6014b2eb2f1db911879bbf5fdc2771a4.ppt
- Количество слайдов: 47
 Unmatched Data, Unsurpassed Patency and Superior TIPS Performance Speaker's Slide Resource Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Unmatched Data, Unsurpassed Patency and Superior TIPS Performance Speaker's Slide Resource Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Table of Contents • Superior TIPS Performance • Unmatched Data • Case Studies • Implantation TIPS for Success • Deployment Animation • Image Resources • Suggested Reading Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Table of Contents • Superior TIPS Performance • Unmatched Data • Case Studies • Implantation TIPS for Success • Deployment Animation • Image Resources • Suggested Reading Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Superior TIPS Performance Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Superior TIPS Performance Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
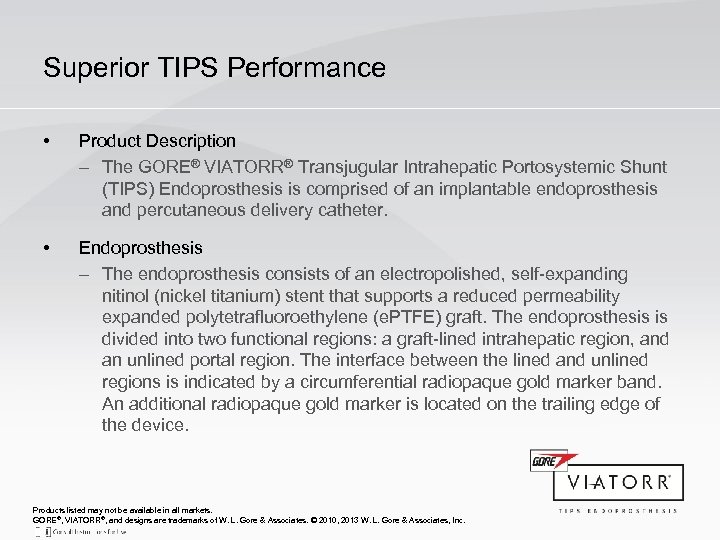 Superior TIPS Performance • Product Description – The GORE® VIATORR® Transjugular Intrahepatic Portosystemic Shunt (TIPS) Endoprosthesis is comprised of an implantable endoprosthesis and percutaneous delivery catheter. • Endoprosthesis – The endoprosthesis consists of an electropolished, self-expanding nitinol (nickel titanium) stent that supports a reduced permeability expanded polytetrafluoroethylene (e. PTFE) graft. The endoprosthesis is divided into two functional regions: a graft-lined intrahepatic region, and an unlined portal region. The interface between the lined and unlined regions is indicated by a circumferential radiopaque gold marker band. An additional radiopaque gold marker is located on the trailing edge of the device. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Superior TIPS Performance • Product Description – The GORE® VIATORR® Transjugular Intrahepatic Portosystemic Shunt (TIPS) Endoprosthesis is comprised of an implantable endoprosthesis and percutaneous delivery catheter. • Endoprosthesis – The endoprosthesis consists of an electropolished, self-expanding nitinol (nickel titanium) stent that supports a reduced permeability expanded polytetrafluoroethylene (e. PTFE) graft. The endoprosthesis is divided into two functional regions: a graft-lined intrahepatic region, and an unlined portal region. The interface between the lined and unlined regions is indicated by a circumferential radiopaque gold marker band. An additional radiopaque gold marker is located on the trailing edge of the device. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Indications • Indications for Use in the US: The GORE® VIATORR® TIPS Endoprosthesis is indicated for use in the de novo and revision treatment of portal hypertension and its complications such as variceal bleeding, gastropathy, refractory ascites, and / or hepatic hydrothorax. • Indications Cleared Under CE Mark: The GORE® VIATORR® TIPS Endoprosthesis is indicated for use in the treatment of portal hypertension and its complications such as: variceal bleeding refractory to, or intolerant of, conventional therapies, inaccessible varices, gastropathy, refractory ascites, and / or hepatic hydrothorax. * * Outside US Only Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Indications • Indications for Use in the US: The GORE® VIATORR® TIPS Endoprosthesis is indicated for use in the de novo and revision treatment of portal hypertension and its complications such as variceal bleeding, gastropathy, refractory ascites, and / or hepatic hydrothorax. • Indications Cleared Under CE Mark: The GORE® VIATORR® TIPS Endoprosthesis is indicated for use in the treatment of portal hypertension and its complications such as: variceal bleeding refractory to, or intolerant of, conventional therapies, inaccessible varices, gastropathy, refractory ascites, and / or hepatic hydrothorax. * * Outside US Only Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
