Certification Bodies & Monitoring Section Testing & Calibration Laboratories & Management Systems Section Medical Testing Laboratories & Liaison Section Inspection Bodies & Competency Section Systems & Product Certification Testing & Calibration Medical Testing & GLP MIBAS, My. PTP & RMP CABs Monitoring Management Systems & Document Control Liaison & Accreditation Affairs Personnel Competence 6
Accreditation Organisation Structure Director General Accreditation Division (Director) Certification Bodies & Monitoring Section Testing & Calibration Laboratories & Management Systems Section Medical Testing Laboratories & Liaison Section Inspection Bodies & Competency Section Systems & Product Certification Testing & Calibration Medical Testing & GLP MIBAS, My. PTP & RMP CABs Monitoring Management Systems & Document Control Liaison & Accreditation Affairs Personnel Competence 6
 Breakdown of Accredited Laboratories as of 31 October 2017 (Active) Type of Government Laboratories Medical Testing 14 Private Public University Private University Total 24 7 0 45 7
Breakdown of Accredited Laboratories as of 31 October 2017 (Active) Type of Government Laboratories Medical Testing 14 Private Public University Private University Total 24 7 0 45 7
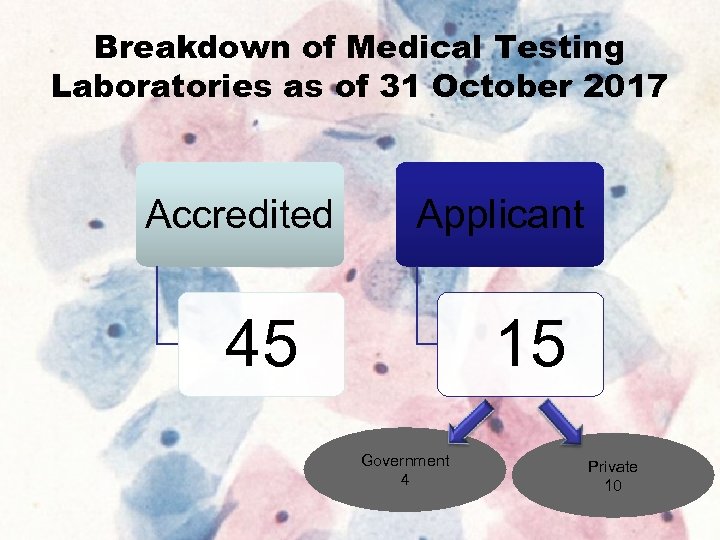 Breakdown of Medical Testing Laboratories as of 31 October 2017 Accredited Applicant 45 15 Government 4 Private 10 8
Breakdown of Medical Testing Laboratories as of 31 October 2017 Accredited Applicant 45 15 Government 4 Private 10 8
 Accreditation process • ACCREDITATION is an evaluation of competence, technical validity and conformity • Questioning of staff, management. • Observation of activities, performances, etc. • Examination of facilities, methodology, sample management records, doc, etc. • Review of proficiency program results & QA program. 15 March 2018 9
Accreditation process • ACCREDITATION is an evaluation of competence, technical validity and conformity • Questioning of staff, management. • Observation of activities, performances, etc. • Examination of facilities, methodology, sample management records, doc, etc. • Review of proficiency program results & QA program. 15 March 2018 9
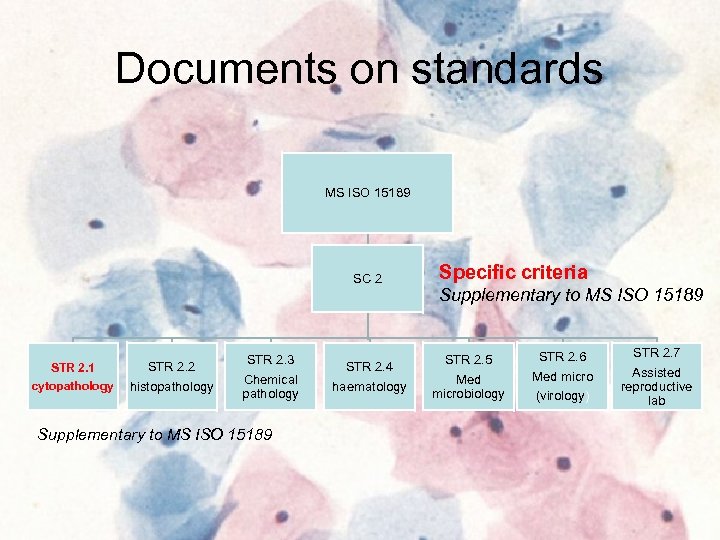 Documents on standards MS ISO 15189 SC 2 STR 2. 1 cytopathology STR 2. 2 histopathology STR 2. 3 Chemical pathology Supplementary to MS ISO 15189 STR 2. 4 haematology Specific criteria Supplementary to MS ISO 15189 STR 2. 5 Med microbiology STR 2. 6 Med micro (virology) STR 2. 7 Assisted reproductive lab
Documents on standards MS ISO 15189 SC 2 STR 2. 1 cytopathology STR 2. 2 histopathology STR 2. 3 Chemical pathology Supplementary to MS ISO 15189 STR 2. 4 haematology Specific criteria Supplementary to MS ISO 15189 STR 2. 5 Med microbiology STR 2. 6 Med micro (virology) STR 2. 7 Assisted reproductive lab
 Accreditation of cytopathology services – STR 2. 1 – Specific Technical Requirements For Accreditation Of Anatomical Pathology (Cytopathology) Laboratories Issue 5, 26 April 2017(Supplementary to MS ISO 15189) shall be read in conjunction with : ü SC 2 – Specific criteria for accreditation in the field of medical testing Issue 4, 31 December 2011(Supplementary to MS ISO 15189) ü Issue 5, 26 th April 2017 ü MS ISO 15189: 2014 Medical laboratories – Requirements for quality and competence (second revision) (ISO 15189 : 2012, IDT)
Accreditation of cytopathology services – STR 2. 1 – Specific Technical Requirements For Accreditation Of Anatomical Pathology (Cytopathology) Laboratories Issue 5, 26 April 2017(Supplementary to MS ISO 15189) shall be read in conjunction with : ü SC 2 – Specific criteria for accreditation in the field of medical testing Issue 4, 31 December 2011(Supplementary to MS ISO 15189) ü Issue 5, 26 th April 2017 ü MS ISO 15189: 2014 Medical laboratories – Requirements for quality and competence (second revision) (ISO 15189 : 2012, IDT)
 MS ISO 15189 SC 2 STR 2. 1
MS ISO 15189 SC 2 STR 2. 1
 REVIEWS MS ISO 15189 • Year 2004 • Year 2008 • Year 2014 • SC 2 Issue 1: 8 Nov 2004 Issue 2: 5 Sept 2006 Issue 3: 5 Jan 2007 Issue 4: 31 Dec 2011 Issue 5: 26 April 2017
REVIEWS MS ISO 15189 • Year 2004 • Year 2008 • Year 2014 • SC 2 Issue 1: 8 Nov 2004 Issue 2: 5 Sept 2006 Issue 3: 5 Jan 2007 Issue 4: 31 Dec 2011 Issue 5: 26 April 2017
 REVIEWS Sc 2 Issue 1: 8 Nov 2004 Issue 2: 5 Sept 2006 Issue 3: 5 Jan 2007 Issue 4: 31 Dec 2011 Issue 5: 26 April 2017 STR 2. 1 Issue 1: 8 Nov 2004 Issue 2: 5 Sept 2006 Issue 3: 5 Jan 2007 Issue 4: 31 Dec 2011 Issue 5: 26 April 2017
REVIEWS Sc 2 Issue 1: 8 Nov 2004 Issue 2: 5 Sept 2006 Issue 3: 5 Jan 2007 Issue 4: 31 Dec 2011 Issue 5: 26 April 2017 STR 2. 1 Issue 1: 8 Nov 2004 Issue 2: 5 Sept 2006 Issue 3: 5 Jan 2007 Issue 4: 31 Dec 2011 Issue 5: 26 April 2017
 STR 2. 1 • Refers to specific requirements to be complied by cytopathology labs • Has to read together with MS ISO 15189: 2014 (Medical laboratories – particular requirements for quality and competence) and SC 2 ( specific criteria for accreditation in the field of medical testing).
