e2e67bd4fd05e15b1bcdbe95e2dd732f.ppt
- Количество слайдов: 15

Update on the BVS-EXTEND Trial Cohort Robert-Jan van Geuns, MD, Ph. D on behalf of the ABSORB EXTEND Investigators Associate Professor, Thoraxcenter, Erasmus Medical Center, Rotterdam, the Netherlands

Robert-Jan J. M. Van Geuns, MD, Ph. D Advisory board and minor honoraria for Abbott Vascular Thoraxcenter Rotterdam: Research grants on BVS by Abbott BVS-EXPAND is an Abbott Vascular initiated trial
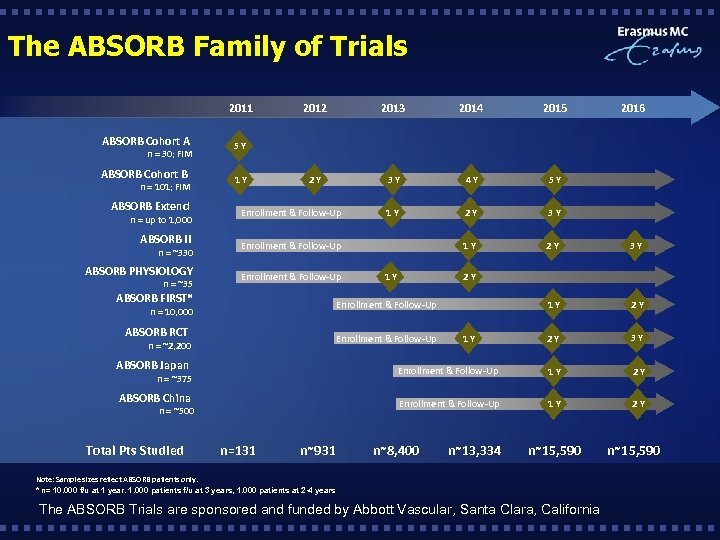
The ABSORB Family of Trials 2011 ABSORB Cohort A 1 Y 2013 2014 2015 2016 2 Y 3 Y 4 Y 5 Y 1 Y 2 Y 3 Y 1 Y 2 Y 2 Y 3 Y 5 Y ABSORB Cohort B 2012 n = 30; FIM n = 101; FIM ABSORB Extend Enrollment & Follow-Up ABSORB II Enrollment & Follow-Up ABSORB PHYSIOLOGY Enrollment & Follow-Up n = up to 1, 000 n = ~330 n = ~35 1 Y 2 Y ABSORB FIRST* Enrollment & Follow-Up ABSORB RCT Enrollment & Follow-Up n = 10, 000 n = ~2, 200 1 Y ABSORB Japan Enrollment & Follow-Up 1 Y 2 Y ABSORB China Enrollment & Follow-Up 1 Y 2 Y n = ~375 n = ~500 Total Pts Studied n=131 n~931 n~8, 400 n~13, 334 n~15, 590 Note: Sample sizes reflect ABSORB patients only. * n= 10. 000 f/u at 1 year. 1. 000 patients f/u at 3 years, 1. 000 patients at 2 -4 years The ABSORB Trials are sponsored and funded by Abbott Vascular, Santa Clara, California n~15, 590
Background • The results from ABSORB Cohort B confirm the performance and safety established in the Cohort A investigation: Ø The 24 -month angiographic results from Cohort B (Group 1) demonstrated an in-scaffold late loss of 0. 27 mm; Ø MACE rate of 9. 0% for the full cohort of 101 patients at 24 months; Ø No scaffold thrombosis events out to 24 months. • The primary objective of the ABSORB EXTEND study is to continue the assessment of the safety and performance of the Absorb BVS across global geographies in a larger patient population with increased lesion length and complexity.