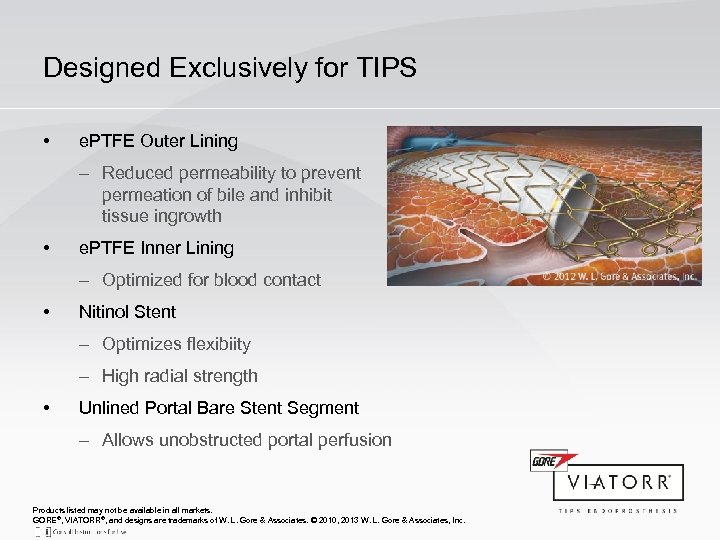 Designed Exclusively for TIPS • e. PTFE Outer Lining – Reduced permeability to prevent permeation of bile and inhibit tissue ingrowth • e. PTFE Inner Lining – Optimized for blood contact • Nitinol Stent – Optimizes flexibiity – High radial strength • Unlined Portal Bare Stent Segment – Allows unobstructed portal perfusion Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Designed Exclusively for TIPS • e. PTFE Outer Lining – Reduced permeability to prevent permeation of bile and inhibit tissue ingrowth • e. PTFE Inner Lining – Optimized for blood contact • Nitinol Stent – Optimizes flexibiity – High radial strength • Unlined Portal Bare Stent Segment – Allows unobstructed portal perfusion Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
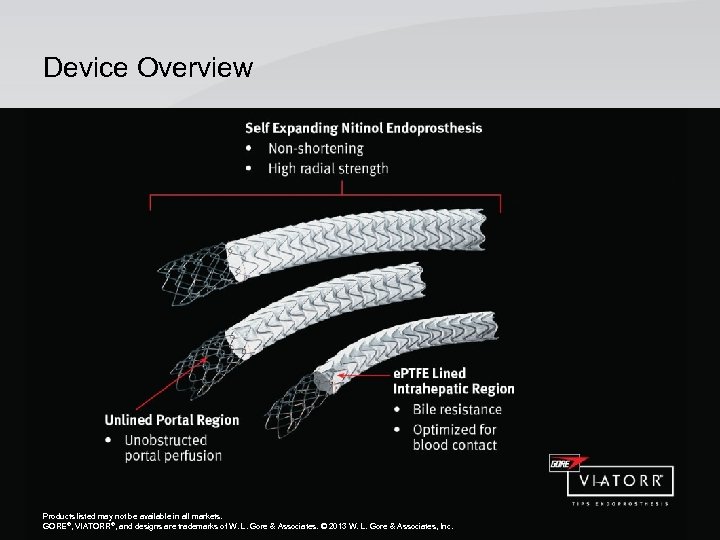 Device Overview Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2013 W. L. Gore & Associates, Inc.
Device Overview Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2013 W. L. Gore & Associates, Inc.
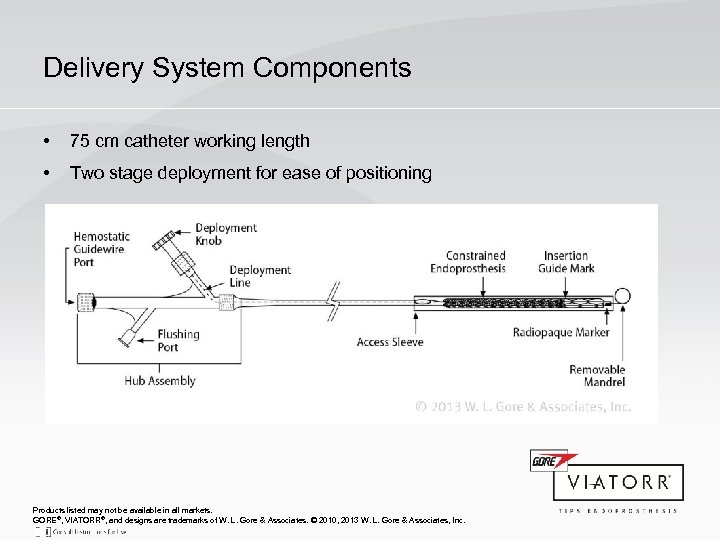 Delivery System Components • 75 cm catheter working length • Two stage deployment for ease of positioning Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Delivery System Components • 75 cm catheter working length • Two stage deployment for ease of positioning Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
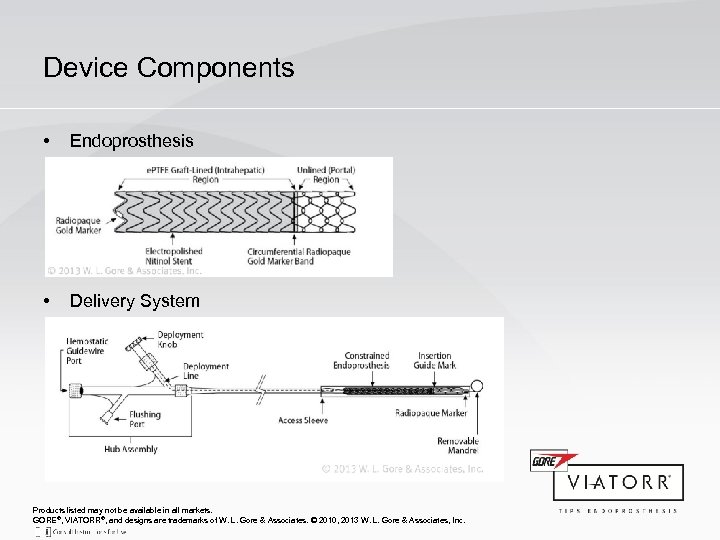 Device Components • Endoprosthesis • Delivery System Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Device Components • Endoprosthesis • Delivery System Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
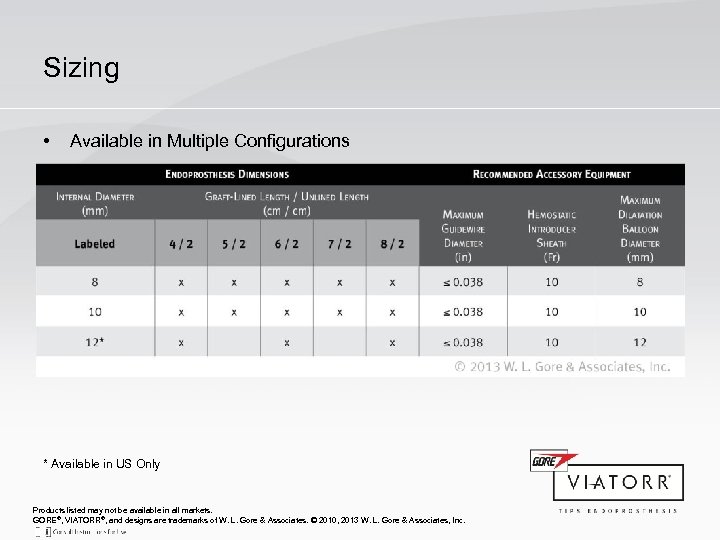 Sizing • Available in Multiple Configurations * Available in US Only Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Sizing • Available in Multiple Configurations * Available in US Only Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Unmatched Data Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Unmatched Data Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
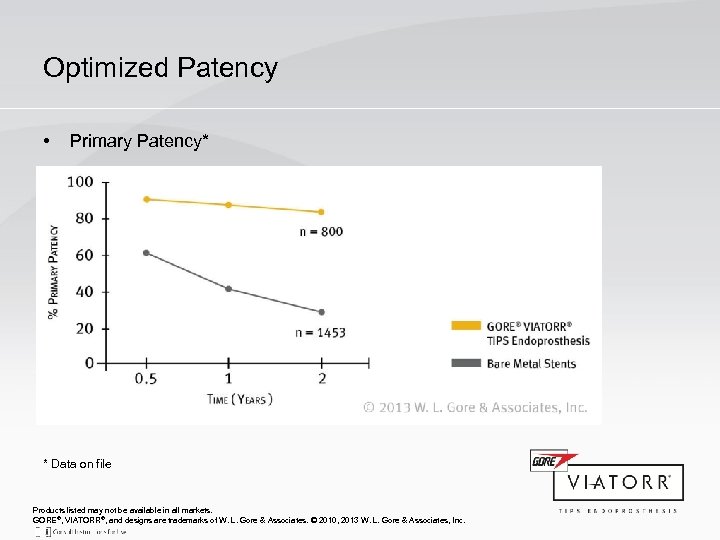 Optimized Patency • Primary Patency* * Data on file Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Optimized Patency • Primary Patency* * Data on file Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
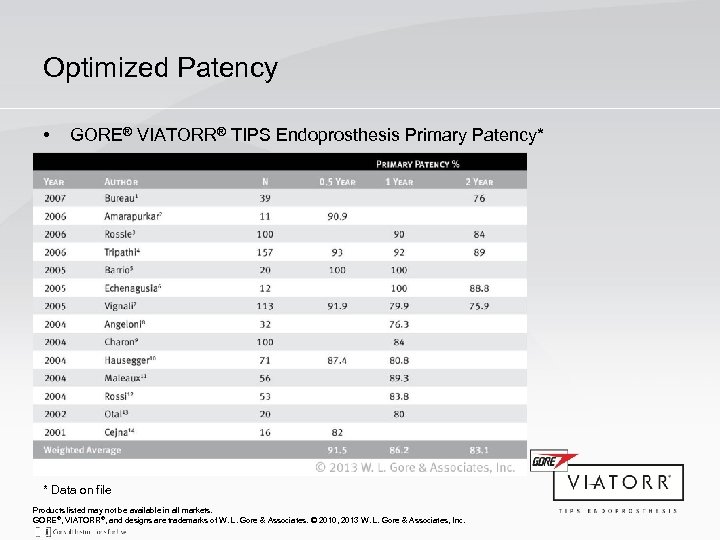 Optimized Patency • GORE® VIATORR® TIPS Endoprosthesis Primary Patency* * Data on file Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Optimized Patency • GORE® VIATORR® TIPS Endoprosthesis Primary Patency* * Data on file Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
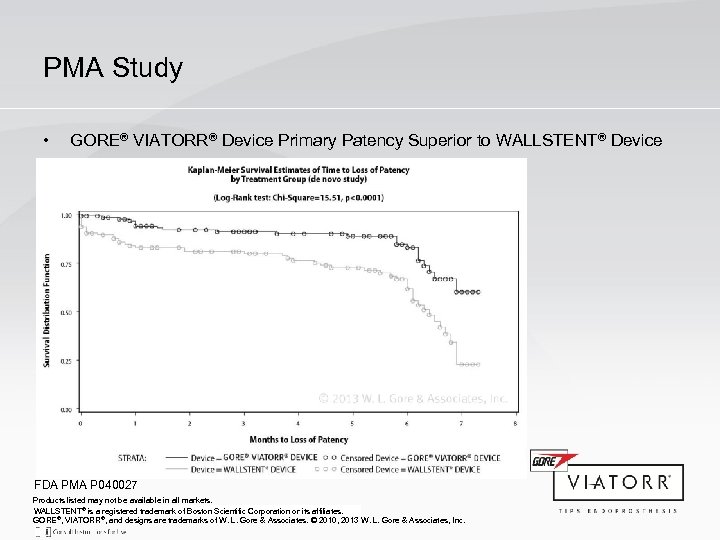 PMA Study • GORE® VIATORR® Device Primary Patency Superior to WALLSTENT® Device FDA PMA P 040027 Products listed may not be available in all markets. WALLSTENT® is a registered trademark of Boston Scientific Corporation or its affiliates. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
PMA Study • GORE® VIATORR® Device Primary Patency Superior to WALLSTENT® Device FDA PMA P 040027 Products listed may not be available in all markets. WALLSTENT® is a registered trademark of Boston Scientific Corporation or its affiliates. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