STR 2. 1 • Refers to specific requirements to be complied by cytopathology labs • Has to read together with MS ISO 15189: 2014 (Medical laboratories – particular requirements for quality and competence) and SC 2 ( specific criteria for accreditation in the field of medical testing).
 Technical requirements 5. 1 Personnel 5. 2 Accommodation and environmental conditions 5. 3 Laboratory equipment, reagents and consumables 5. 4 Pre-examination processes 5. 5 Exa mination processes 5. 6 Assuring quality of examination results 5. 7 Post-environment processes 5. 8 Reporting of results 5. 9 Release of results 5. 10 Lab information management
Technical requirements 5. 1 Personnel 5. 2 Accommodation and environmental conditions 5. 3 Laboratory equipment, reagents and consumables 5. 4 Pre-examination processes 5. 5 Exa mination processes 5. 6 Assuring quality of examination results 5. 7 Post-environment processes 5. 8 Reporting of results 5. 9 Release of results 5. 10 Lab information management
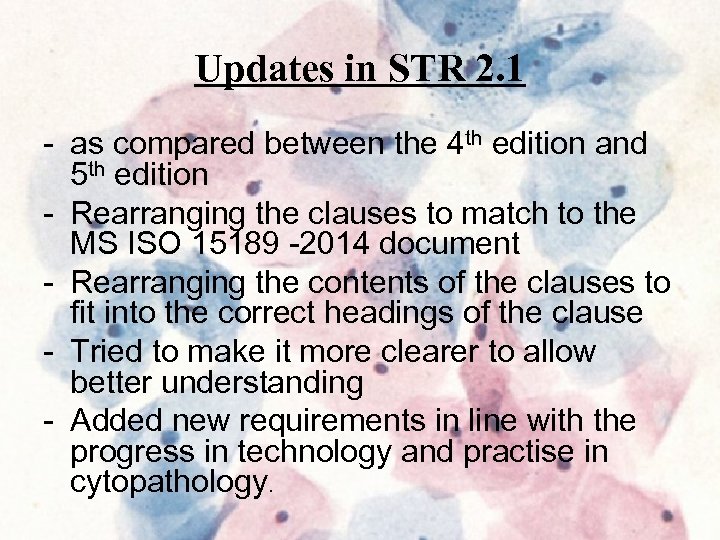 Updates in STR 2. 1 - as compared between the 4 th edition and 5 th edition - Rearranging the clauses to match to the MS ISO 15189 -2014 document - Rearranging the contents of the clauses to fit into the correct headings of the clause - Tried to make it more clearer to allow better understanding - Added new requirements in line with the progress in technology and practise in cytopathology.
Updates in STR 2. 1 - as compared between the 4 th edition and 5 th edition - Rearranging the clauses to match to the MS ISO 15189 -2014 document - Rearranging the contents of the clauses to fit into the correct headings of the clause - Tried to make it more clearer to allow better understanding - Added new requirements in line with the progress in technology and practise in cytopathology.
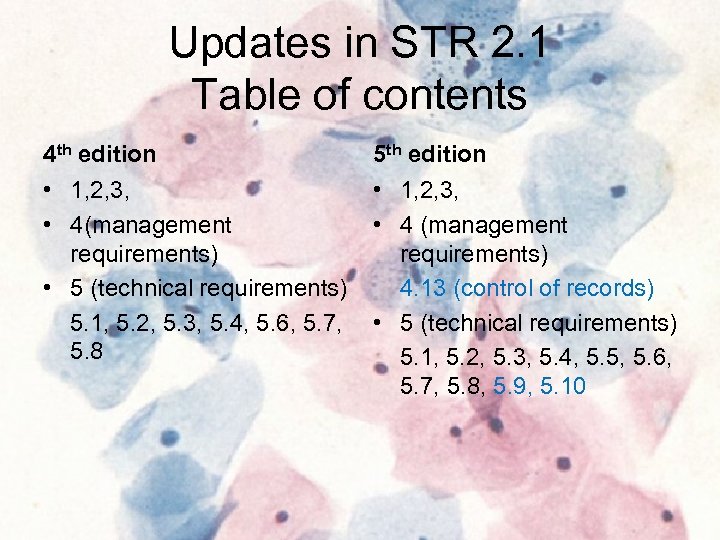 Updates in STR 2. 1 Table of contents 4 th edition 5 th edition • 1, 2, 3, • 4(management requirements) • 5 (technical requirements) 5. 1, 5. 2, 5. 3, 5. 4, 5. 6, 5. 7, 5. 8 • 1, 2, 3, • 4 (management requirements) 4. 13 (control of records) • 5 (technical requirements) 5. 1, 5. 2, 5. 3, 5. 4, 5. 5, 5. 6, 5. 7, 5. 8, 5. 9, 5. 10
Updates in STR 2. 1 Table of contents 4 th edition 5 th edition • 1, 2, 3, • 4(management requirements) • 5 (technical requirements) 5. 1, 5. 2, 5. 3, 5. 4, 5. 6, 5. 7, 5. 8 • 1, 2, 3, • 4 (management requirements) 4. 13 (control of records) • 5 (technical requirements) 5. 1, 5. 2, 5. 3, 5. 4, 5. 5, 5. 6, 5. 7, 5. 8, 5. 9, 5. 10
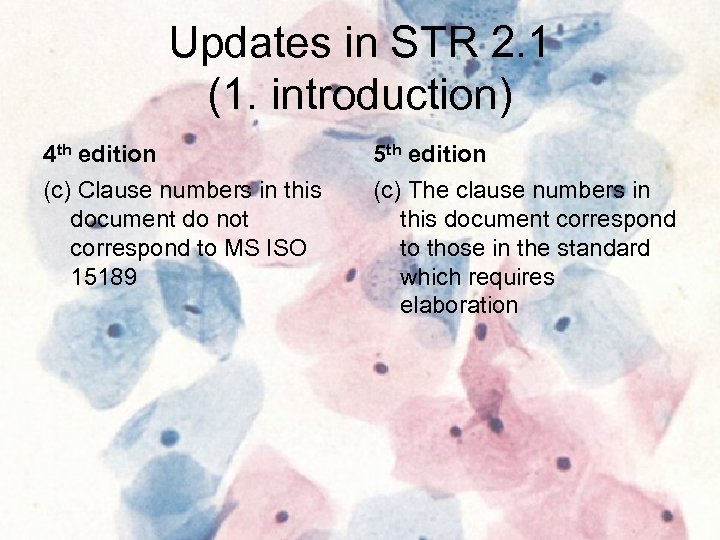 Updates in STR 2. 1 (1. introduction) 4 th edition 5 th edition (c) Clause numbers in this document do not correspond to MS ISO 15189 (c) The clause numbers in this document correspond to those in the standard which requires elaboration
Updates in STR 2. 1 (1. introduction) 4 th edition 5 th edition (c) Clause numbers in this document do not correspond to MS ISO 15189 (c) The clause numbers in this document correspond to those in the standard which requires elaboration
 Updates in STR 2. 1 2. Scope of accreditation 2. 1 Gynaecological cytopathology a. Conventional b. Liquid-based 2. 2 Non-gynaecological cytopathology 2. 3 Fine needle aspiration cytology 2. 4 Specialised tests such as special stains, immunohistochemical stains and molecular testing (HPV DNA) if performed in the cytopathology laboratory shall be included in the scope of accreditation
Updates in STR 2. 1 2. Scope of accreditation 2. 1 Gynaecological cytopathology a. Conventional b. Liquid-based 2. 2 Non-gynaecological cytopathology 2. 3 Fine needle aspiration cytology 2. 4 Specialised tests such as special stains, immunohistochemical stains and molecular testing (HPV DNA) if performed in the cytopathology laboratory shall be included in the scope of accreditation
 STR 2. 1 Gynaecological cytopathology • 3 Terms and definitions 3. 4 Liquid-based cytology (LBC) cytologic samples collected in appropiate liquid medium for processing into smears 3. 6 Abnormal gynaecological smear Any cytological abnormality that falls under the category of epithelial cell abnormality as in The Bethesda classification 3. 7 Negative gynaecological smear Any cytological changes that do not fall under the category of abnormal gynaecological smears
STR 2. 1 Gynaecological cytopathology • 3 Terms and definitions 3. 4 Liquid-based cytology (LBC) cytologic samples collected in appropiate liquid medium for processing into smears 3. 6 Abnormal gynaecological smear Any cytological abnormality that falls under the category of epithelial cell abnormality as in The Bethesda classification 3. 7 Negative gynaecological smear Any cytological changes that do not fall under the category of abnormal gynaecological smears