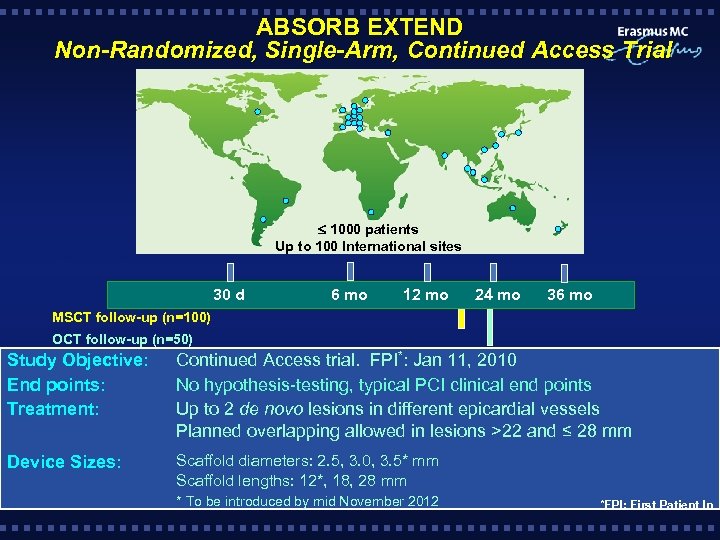
ABSORB EXTEND Non-Randomized, Single-Arm, Continued Access Trial 1000 patients Up to 100 International sites Clinical follow-up 30 d 6 mo 12 mo 24 mo 36 mo MSCT follow-up (n=100) OCT follow-up (n=50) Study Objective: End points: Treatment: Continued Access trial. FPI*: Jan 11, 2010 No hypothesis-testing, typical PCI clinical end points Up to 2 de novo lesions in different epicardial vessels Planned overlapping allowed in lesions >22 and ≤ 28 mm Device Sizes: Scaffold diameters: 2. 5, 3. 0, 3. 5* mm Scaffold lengths: 12*, 18, 28 mm * To be introduced by mid November 2012 *FPI: First Patient In
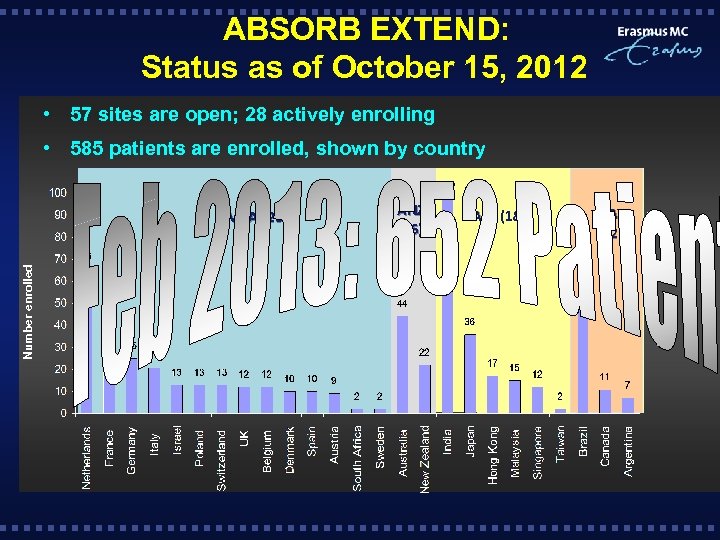
ABSORB EXTEND: Status as of October 15, 2012 • 57 sites are open; 28 actively enrolling • 585 patients are enrolled, shown by country Number enrolled EMEA (265) ANZ (66) APJ (182) LA (72)
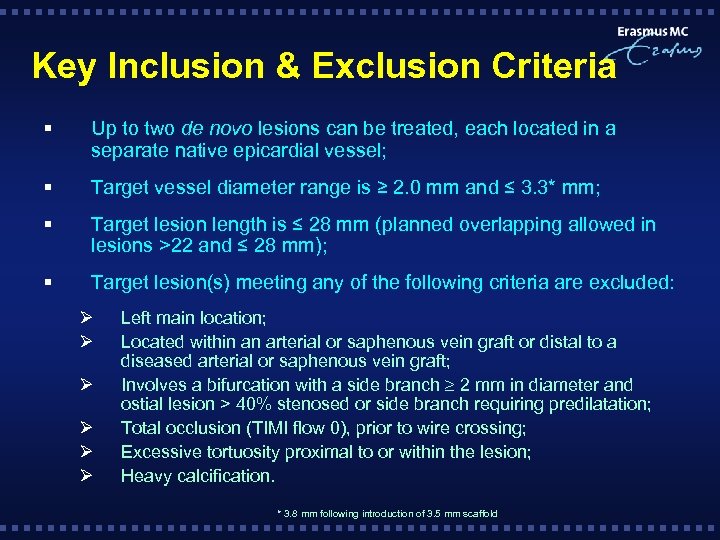
Key Inclusion & Exclusion Criteria § Up to two de novo lesions can be treated, each located in a separate native epicardial vessel; § Target vessel diameter range is ≥ 2. 0 mm and ≤ 3. 