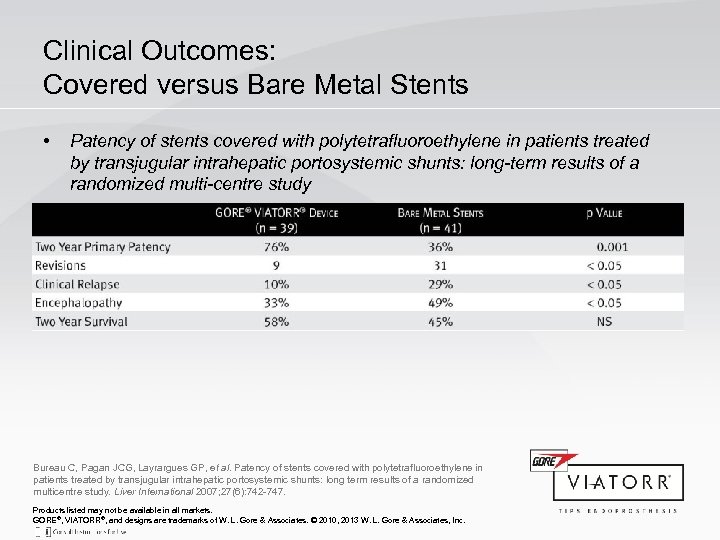 Clinical Outcomes: Covered versus Bare Metal Stents • Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multi-centre study Bureau C, Pagan JCG, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long term results of a randomized multicentre study. Liver International 2007; 27(6): 742 -747. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Clinical Outcomes: Covered versus Bare Metal Stents • Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multi-centre study Bureau C, Pagan JCG, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long term results of a randomized multicentre study. Liver International 2007; 27(6): 742 -747. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
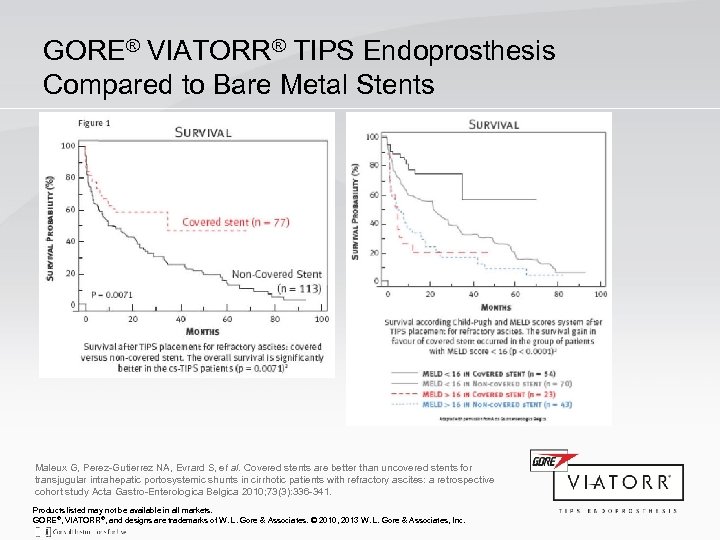 GORE® VIATORR® TIPS Endoprosthesis Compared to Bare Metal Stents Maleux G, Perez-Gutierrez NA, Evrard S, et al. Covered stents are better than uncovered stents for transjugular intrahepatic portosystemic shunts in cirrhotic patients with refractory ascites: a retrospective cohort study Acta Gastro-Enterologica Belgica 2010; 73(3): 336 -341. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
GORE® VIATORR® TIPS Endoprosthesis Compared to Bare Metal Stents Maleux G, Perez-Gutierrez NA, Evrard S, et al. Covered stents are better than uncovered stents for transjugular intrahepatic portosystemic shunts in cirrhotic patients with refractory ascites: a retrospective cohort study Acta Gastro-Enterologica Belgica 2010; 73(3): 336 -341. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
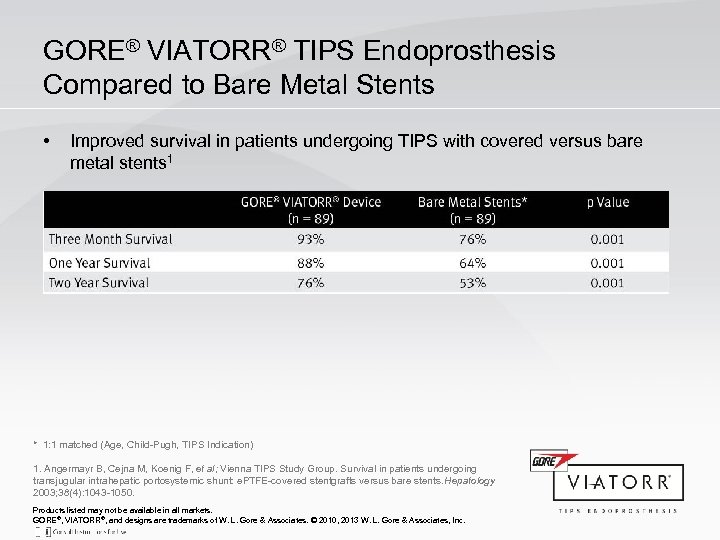 GORE® VIATORR® TIPS Endoprosthesis Compared to Bare Metal Stents • Improved survival in patients undergoing TIPS with covered versus bare metal stents 1 * 1: 1 matched (Age, Child-Pugh, TIPS Indication) 1. Angermayr B, Cejna M, Koenig F, et al; Vienna TIPS Study Group. Survival in patients undergoing transjugular intrahepatic portosystemic shunt: e. PTFE-covered stentgrafts versus bare stents. Hepatology 2003; 38(4): 1043 -1050. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
GORE® VIATORR® TIPS Endoprosthesis Compared to Bare Metal Stents • Improved survival in patients undergoing TIPS with covered versus bare metal stents 1 * 1: 1 matched (Age, Child-Pugh, TIPS Indication) 1. Angermayr B, Cejna M, Koenig F, et al; Vienna TIPS Study Group. Survival in patients undergoing transjugular intrahepatic portosystemic shunt: e. PTFE-covered stentgrafts versus bare stents. Hepatology 2003; 38(4): 1043 -1050. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
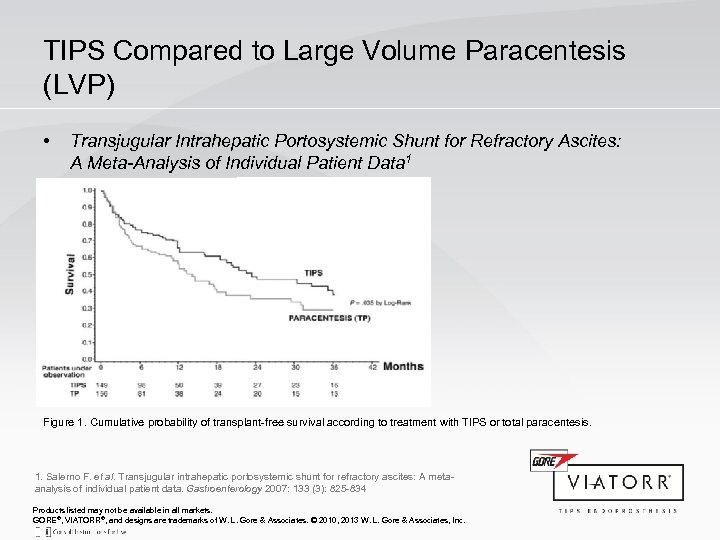 TIPS Compared to Large Volume Paracentesis (LVP) • Transjugular Intrahepatic Portosystemic Shunt for Refractory Ascites: A Meta-Analysis of Individual Patient Data 1 Figure 1. Cumulative probability of transplant-free survival according to treatment with TIPS or total paracentesis. 1. Salerno F. et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: A metaanalysis of individual patient data. Gastroenterology 2007: 133 (3): 825 -834 Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
TIPS Compared to Large Volume Paracentesis (LVP) • Transjugular Intrahepatic Portosystemic Shunt for Refractory Ascites: A Meta-Analysis of Individual Patient Data 1 Figure 1. Cumulative probability of transplant-free survival according to treatment with TIPS or total paracentesis. 1. Salerno F. et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: A metaanalysis of individual patient data. Gastroenterology 2007: 133 (3): 825 -834 Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