 • 4. Management requirement As in MS ISO 15189 and Specific Criteria 2 During assessment this element is normally assessed by the lead assessor but technical assessor has to also check on 4. 5 (exam of referral lab), 4. 6 (external services and supplies, 4. 7 (advisory services), 4. 9 (identification & control of non conformities, 4. 12 (continual improvement and 4. 13 (control of records)
• 4. Management requirement As in MS ISO 15189 and Specific Criteria 2 During assessment this element is normally assessed by the lead assessor but technical assessor has to also check on 4. 5 (exam of referral lab), 4. 6 (external services and supplies, 4. 7 (advisory services), 4. 9 (identification & control of non conformities, 4. 12 (continual improvement and 4. 13 (control of records)
 4. 13 Control of records The laboratory shall maintain statistics of the number of samples handled in the laboratory classified under the following headings: a) Gynaecological; b) General (non-gynaecological) cytology; c) Fine needle aspirations. If any relevant records or slides are removed from a file, this shall be traceable.
4. 13 Control of records The laboratory shall maintain statistics of the number of samples handled in the laboratory classified under the following headings: a) Gynaecological; b) General (non-gynaecological) cytology; c) Fine needle aspirations. If any relevant records or slides are removed from a file, this shall be traceable.
 STR 2. 1 Gynaecological cytopathology • 5. 1 Personnel 5. 1. 1 General A cytopathology laboratory shall have at least a cytoscreener and a cytopathologist Key personnel in cytopathology laboratory shall include, cytopathologist, supervisory cytoscientist and/or supervisory cytotechnologist
STR 2. 1 Gynaecological cytopathology • 5. 1 Personnel 5. 1. 1 General A cytopathology laboratory shall have at least a cytoscreener and a cytopathologist Key personnel in cytopathology laboratory shall include, cytopathologist, supervisory cytoscientist and/or supervisory cytotechnologist
 STR 2. 1 Gynaecological cytopathology • 5. 1. 2 Qualifications and workload policy a. Clinical personnel i. A qualified cytopathologist shall be a medical practitioner registered with the Malaysian Medical Council and an Anatomical Pathologist with additional training or working experience in cytopathology for at least 6 months. Registration with National Specialist register and additional certification in cytopathology are desirable. ii. A cytopathology trainee is a medical practitioner working in a cytopathology laboratory under the supervision of a cytopathologist.
STR 2. 1 Gynaecological cytopathology • 5. 1. 2 Qualifications and workload policy a. Clinical personnel i. A qualified cytopathologist shall be a medical practitioner registered with the Malaysian Medical Council and an Anatomical Pathologist with additional training or working experience in cytopathology for at least 6 months. Registration with National Specialist register and additional certification in cytopathology are desirable. ii. A cytopathology trainee is a medical practitioner working in a cytopathology laboratory under the supervision of a cytopathologist.
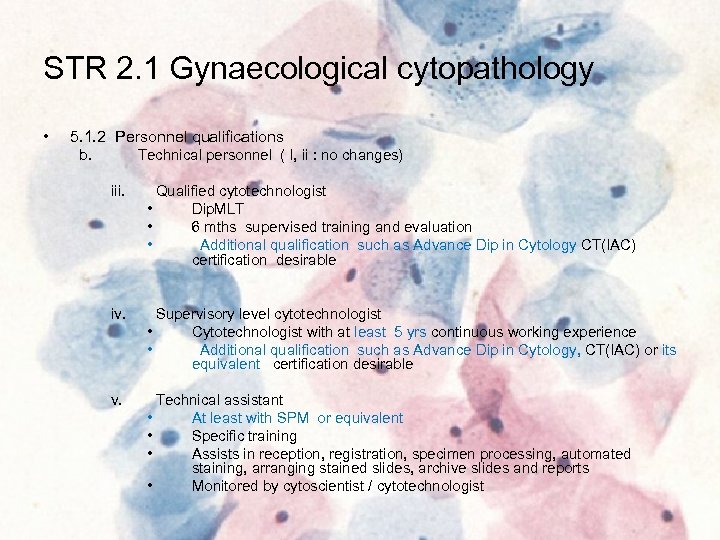 STR 2. 1 Gynaecological cytopathology • 5. 1. 2 Personnel qualifications b. Technical personnel ( I, ii : no changes) iii. Qualified cytotechnologist • Dip. MLT • 6 mths supervised training and evaluation • Additional qualification such as Advance Dip in Cytology CT(IAC) certification desirable iv. Supervisory level cytotechnologist • Cytotechnologist with at least 5 yrs continuous working experience • Additional qualification such as Advance Dip in Cytology, CT(IAC) or its equivalent certification desirable v. Technical assistant • At least with SPM or equivalent • Specific training • Assists in reception, registration, specimen processing, automated staining, arranging stained slides, archive slides and reports • Monitored by cytoscientist / cytotechnologist
STR 2. 1 Gynaecological cytopathology • 5. 1. 2 Personnel qualifications b. Technical personnel ( I, ii : no changes) iii. Qualified cytotechnologist • Dip. MLT • 6 mths supervised training and evaluation • Additional qualification such as Advance Dip in Cytology CT(IAC) certification desirable iv. Supervisory level cytotechnologist • Cytotechnologist with at least 5 yrs continuous working experience • Additional qualification such as Advance Dip in Cytology, CT(IAC) or its equivalent certification desirable v. Technical assistant • At least with SPM or equivalent • Specific training • Assists in reception, registration, specimen processing, automated staining, arranging stained slides, archive slides and reports • Monitored by cytoscientist / cytotechnologist
 5. 1. 3 Job descriptions • Given the duties of a cytopathologist (resident and visiting) includes direct supervision of personnel, processes and quality control, it is strongly advised that the cytopathologist is on site to perform the above duties. The frequency and duration of visits are defined by the volume and scope of work undertaken by the cytopathologist.