3* mm; § Target lesion length is ≤ 28 mm (planned overlapping allowed in lesions >22 and ≤ 28 mm); § Target lesion(s) meeting any of the following criteria are excluded: Ø Ø Ø Left main location; Located within an arterial or saphenous vein graft or distal to a diseased arterial or saphenous vein graft; Involves a bifurcation with a side branch 2 mm in diameter and ostial lesion > 40% stenosed or side branch requiring predilatation; Total occlusion (TIMI flow 0), prior to wire crossing; Excessive tortuosity proximal to or within the lesion; Heavy calcification. * 3. 8 mm following introduction of 3. 5 mm scaffold
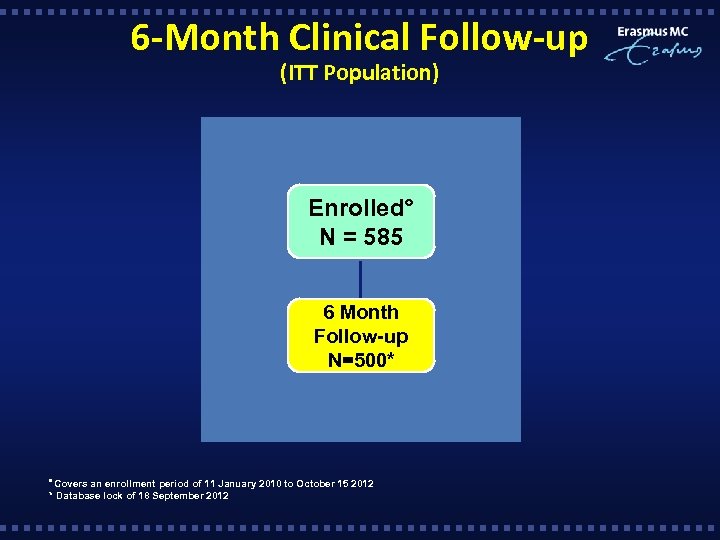
6 -Month Clinical Follow-up (ITT Population) Enrolled° N = 585 6 Month Follow-up N=500* Covers an enrollment period of 11 January 2010 to October 15 2012 * Database lock of 18 September 2012

ABSORB EXTEND - Baseline Demographics (ITT) EXTEND (N = 500) Male (%) 74. 4 Mean age (years) 62 Prior Cardiac Intervention on Target Vessel (%) 4. 6 Previous MI (%) 28. 5 Unstable Angina (%) 31. 2 Diabetes mellitus (%) 26. 2 Dyslipidemia req. med. (%) 62. 6 Hypertension req. med. (%) 65. 4 Current smoker (%) 22. 6
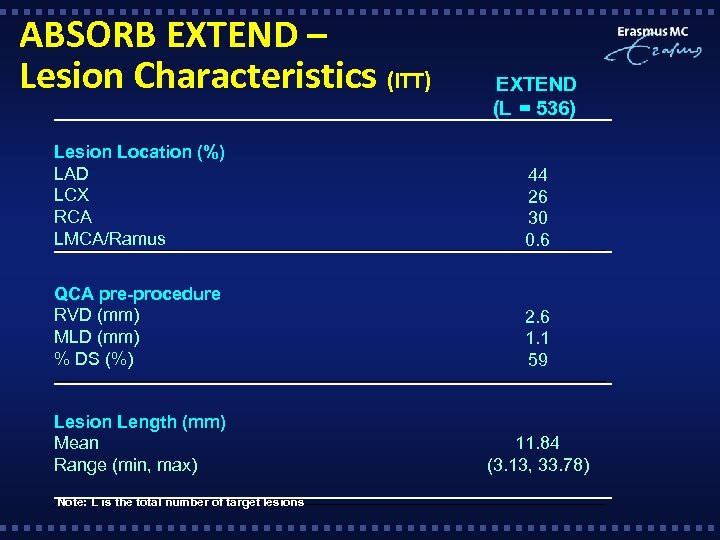
ABSORB EXTEND – Lesion Characteristics (ITT) EXTEND (L = 536) Lesion Location (%) LAD LCX RCA LMCA/Ramus 44 26 30 0. 6 QCA pre-procedure RVD (mm) MLD (mm) % DS (%) 2. 6 1. 1 59 Lesion Length (mm) Mean Range (min, max) 11. 84 (3. 13, 33. 78) Note: L is the total number of target lesions