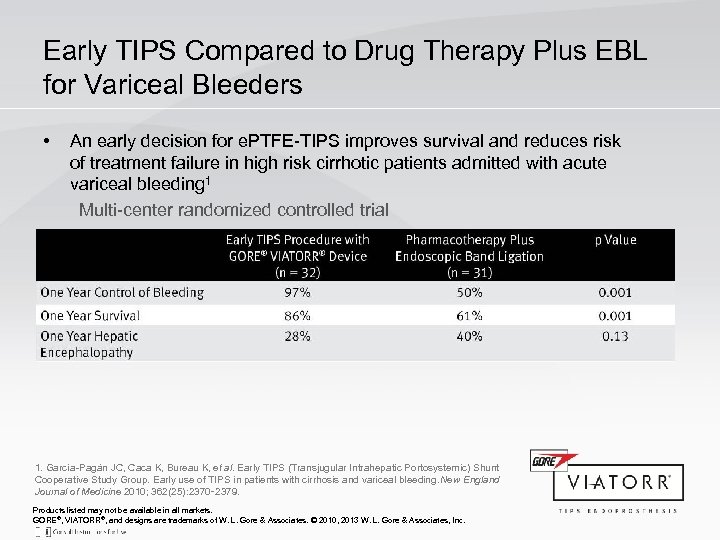 Early TIPS Compared to Drug Therapy Plus EBL for Variceal Bleeders • An early decision for e. PTFE-TIPS improves survival and reduces risk of treatment failure in high risk cirrhotic patients admitted with acute variceal bleeding 1 Multi-center randomized controlled trial 1. García-Pagán JC, Caca K, Bureau K, et al. Early TIPS (Transjugular Intrahepatic Portosystemic) Shunt Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. ew England N Journal of Medicine 2010; 362(25): 2370‑ 2379. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Early TIPS Compared to Drug Therapy Plus EBL for Variceal Bleeders • An early decision for e. PTFE-TIPS improves survival and reduces risk of treatment failure in high risk cirrhotic patients admitted with acute variceal bleeding 1 Multi-center randomized controlled trial 1. García-Pagán JC, Caca K, Bureau K, et al. Early TIPS (Transjugular Intrahepatic Portosystemic) Shunt Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. ew England N Journal of Medicine 2010; 362(25): 2370‑ 2379. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Case Studies Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Case Studies Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
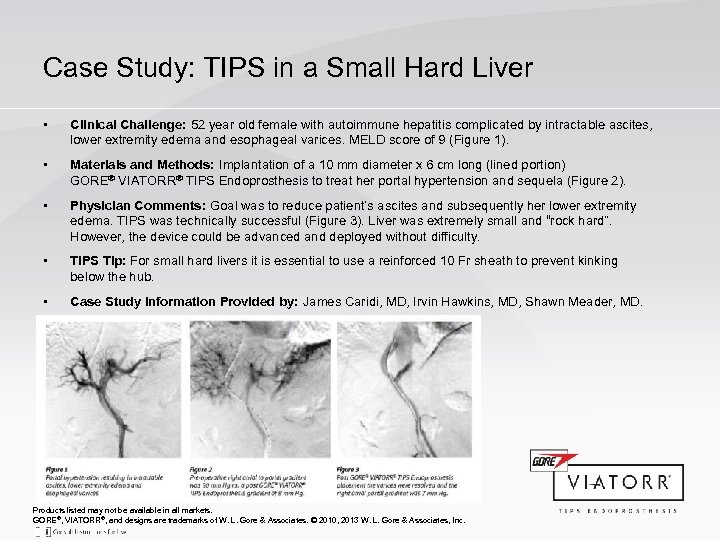 Case Study: TIPS in a Small Hard Liver • Clinical Challenge: 52 year old female with autoimmune hepatitis complicated by intractable ascites, lower extremity edema and esophageal varices. MELD score of 9 (Figure 1). • Materials and Methods: Implantation of a 10 mm diameter x 6 cm long (lined portion) GORE® VIATORR® TIPS Endoprosthesis to treat her portal hypertension and sequela (Figure 2). • Physician Comments: Goal was to reduce patient’s ascites and subsequently her lower extremity edema. TIPS was technically successful (Figure 3). Liver was extremely small and “rock hard”. However, the device could be advanced and deployed without difficulty. • TIPS Tip: For small hard livers it is essential to use a reinforced 10 Fr sheath to prevent kinking below the hub. • Case Study Information Provided by: James Caridi, MD, Irvin Hawkins, MD, Shawn Meader, MD. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Case Study: TIPS in a Small Hard Liver • Clinical Challenge: 52 year old female with autoimmune hepatitis complicated by intractable ascites, lower extremity edema and esophageal varices. MELD score of 9 (Figure 1). • Materials and Methods: Implantation of a 10 mm diameter x 6 cm long (lined portion) GORE® VIATORR® TIPS Endoprosthesis to treat her portal hypertension and sequela (Figure 2). • Physician Comments: Goal was to reduce patient’s ascites and subsequently her lower extremity edema. TIPS was technically successful (Figure 3). Liver was extremely small and “rock hard”. However, the device could be advanced and deployed without difficulty. • TIPS Tip: For small hard livers it is essential to use a reinforced 10 Fr sheath to prevent kinking below the hub. • Case Study Information Provided by: James Caridi, MD, Irvin Hawkins, MD, Shawn Meader, MD. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