5. 1. 3 Job descriptions • Given the duties of a cytopathologist (resident and visiting) includes direct supervision of personnel, processes and quality control, it is strongly advised that the cytopathologist is on site to perform the above duties. The frequency and duration of visits are defined by the volume and scope of work undertaken by the cytopathologist.
 5. 1. 6 Competence assessment Personnel Competency a) A cytoscreener shall perform screening of at least 3000 gynaecological smears per year to maintain competency. b) A cytopathologist/ cytoscientist/ cytotechnologist shall screen a minimum of 20 abnormal gynaecological smears per month to maintain competency. If the number of abnormal cases reported is insufficient, the laboratory shall take part in documented supplementary activities designed to maintain expertise. c) A cytopathologist shall report no less than 750 cases (gynaecological and/or nongynaecological and/or FNAC) per year to maintain competency. Under SC 2: Competency assessment shall be done at least once in two years
5. 1. 6 Competence assessment Personnel Competency a) A cytoscreener shall perform screening of at least 3000 gynaecological smears per year to maintain competency. b) A cytopathologist/ cytoscientist/ cytotechnologist shall screen a minimum of 20 abnormal gynaecological smears per month to maintain competency. If the number of abnormal cases reported is insufficient, the laboratory shall take part in documented supplementary activities designed to maintain expertise. c) A cytopathologist shall report no less than 750 cases (gynaecological and/or nongynaecological and/or FNAC) per year to maintain competency. Under SC 2: Competency assessment shall be done at least once in two years
 Workload policy (5. 1. 6 contd) i) A cytoscreener with no other duties shall screen: a) no more than 70 conventional gynaecological smears per 24 hours. For screening of gynaecological smears, the maximum rate shall not exceed 10 smears per hour. b) no more than 100 liquid-based gynaecological smears per 24 hours. c) no more than 140 slides per 24 hours for smears prepared by liquidbased method and pre-screened using automated devices. ii) A cytoscreener performing full re-screening shall screen no more than 20 conventional gynaecological smears per hour or no more than 25 smears per hour for LBC. iii) If anatomical pathologist performs primary screening as well as reporting, he or she shall be bound by the same workload limits as for cytotechnologist screeners. There shall be a system for rescreening.
Workload policy (5. 1. 6 contd) i) A cytoscreener with no other duties shall screen: a) no more than 70 conventional gynaecological smears per 24 hours. For screening of gynaecological smears, the maximum rate shall not exceed 10 smears per hour. b) no more than 100 liquid-based gynaecological smears per 24 hours. c) no more than 140 slides per 24 hours for smears prepared by liquidbased method and pre-screened using automated devices. ii) A cytoscreener performing full re-screening shall screen no more than 20 conventional gynaecological smears per hour or no more than 25 smears per hour for LBC. iii) If anatomical pathologist performs primary screening as well as reporting, he or she shall be bound by the same workload limits as for cytotechnologist screeners. There shall be a system for rescreening.
 Workload policy (5. 1. 6 contd) • iv) A part-time cytotechnologist shall observe the same workload limits. • Note: The number of slides screened by an individual should be governed by the relative skill and experience of the screener. • All cytology staff using liquid-based method and automated screening techniques shall be trained and qualified to operate the device, and to interpret results obtained from those technologies. • Important requirement that need to be comply by vendor/supplier
Workload policy (5. 1. 6 contd) • iv) A part-time cytotechnologist shall observe the same workload limits. • Note: The number of slides screened by an individual should be governed by the relative skill and experience of the screener. • All cytology staff using liquid-based method and automated screening techniques shall be trained and qualified to operate the device, and to interpret results obtained from those technologies. • Important requirement that need to be comply by vendor/supplier
 5. 2. accomodation and environmental conditions 5. 2. 2 Lab and office facilities: For laboratories providing FNAC services, the following shall be in place: • a) a procedure on how to handle medical emergencies; • b) trained personnel to handle medical emergencies; and • c) simple resuscitation equipment such as AMBU bag and intravenous drip set.
5. 2. accomodation and environmental conditions 5. 2. 2 Lab and office facilities: For laboratories providing FNAC services, the following shall be in place: • a) a procedure on how to handle medical emergencies; • b) trained personnel to handle medical emergencies; and • c) simple resuscitation equipment such as AMBU bag and intravenous drip set.
 STR 2. 1 Gynaecological cytopathology • 5. 2. 6 Facility maintenance and environmental conditions a) Cytoscreening shall be carried out in a separate room, free from noise and distraction. b) An appropriate extraction system shall be in place in the specimen processing area to minimise the level of noxious vapours.
STR 2. 1 Gynaecological cytopathology • 5. 2. 6 Facility maintenance and environmental conditions a) Cytoscreening shall be carried out in a separate room, free from noise and distraction. b) An appropriate extraction system shall be in place in the specimen processing area to minimise the level of noxious vapours.
 STR 2. 1(5. 2. 2 contd) • For safety and security of personnel in health care facilities, reference may be made to the following documents: • i) Occupational Safety and Health (Use and Standard of Exposure Chemical Hazardous to Health) Regulations 2000 (USECHH Regulations); • ii) Occupational Safety and Health (Classification, Labelling and Safety Data Sheet of Hazardous Chemicals) Regulations 2013 (CLASS Regulations) • iii) Guidelines on Chemical Management in Health Care Facilities Ministry of Health 2010.
STR 2. 1(5. 2. 2 contd) • For safety and security of personnel in health care facilities, reference may be made to the following documents: • i) Occupational Safety and Health (Use and Standard of Exposure Chemical Hazardous to Health) Regulations 2000 (USECHH Regulations); • ii) Occupational Safety and Health (Classification, Labelling and Safety Data Sheet of Hazardous Chemicals) Regulations 2013 (CLASS Regulations) • iii) Guidelines on Chemical Management in Health Care Facilities Ministry of Health 2010.