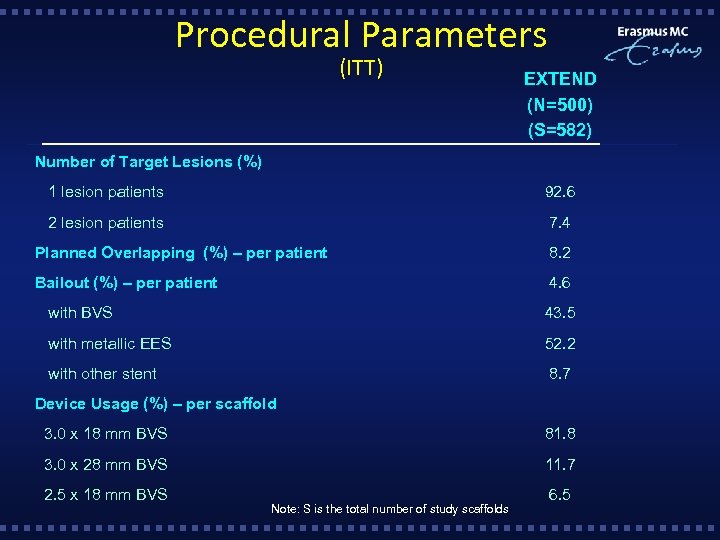
Procedural Parameters (ITT) EXTEND (N=500) (S=582) Number of Target Lesions (%) 1 lesion patients 92. 6 2 lesion patients 7. 4 Planned Overlapping (%) – per patient 8. 2 Bailout (%) – per patient 4. 6 with BVS 43. 5 with metallic EES 52. 2 with other stent 8. 7 Device Usage (%) – per scaffold 3. 0 x 18 mm BVS 81. 8 3. 0 x 28 mm BVS 11. 7 2. 5 x 18 mm BVS 6. 5 Note: S is the total number of study scaffolds
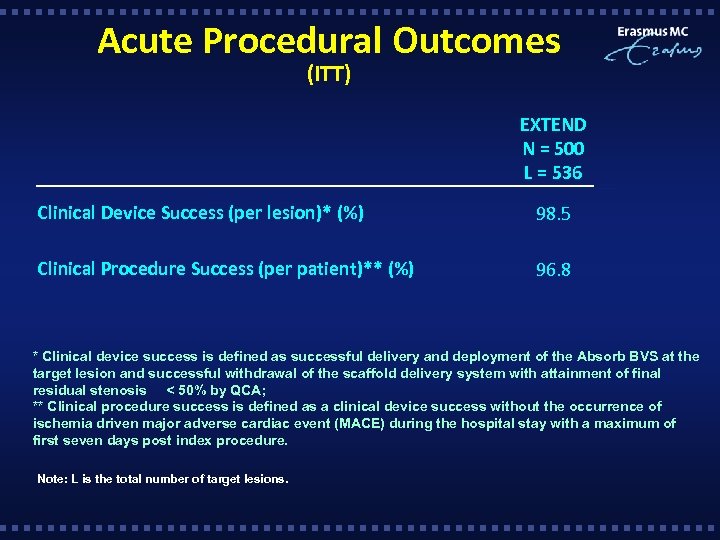
Acute Procedural Outcomes (ITT) EXTEND N = 500 L = 536 Clinical Device Success (per lesion)* (%) 98. 5 Clinical Procedure Success (per patient)** (%) 96. 8 * Clinical device success is defined as successful delivery and deployment of the Absorb BVS at the target lesion and successful withdrawal of the scaffold delivery system with attainment of final residual stenosis < 50% by QCA; ** Clinical procedure success is defined as a clinical device success without the occurrence of ischemia driven major adverse cardiac event (MACE) during the hospital stay with a maximum of first seven days post index procedure. Note: L is the total number of target lesions.