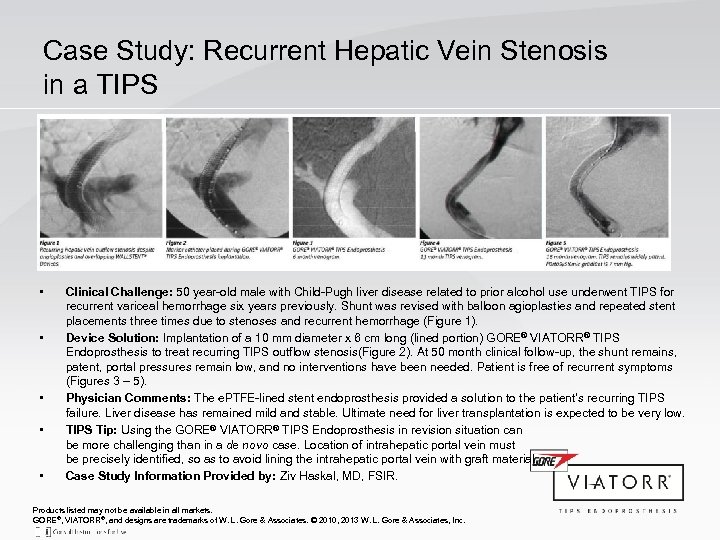 Case Study: Recurrent Hepatic Vein Stenosis in a TIPS • • • Clinical Challenge: 50 year-old male with Child-Pugh liver disease related to prior alcohol use underwent TIPS for recurrent variceal hemorrhage six years previously. Shunt was revised with balloon agioplasties and repeated stent placements three times due to stenoses and recurrent hemorrhage (Figure 1). Device Solution: Implantation of a 10 mm diameter x 6 cm long (lined portion) GORE® VIATORR® TIPS Endoprosthesis to treat recurring TIPS outflow stenosis(Figure 2). At 50 month clinical follow-up, the shunt remains, patent, portal pressures remain low, and no interventions have been needed. Patient is free of recurrent symptoms (Figures 3 – 5). Physician Comments: The e. PTFE-lined stent endoprosthesis provided a solution to the patient’s recurring TIPS failure. Liver disease has remained mild and stable. Ultimate need for liver transplantation is expected to be very low. TIPS Tip: Using the GORE® VIATORR® TIPS Endoprosthesis in revision situation can be more challenging than in a de novo case. Location of intrahepatic portal vein must be precisely identified, so as to avoid lining the intrahepatic portal vein with graft material. Case Study Information Provided by: Ziv Haskal, MD, FSIR. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Case Study: Recurrent Hepatic Vein Stenosis in a TIPS • • • Clinical Challenge: 50 year-old male with Child-Pugh liver disease related to prior alcohol use underwent TIPS for recurrent variceal hemorrhage six years previously. Shunt was revised with balloon agioplasties and repeated stent placements three times due to stenoses and recurrent hemorrhage (Figure 1). Device Solution: Implantation of a 10 mm diameter x 6 cm long (lined portion) GORE® VIATORR® TIPS Endoprosthesis to treat recurring TIPS outflow stenosis(Figure 2). At 50 month clinical follow-up, the shunt remains, patent, portal pressures remain low, and no interventions have been needed. Patient is free of recurrent symptoms (Figures 3 – 5). Physician Comments: The e. PTFE-lined stent endoprosthesis provided a solution to the patient’s recurring TIPS failure. Liver disease has remained mild and stable. Ultimate need for liver transplantation is expected to be very low. TIPS Tip: Using the GORE® VIATORR® TIPS Endoprosthesis in revision situation can be more challenging than in a de novo case. Location of intrahepatic portal vein must be precisely identified, so as to avoid lining the intrahepatic portal vein with graft material. Case Study Information Provided by: Ziv Haskal, MD, FSIR. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Implantation Tips for Success Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
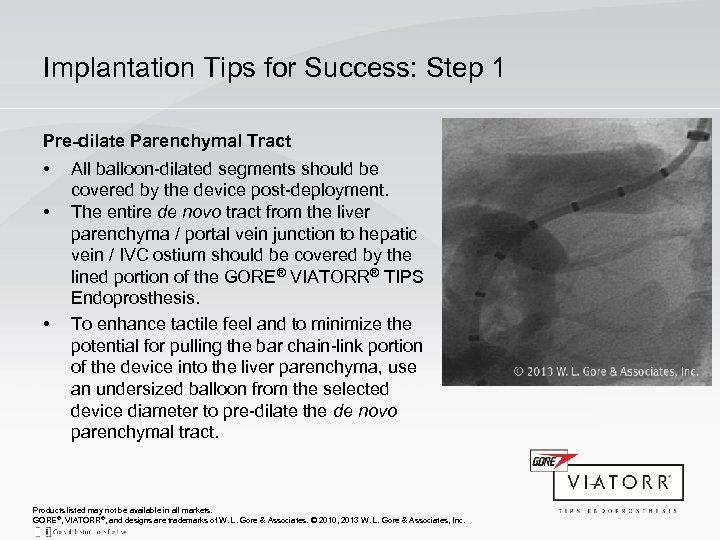 Implantation Tips for Success: Step 1 Pre-dilate Parenchymal Tract • • • All balloon-dilated segments should be covered by the device post-deployment. The entire de novo tract from the liver parenchyma / portal vein junction to hepatic vein / IVC ostium should be covered by the lined portion of the GORE® VIATORR® TIPS Endoprosthesis. To enhance tactile feel and to minimize the potential for pulling the bar chain-link portion of the device into the liver parenchyma, use an undersized balloon from the selected device diameter to pre-dilate the de novo parenchymal tract. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 1 Pre-dilate Parenchymal Tract • • • All balloon-dilated segments should be covered by the device post-deployment. The entire de novo tract from the liver parenchyma / portal vein junction to hepatic vein / IVC ostium should be covered by the lined portion of the GORE® VIATORR® TIPS Endoprosthesis. To enhance tactile feel and to minimize the potential for pulling the bar chain-link portion of the device into the liver parenchyma, use an undersized balloon from the selected device diameter to pre-dilate the de novo parenchymal tract. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