 5. 3 Laboratory equipment, reagents and consumables • 5. 3. 1. 1 General -All cytopathology specimen preparation shall be carried out in a biosafety cabinet that provides protection for the operator and the environment. -Manual preparation of smears which include staining, clearing and mounting shall be carried out in a fume cabinet. -High quality binocular microscopes shall be available to all cytoscreeners. Microscope should include 4 x, 10 x, 20 x and 40 x objectives. -Multi-headed microscope or other similar devices should be available for teaching and training.
5. 3 Laboratory equipment, reagents and consumables • 5. 3. 1. 1 General -All cytopathology specimen preparation shall be carried out in a biosafety cabinet that provides protection for the operator and the environment. -Manual preparation of smears which include staining, clearing and mounting shall be carried out in a fume cabinet. -High quality binocular microscopes shall be available to all cytoscreeners. Microscope should include 4 x, 10 x, 20 x and 40 x objectives. -Multi-headed microscope or other similar devices should be available for teaching and training.
 5. 4 Pre-examination processes 5. 4. 3 Request form information Information needed in the request form for gynecological cytopathology should include the following: (a) Last Menstrual Period (LMP) (b) Previous surgery (GYN) (c) Hormonal/Oral Contraceptive (OCP) 5. 4. 4 Primary sample 5. 4. 4. 1 In laboratories that provide FNAC services, a signed consent from the patient shall be obtained by the person performing the procedure
5. 4 Pre-examination processes 5. 4. 3 Request form information Information needed in the request form for gynecological cytopathology should include the following: (a) Last Menstrual Period (LMP) (b) Previous surgery (GYN) (c) Hormonal/Oral Contraceptive (OCP) 5. 4. 4 Primary sample 5. 4. 4. 1 In laboratories that provide FNAC services, a signed consent from the patient shall be obtained by the person performing the procedure
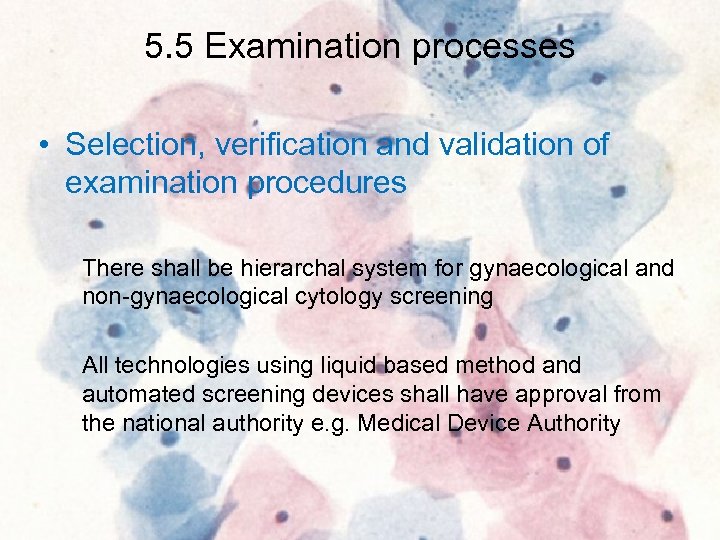 5. 5 Examination processes • Selection, verification and validation of examination procedures There shall be hierarchal system for gynaecological and non-gynaecological cytology screening All technologies using liquid based method and automated screening devices shall have approval from the national authority e. g. Medical Device Authority
5. 5 Examination processes • Selection, verification and validation of examination procedures There shall be hierarchal system for gynaecological and non-gynaecological cytology screening All technologies using liquid based method and automated screening devices shall have approval from the national authority e. g. Medical Device Authority
 5. 6 Assuring quality of examination results 5. 6. 1 General There shall be a mechanism for feedback to the cytoscreener when the final diagnosis in the report is different from the cytoscreener’s interpretation 5. 6. 2 Quality Control The laboratory shall also carry out internal quality control activities which may include the following activities: i) slide staining quality ii) steps to prevent cross contamination Ø Ø Run quality control PAP staining – at least once a day Filter reagents – haematoxylin, OG 6, EA 50 Regular change of reagents – scheduled and documented Separation of staining of Non-gynae, FNA slides from gynae slides
5. 6 Assuring quality of examination results 5. 6. 1 General There shall be a mechanism for feedback to the cytoscreener when the final diagnosis in the report is different from the cytoscreener’s interpretation 5. 6. 2 Quality Control The laboratory shall also carry out internal quality control activities which may include the following activities: i) slide staining quality ii) steps to prevent cross contamination Ø Ø Run quality control PAP staining – at least once a day Filter reagents – haematoxylin, OG 6, EA 50 Regular change of reagents – scheduled and documented Separation of staining of Non-gynae, FNA slides from gynae slides
 5. 6. 3 Interlaboratory comparison The lab shall participate in Interlaboratory comparison programme (ILC) such as EQA national and/international programme which address its diagnostic and technical activities. The lab shall monitor individual and overall performance and implement corrective action where necessary. Record of these activities shall be maintained. • Where a cytopathologist is providing service in more than one laboratoty, he/she is required to participate in the appropriate module(s) of the EQA programme.
5. 6. 3 Interlaboratory comparison The lab shall participate in Interlaboratory comparison programme (ILC) such as EQA national and/international programme which address its diagnostic and technical activities. The lab shall monitor individual and overall performance and implement corrective action where necessary. Record of these activities shall be maintained. • Where a cytopathologist is providing service in more than one laboratoty, he/she is required to participate in the appropriate module(s) of the EQA programme.