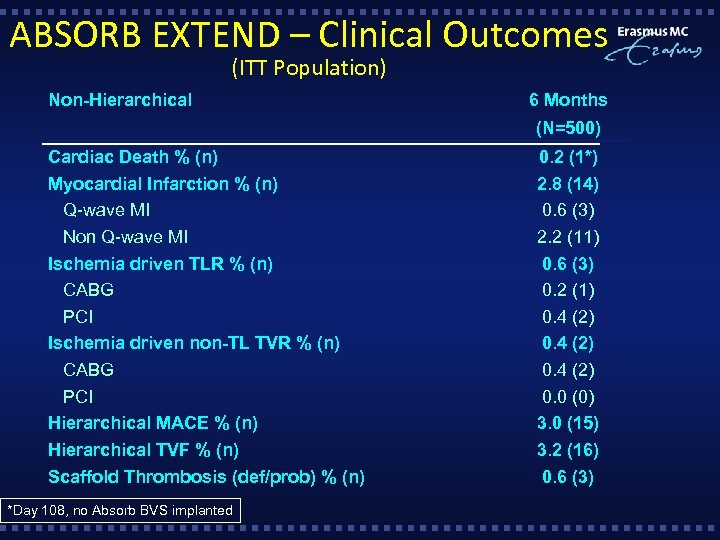
ABSORB EXTEND – Clinical Outcomes (ITT Population) Non-Hierarchical 6 Months (N=500) Cardiac Death % (n) Myocardial Infarction % (n) Q-wave MI Non Q-wave MI Ischemia driven TLR % (n) CABG PCI Ischemia driven non-TL TVR % (n) CABG PCI Hierarchical MACE % (n) Hierarchical TVF % (n) Scaffold Thrombosis (def/prob) % (n) *Day 108, no Absorb BVS implanted 0. 2 (1*) 2. 8 (14) 0. 6 (3) 2. 2 (11) 0. 6 (3) 0. 2 (1) 0. 4 (2) 0. 0 (0) 3. 0 (15) 3. 2 (16) 0. 6 (3)
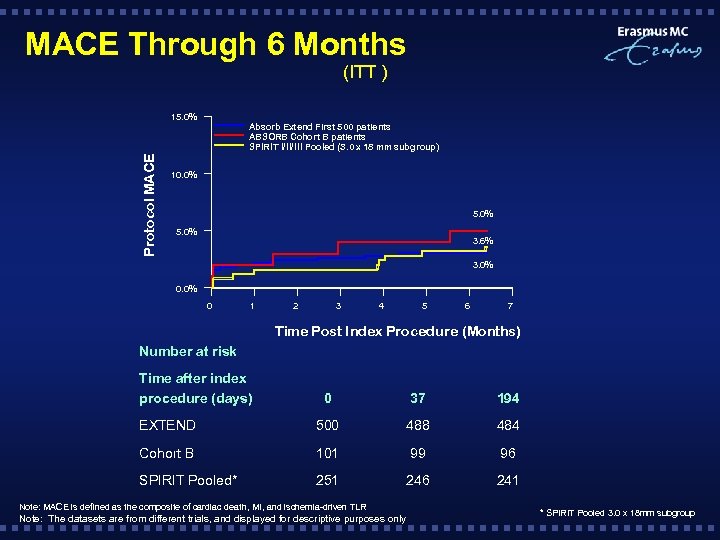
MACE Through 6 Months (ITT ) Protocol MACE 15. 0% Absorb Extend First 500 patients ABSORB Cohort B patients SPIRIT I/II/III Pooled (3. 0 x 18 mm subgroup) 10. 0% 5. 0% 3. 6% 3. 0% 0 1 2 3 4 5 6 7 Time Post Index Procedure (Months) Number at risk Time after index procedure (days) 0 37 194 EXTEND 500 488 484 Cohort B 101 99 96 SPIRIT Pooled* 251 246 241 Note: MACE is defined as the composite of cardiac death, MI, and ischemia-driven TLR Note: The datasets are from different trials, and displayed for descriptive purposes only * SPIRIT Pooled 3. 0 x 18 mm subgroup

Conclusions § This overview of 500 patients from ABSORB EXTEND included follow -up data out to 6 months; § Findings on the 6 -month outcomes in 500 patients include: Ø Low event rates including MACE (3. 0%) and ST (0. 6%) out to 6 months Ø MACE rate shown at 6 months (3. 0%) are sustained with the first 250 patients enrolled through 12 -month follow-up (4. 4%) § Despite an increase in complexity in ABSORB EXTEND, the data to date demonstrates the consistency in clinical outcomes between ABSORB EXTEND, ABSORB Cohort B and the SPIRIT Pooled Population.
e2e67bd4fd05e15b1bcdbe95e2dd732f.ppt