 STR 2. 1 (5. 6. 3 contd) a) The laboratory shall establish criteria for review of cases by the cytopathologist. Shall include but not limited to abnormal and unsatisfactory smears Ø Ø Ø Abnormal clinical history IUCD cells Irradiation Endomerial cells in more than 45 years Uncommon organi b) The rates of unsatisfactory smears and those without endocervical or squamous metaplastic cells shall be monitored and feedback given to smear takers at least every six months
STR 2. 1 (5. 6. 3 contd) a) The laboratory shall establish criteria for review of cases by the cytopathologist. Shall include but not limited to abnormal and unsatisfactory smears Ø Ø Ø Abnormal clinical history IUCD cells Irradiation Endomerial cells in more than 45 years Uncommon organi b) The rates of unsatisfactory smears and those without endocervical or squamous metaplastic cells shall be monitored and feedback given to smear takers at least every six months
 STR 2. 1 (5. 6. 3 contd) c) Cyto-histopathology correlation of High Grade Squamous Intraepithelial Lesion (HSIL) and more severe lesion is recommended d) There shall be a system to review the previous cytology smears of current abnormal smears. The recommendation for review is slide/s within preceding 3 yrs
STR 2. 1 (5. 6. 3 contd) c) Cyto-histopathology correlation of High Grade Squamous Intraepithelial Lesion (HSIL) and more severe lesion is recommended d) There shall be a system to review the previous cytology smears of current abnormal smears. The recommendation for review is slide/s within preceding 3 yrs
 5. 6. 3 contd e) Lab shall establish a system of rescreening of negative gynae smears (manual & automated system). A minimum of 10% rescreening of negative smears and all targeted cases shall be carried out. Labs are encouraged to achieve 100% rescreening. Target cases : • positive clinical history (pv bleeding/discharge) f) Lab shall monitor (at least annually) its performance as a whole, these activities shall include the following: i. Rate of unsatisfactory smears ii. Rate of negative smear iii. Rate of abnormal smears (for each category) iv. False positive and false negative rates
5. 6. 3 contd e) Lab shall establish a system of rescreening of negative gynae smears (manual & automated system). A minimum of 10% rescreening of negative smears and all targeted cases shall be carried out. Labs are encouraged to achieve 100% rescreening. Target cases : • positive clinical history (pv bleeding/discharge) f) Lab shall monitor (at least annually) its performance as a whole, these activities shall include the following: i. Rate of unsatisfactory smears ii. Rate of negative smear iii. Rate of abnormal smears (for each category) iv. False positive and false negative rates
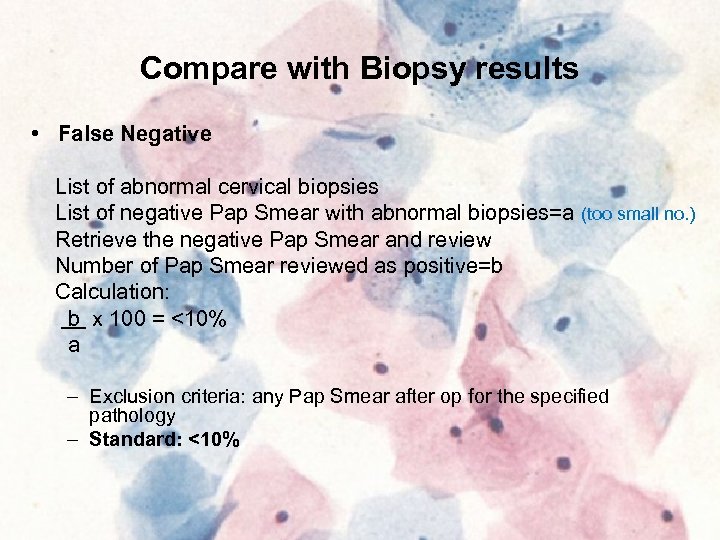 Compare with Biopsy results • False Negative List of abnormal cervical biopsies List of negative Pap Smear with abnormal biopsies=a (too small no. ) Retrieve the negative Pap Smear and review Number of Pap Smear reviewed as positive=b Calculation: b x 100 = <10% a – Exclusion criteria: any Pap Smear after op for the specified pathology – Standard: <10%
Compare with Biopsy results • False Negative List of abnormal cervical biopsies List of negative Pap Smear with abnormal biopsies=a (too small no. ) Retrieve the negative Pap Smear and review Number of Pap Smear reviewed as positive=b Calculation: b x 100 = <10% a – Exclusion criteria: any Pap Smear after op for the specified pathology – Standard: <10%
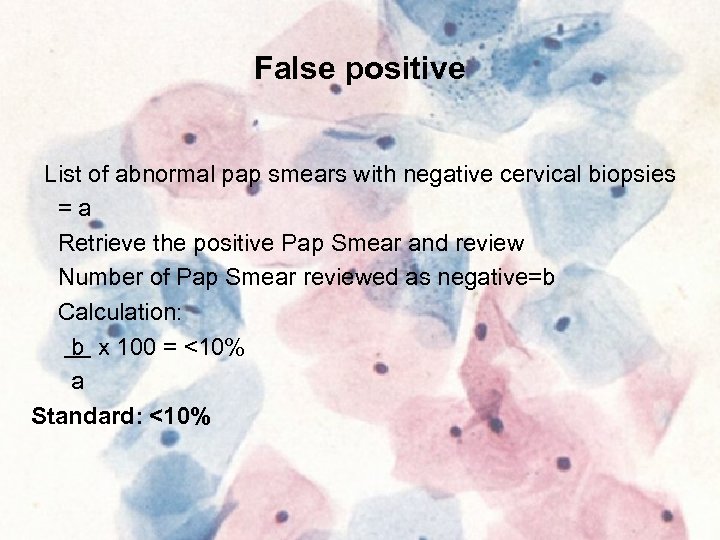 False positive List of abnormal pap smears with negative cervical biopsies =a Retrieve the positive Pap Smear and review Number of Pap Smear reviewed as negative=b Calculation: b x 100 = <10% a Standard: <10%
False positive List of abnormal pap smears with negative cervical biopsies =a Retrieve the positive Pap Smear and review Number of Pap Smear reviewed as negative=b Calculation: b x 100 = <10% a Standard: <10%
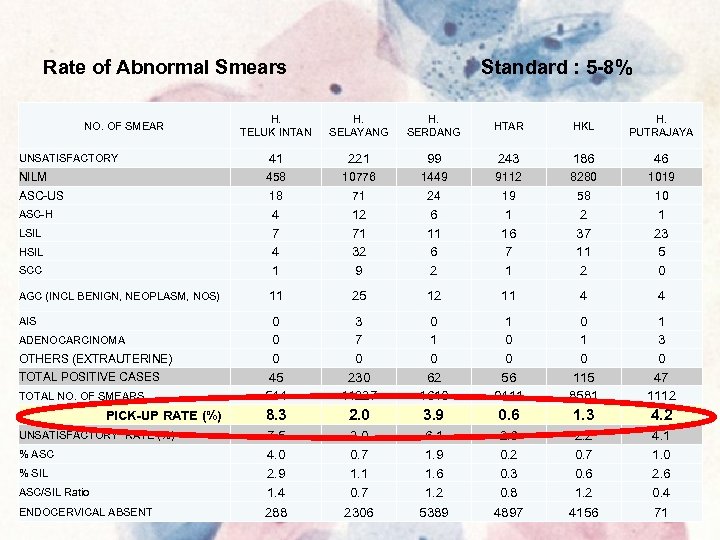 Rate of Abnormal Smears Standard : 5 -8% H. TELUK INTAN H. SELAYANG H. SERDANG HTAR HKL H. PUTRAJAYA UNSATISFACTORY 41 221 99 243 186 46 NILM 458 10776 1449 9112 8280 1019 ASC-US 18 71 24 19 58 10 ASC-H 4 7 4 1 12 71 32 9 6 11 6 2 1 16 7 1 2 37 11 2 1 23 5 0 11 25 12 11 4 4 0 0 0 45 544 3 7 0 230 11227 0 1 0 62 1610 1 0 0 56 9411 0 115 8581 1 3 0 47 1112 8. 3 2. 0 3. 9 0. 6 1. 3 4. 2 ASC/SIL Ratio 7. 5 4. 0 2. 9 1. 4 2. 0 0. 7 1. 1 0. 7 6. 1 1. 9 1. 6 1. 2 2. 6 0. 2 0. 3 0. 8 2. 2 0. 7 0. 6 1. 2 4. 1 1. 0 2. 6 0. 4 ENDOCERVICAL ABSENT 288 2306 5389 4897 4156 71 NO. OF SMEAR LSIL HSIL SCC AGC (INCL BENIGN, NEOPLASM, NOS) AIS ADENOCARCINOMA OTHERS (EXTRAUTERINE) TOTAL POSITIVE CASES TOTAL NO. OF SMEARS PICK-UP RATE (%) UNSATISFACTORY RATE (%) % ASC % SIL
Rate of Abnormal Smears Standard : 5 -8% H. TELUK INTAN H. SELAYANG H. SERDANG HTAR HKL H. PUTRAJAYA UNSATISFACTORY 41 221 99 243 186 46 NILM 458 10776 1449 9112 8280 1019 ASC-US 18 71 24 19 58 10 ASC-H 4 7 4 1 12 71 32 9 6 11 6 2 1 16 7 1 2 37 11 2 1 23 5 0 11 25 12 11 4 4 0 0 0 45 544 3 7 0 230 11227 0 1 0 62 1610 1 0 0 56 9411 0 115 8581 1 3 0 47 1112 8. 3 2. 0 3. 9 0. 6 1. 3 4. 2 ASC/SIL Ratio 7. 5 4. 0 2. 9 1. 4 2. 0 0. 7 1. 1 0. 7 6. 1 1. 9 1. 6 1. 2 2. 6 0. 2 0. 3 0. 8 2. 2 0. 7 0. 6 1. 2 4. 1 1. 0 2. 6 0. 4 ENDOCERVICAL ABSENT 288 2306 5389 4897 4156 71 NO. OF SMEAR LSIL HSIL SCC AGC (INCL BENIGN, NEOPLASM, NOS) AIS ADENOCARCINOMA OTHERS (EXTRAUTERINE) TOTAL POSITIVE CASES TOTAL NO. OF SMEARS PICK-UP RATE (%) UNSATISFACTORY RATE (%) % ASC % SIL
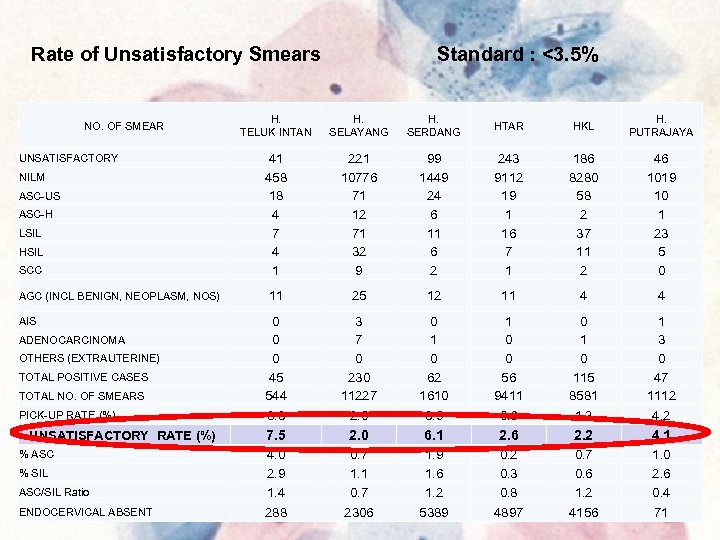 Rate of Unsatisfactory Smears Standard : <3. 5% H. TELUK INTAN H. SELAYANG H. SERDANG HTAR HKL H. PUTRAJAYA 41 458 18 4 7 4 1 221 10776 71 12 71 32 9 99 1449 24 6 11 6 2 243 9112 19 1 16 7 1 186 8280 58 2 37 11 2 46 1019 10 1 23 5 0 11 25 12 11 4 4 0 0 0 45 544 8. 3 3 7 0 230 11227 2. 0 0 1 0 62 1610 3. 9 1 0 0 56 9411 0. 6 0 115 8581 1. 3 1 3 0 47 1112 4. 2 7. 5 2. 0 6. 1 2. 6 2. 2 4. 1 ASC/SIL Ratio 4. 0 2. 9 1. 4 0. 7 1. 1 0. 7 1. 9 1. 6 1. 2 0. 3 0. 8 0. 7 0. 6 1. 2 1. 0 2. 6 0. 4 ENDOCERVICAL ABSENT 288 2306 5389 4897 4156 71 NO. OF SMEAR UNSATISFACTORY NILM ASC-US ASC-H LSIL HSIL SCC AGC (INCL BENIGN, NEOPLASM, NOS) AIS ADENOCARCINOMA OTHERS (EXTRAUTERINE) TOTAL POSITIVE CASES TOTAL NO. OF SMEARS PICK-UP RATE (%) UNSATISFACTORY RATE (%) % ASC % SIL
Rate of Unsatisfactory Smears Standard : <3. 5% H. TELUK INTAN H. SELAYANG H. SERDANG HTAR HKL H. PUTRAJAYA 41 458 18 4 7 4 1 221 10776 71 12 71 32 9 99 1449 24 6 11 6 2 243 9112 19 1 16 7 1 186 8280 58 2 37 11 2 46 1019 10 1 23 5 0 11 25 12 11 4 4 0 0 0 45 544 8. 3 3 7 0 230 11227 2. 0 0 1 0 62 1610 3. 9 1 0 0 56 9411 0. 6 0 115 8581 1. 3 1 3 0 47 1112 4. 2 7. 5 2. 0 6. 1 2. 6 2. 2 4. 1 ASC/SIL Ratio 4. 0 2. 9 1. 4 0. 7 1. 1 0. 7 1. 9 1. 6 1. 2 0. 3 0. 8 0. 7 0. 6 1. 2 1. 0 2. 6 0. 4 ENDOCERVICAL ABSENT 288 2306 5389 4897 4156 71 NO. OF SMEAR UNSATISFACTORY NILM ASC-US ASC-H LSIL HSIL SCC AGC (INCL BENIGN, NEOPLASM, NOS) AIS ADENOCARCINOMA OTHERS (EXTRAUTERINE) TOTAL POSITIVE CASES TOTAL NO. OF SMEARS PICK-UP RATE (%) UNSATISFACTORY RATE (%) % ASC % SIL
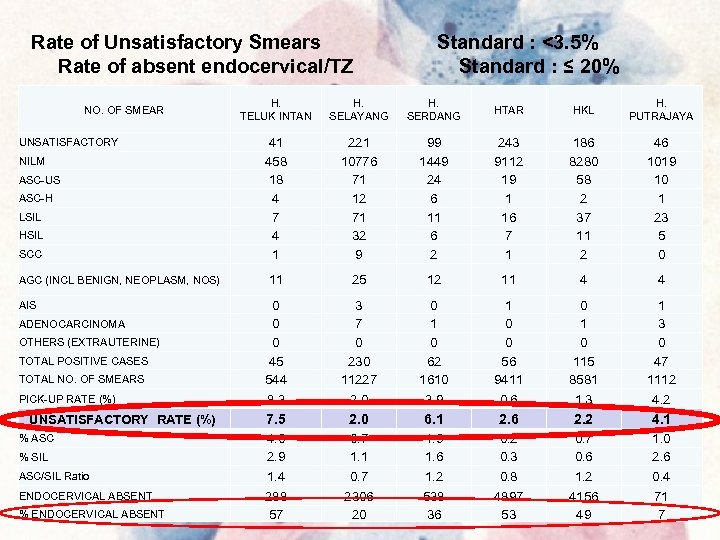 Rate of Unsatisfactory Smears Rate of absent endocervical/TZ NO. OF SMEAR UNSATISFACTORY NILM ASC-US ASC-H LSIL HSIL SCC AGC (INCL BENIGN, NEOPLASM, NOS) AIS ADENOCARCINOMA OTHERS (EXTRAUTERINE) TOTAL POSITIVE CASES TOTAL NO. OF SMEARS PICK-UP RATE (%) UNSATISFACTORY RATE (%) % ASC % SIL ASC/SIL Ratio ENDOCERVICAL ABSENT % ENDOCERVICAL ABSENT Standard : <3. 5% Standard : ≤ 20% H. TELUK INTAN H. SELAYANG H. SERDANG HTAR HKL H. PUTRAJAYA 41 458 18 4 7 4 1 221 10776 71 12 71 32 9 99 1449 24 6 11 6 2 243 9112 19 1 16 7 1 186 8280 58 2 37 11 2 46 1019 10 1 23 5 0 11 25 12 11 4 4 0 0 0 45 544 8. 3 3 7 0 230 11227 2. 0 0 1 0 62 1610 3. 9 1 0 0 56 9411 0. 6 0 115 8581 1. 3 1 3 0 47 1112 4. 2 7. 5 2. 0 6. 1 2. 6 2. 2 4. 1 4. 0 2. 9 1. 4 0. 7 1. 1 0. 7 1. 9 1. 6 1. 2 0. 3 0. 8 0. 7 0. 6 1. 2 1. 0 2. 6 0. 4 288 57 2306 20 538 36 4897 53 4156 49 71 7
Rate of Unsatisfactory Smears Rate of absent endocervical/TZ NO. OF SMEAR UNSATISFACTORY NILM ASC-US ASC-H LSIL HSIL SCC AGC (INCL BENIGN, NEOPLASM, NOS) AIS ADENOCARCINOMA OTHERS (EXTRAUTERINE) TOTAL POSITIVE CASES TOTAL NO. OF SMEARS PICK-UP RATE (%) UNSATISFACTORY RATE (%) % ASC % SIL ASC/SIL Ratio ENDOCERVICAL ABSENT % ENDOCERVICAL ABSENT Standard : <3. 5% Standard : ≤ 20% H. TELUK INTAN H. SELAYANG H. SERDANG HTAR HKL H. PUTRAJAYA 41 458 18 4 7 4 1 221 10776 71 12 71 32 9 99 1449 24 6 11 6 2 243 9112 19 1 16 7 1 186 8280 58 2 37 11 2 46 1019 10 1 23 5 0 11 25 12 11 4 4 0 0 0 45 544 8. 3 3 7 0 230 11227 2. 0 0 1 0 62 1610 3. 9 1 0 0 56 9411 0. 6 0 115 8581 1. 3 1 3 0 47 1112 4. 2 7. 5 2. 0 6. 1 2. 6 2. 2 4. 1 4. 0 2. 9 1. 4 0. 7 1. 1 0. 7 1. 9 1. 6 1. 2 0. 3 0. 8 0. 7 0. 6 1. 2 1. 0 2. 6 0. 4 288 57 2306 20 538 36 4897 53 4156 49 71 7
 5. 6. 3 cont Non-gynaecolological cytopathology a) There shall be a system to review the previous cytology sears of current abnormal smears b) Cyto-histopathologic correlation should be carried out in laboratory that provide both cytology and histopathology services. FNA cytopathology a) Cyto-histopathologic correlation should be carried out in laboratory that provide both cytology and histopathology services.
5. 6. 3 cont Non-gynaecolological cytopathology a) There shall be a system to review the previous cytology sears of current abnormal smears b) Cyto-histopathologic correlation should be carried out in laboratory that provide both cytology and histopathology services. FNA cytopathology a) Cyto-histopathologic correlation should be carried out in laboratory that provide both cytology and histopathology services.
 5. 7 Post-examination processes 5. 7. 1 In the event of discrepancy in diagnosis with prior samples examined by the laboratory, previous cytopathologic and histopathologic result shall be reviewed.
5. 7 Post-examination processes 5. 7. 1 In the event of discrepancy in diagnosis with prior samples examined by the laboratory, previous cytopathologic and histopathologic result shall be reviewed.
 5. 8 Reporting results Peer consultation of difficult cases should be encourage before the final report is issued. Gynaecological cytopathology a) Negative smears may be reported by authorised cytoscientist, cytotechnologist or pathology trainee in cytopathology. All other smears shall be reported by a cytopathologist b) The current Bathesda system shall be used for reporting Non. Gynaecological cytopathology Negative sputum smears may be reported by authorised cytoscientist, cytotechnologist or pathology trainee in cytopathology. All other smears shall be reported by a cytopathologist FNA cytopathology a) All cases shall be reported by cytopathologist
5. 8 Reporting results Peer consultation of difficult cases should be encourage before the final report is issued. Gynaecological cytopathology a) Negative smears may be reported by authorised cytoscientist, cytotechnologist or pathology trainee in cytopathology. All other smears shall be reported by a cytopathologist b) The current Bathesda system shall be used for reporting Non. Gynaecological cytopathology Negative sputum smears may be reported by authorised cytoscientist, cytotechnologist or pathology trainee in cytopathology. All other smears shall be reported by a cytopathologist FNA cytopathology a) All cases shall be reported by cytopathologist
 5. 9 Releases of results The laboratory shall establish an “alert” or “critical” result eg High grade lesion in Pap smear from an Asymptomatic woman
5. 9 Releases of results The laboratory shall establish an “alert” or “critical” result eg High grade lesion in Pap smear from an Asymptomatic woman
 5. 10 LIS • If the LIS does not meet the requirement of clause 5. 10 MS 15189 or the system has limitation (e. g tracibility, accessibility) laboratory is required to have appropriate procedure to address the limitation. • List of References
5. 10 LIS • If the LIS does not meet the requirement of clause 5. 10 MS 15189 or the system has limitation (e. g tracibility, accessibility) laboratory is required to have appropriate procedure to address the limitation. • List of References
 Medical Laboratory Accreditation – what does it mean? • The laboratory has competent & experienced staff • Integrity and traceability of equipment and material • Technical validity of methods • Validity and suitability of results • Compliance with ISO management system standards
Medical Laboratory Accreditation – what does it mean? • The laboratory has competent & experienced staff • Integrity and traceability of equipment and material • Technical validity of methods • Validity and suitability of results • Compliance with ISO management system standards
 Accreditation – benefits to the laboratory • Competitive advantage – Mark of quality & competence – Credibility & recognition – Public and industry acceptance – Reimbursement of fees in healthcare financing/insurance schemes – Increase prospects for international market (AFTA & WTO)
Accreditation – benefits to the laboratory • Competitive advantage – Mark of quality & competence – Credibility & recognition – Public and industry acceptance – Reimbursement of fees in healthcare financing/insurance schemes – Increase prospects for international market (AFTA & WTO)
 Accreditation – benefits to the laboratory • Minimise possibility of mistakes – Identify & correct deficiencies • Improved staff motivation and esteem • Job satisfaction & pride • Improve working environment • Ensures better support in the event of legal challenge • Saves money by getting it right the first time.
Accreditation – benefits to the laboratory • Minimise possibility of mistakes – Identify & correct deficiencies • Improved staff motivation and esteem • Job satisfaction & pride • Improve working environment • Ensures better support in the event of legal challenge • Saves money by getting it right the first time.
 Thank You
Thank You