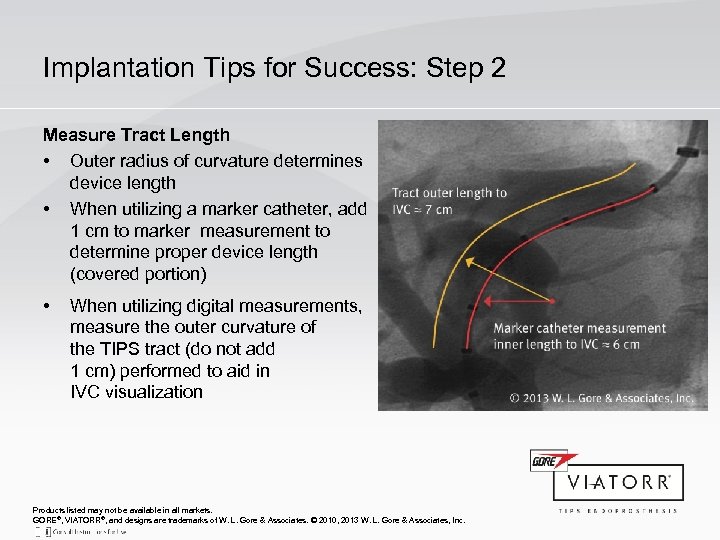 Implantation Tips for Success: Step 2 Measure Tract Length • Outer radius of curvature determines device length • When utilizing a marker catheter, add 1 cm to marker measurement to determine proper device length (covered portion) • When utilizing digital measurements, measure the outer curvature of the TIPS tract (do not add 1 cm) performed to aid in IVC visualization Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 2 Measure Tract Length • Outer radius of curvature determines device length • When utilizing a marker catheter, add 1 cm to marker measurement to determine proper device length (covered portion) • When utilizing digital measurements, measure the outer curvature of the TIPS tract (do not add 1 cm) performed to aid in IVC visualization Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Implantation Tips for Success: Step 3 Device Selection • Select the appropriate device (diameter and length) from the sizing table. • Select the appropriate accessory equipment. Table 1: Dimensions and Recommended Accessories (12 mm device is not available in all countries) 1 Lengths may vary by ± 0. 5 cm. 2 A stiff guidewire, having a length of at least 180 cm and a maximum diameter ≤ 0. 038" (0. 97 mm), is required. Delivery catheter working length is 75 cm for all endoprosthesis configurations. 3 Introducer sheath length must be sufficient to be delivered into the portal circulation by ≥ 3 cm. It is recommended that a wall-reinforced TIPS introducer sheath (such as COOK® FLEXOR®, CHECK-FLO® II Introducer Sheath. or equivalent) with an integral radiopaque marker band a length of approximately 40– 45 cm be used. 4 The same balloon dilatation device can be used for TIPS dilatation and dilatation of the endoprosthesis following implantation. Products listed may not be available in all markets. COOK®, FLEXOR®, and CHECK-FLO ® are registered trademarks of Cook, Inc. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 3 Device Selection • Select the appropriate device (diameter and length) from the sizing table. • Select the appropriate accessory equipment. Table 1: Dimensions and Recommended Accessories (12 mm device is not available in all countries) 1 Lengths may vary by ± 0. 5 cm. 2 A stiff guidewire, having a length of at least 180 cm and a maximum diameter ≤ 0. 038" (0. 97 mm), is required. Delivery catheter working length is 75 cm for all endoprosthesis configurations. 3 Introducer sheath length must be sufficient to be delivered into the portal circulation by ≥ 3 cm. It is recommended that a wall-reinforced TIPS introducer sheath (such as COOK® FLEXOR®, CHECK-FLO® II Introducer Sheath. or equivalent) with an integral radiopaque marker band a length of approximately 40– 45 cm be used. 4 The same balloon dilatation device can be used for TIPS dilatation and dilatation of the endoprosthesis following implantation. Products listed may not be available in all markets. COOK®, FLEXOR®, and CHECK-FLO ® are registered trademarks of Cook, Inc. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Implantation Tips for Success: Step 4 Prepare the Delivery System • Do not displace or remove the access sleeve. • Inspect for damage. • Flush guidewire lumen and flushing port. To ensure complete endoprosthesis flush, place finger over end of access sleeve and flush until liquid emerges from proximal end of access sleeve. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 4 Prepare the Delivery System • Do not displace or remove the access sleeve. • Inspect for damage. • Flush guidewire lumen and flushing port. To ensure complete endoprosthesis flush, place finger over end of access sleeve and flush until liquid emerges from proximal end of access sleeve. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Implantation Tips for Success: Step 5 Insert the Device into Introducer Sheath • Insertion of Access Sleeve: – Recommended accessories: 0. 035" x 180 cm stiff guidewire; 10 Fr x 40 cm reinforced wall introducer sheath (Table ). – Hold access sleeve in center and push straight through hemostatic valve in introducer sheath. – Advance to the stop bottom of hemostatic valve body and maintain access sleeve position during process of device transfer into introducer sheath tube. • Insertion of Delivery Catheter: – Hold access sleeve within bottom of introducer sheath to prevent ‘backing out’. – Hold delivery catheter near the end of the access sleeve. – Advance delivery catheter through access sleeve in small (approximately 5 mm) increments until entire device has cleared the hemostatic valve body and has advanced at least 5 cm into the introducer sheath tube. – Once device transfer is complete, access sleeve may be retracted from hemostatic valve loading. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 5 Insert the Device into Introducer Sheath • Insertion of Access Sleeve: – Recommended accessories: 0. 035" x 180 cm stiff guidewire; 10 Fr x 40 cm reinforced wall introducer sheath (Table ). – Hold access sleeve in center and push straight through hemostatic valve in introducer sheath. – Advance to the stop bottom of hemostatic valve body and maintain access sleeve position during process of device transfer into introducer sheath tube. • Insertion of Delivery Catheter: – Hold access sleeve within bottom of introducer sheath to prevent ‘backing out’. – Hold delivery catheter near the end of the access sleeve. – Advance delivery catheter through access sleeve in small (approximately 5 mm) increments until entire device has cleared the hemostatic valve body and has advanced at least 5 cm into the introducer sheath tube. – Once device transfer is complete, access sleeve may be retracted from hemostatic valve loading. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
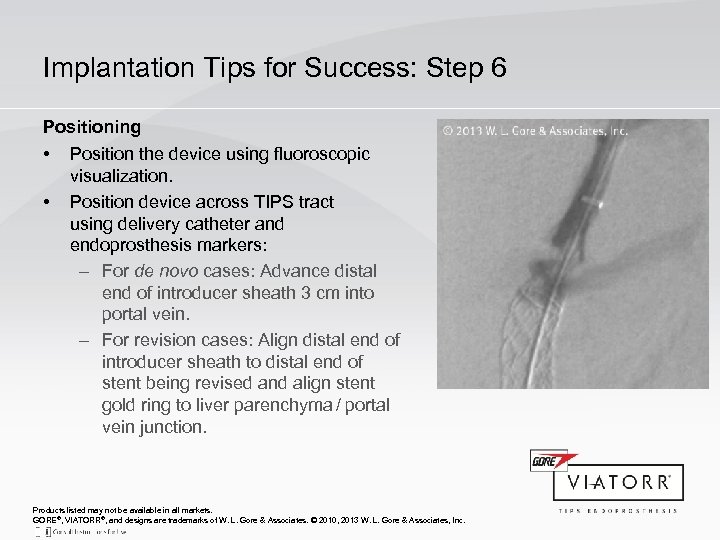 Implantation Tips for Success: Step 6 Positioning • • Position the device using fluoroscopic visualization. Position device across TIPS tract using delivery catheter and endoprosthesis markers: – For de novo cases: Advance distal end of introducer sheath 3 cm into portal vein. – For revision cases: Align distal end of introducer sheath to distal end of stent being revised and align stent gold ring to liver parenchyma / portal vein junction. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 6 Positioning • • Position the device using fluoroscopic visualization. Position device across TIPS tract using delivery catheter and endoprosthesis markers: – For de novo cases: Advance distal end of introducer sheath 3 cm into portal vein. – For revision cases: Align distal end of introducer sheath to distal end of stent being revised and align stent gold ring to liver parenchyma / portal vein junction. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Implantation Tips for Success: Step 7 Deployment • Consider utilizing a two-operator approach to device deployment. • Fluoroscopic guidance is required. • While maintaining the delivery system position, retract the sheath and deploy the chain-link segment of the device into the portal vein. Completely retract the hemostatic introducer sheath beyond the trailing end of the endoprosthesis and into the IVC: – For de novo procedures, once the chain-link portion is deployed (3 cm) into the portal vein, gently pull back on the delivery catheter until resistance is felt. This will seal the distal end of the covered device portion to the liver parenchyma / portal vein junction. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 7 Deployment • Consider utilizing a two-operator approach to device deployment. • Fluoroscopic guidance is required. • While maintaining the delivery system position, retract the sheath and deploy the chain-link segment of the device into the portal vein. Completely retract the hemostatic introducer sheath beyond the trailing end of the endoprosthesis and into the IVC: – For de novo procedures, once the chain-link portion is deployed (3 cm) into the portal vein, gently pull back on the delivery catheter until resistance is felt. This will seal the distal end of the covered device portion to the liver parenchyma / portal vein junction. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Implantation Tips for Success: Step 7 continued. . . Deployment • While maintaining delivery system traction and the delivery system position relative to the sheath, keep the delivery system straight and loosen the GORE® SIM‑PULL Deployment System knob. – Pull the deployment knob with smooth, controlled tension. – Do not attempt repositioning during deployment. NOTE: • Do not attempt to recapture once deployment is initiated. • Device cannot be repositioned once deployment is complete. • For de novo cases: Advance distal end of introducer sheath 3 cm into portal vein. • For revision cases: Align distal end of introducer sheath to distal end of stent being revised. Do not advance chain-link beyond end of bare stent as pulling back the GORE® VIATORR® TIPS Endoprosthesis may result in device or deployment line entanglement on the distal end of bare stent. Exercise care when positioning device while patient is breathing. Products listed may not be available in all markets. GORE®, SIM-PULL, VIATORR ®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 7 continued. . . Deployment • While maintaining delivery system traction and the delivery system position relative to the sheath, keep the delivery system straight and loosen the GORE® SIM‑PULL Deployment System knob. – Pull the deployment knob with smooth, controlled tension. – Do not attempt repositioning during deployment. NOTE: • Do not attempt to recapture once deployment is initiated. • Device cannot be repositioned once deployment is complete. • For de novo cases: Advance distal end of introducer sheath 3 cm into portal vein. • For revision cases: Align distal end of introducer sheath to distal end of stent being revised. Do not advance chain-link beyond end of bare stent as pulling back the GORE® VIATORR® TIPS Endoprosthesis may result in device or deployment line entanglement on the distal end of bare stent. Exercise care when positioning device while patient is breathing. Products listed may not be available in all markets. GORE®, SIM-PULL, VIATORR ®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Implantation Tips for Success: Step 8 Remove Delivery Catheter • Gently remove the spent delivery catheter. • Stop if resistance is felt when removing the catheter. • Maintain guidewire access. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 8 Remove Delivery Catheter • Gently remove the spent delivery catheter. • Stop if resistance is felt when removing the catheter. • Maintain guidewire access. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Implantation Tips for Success: Step 9 Post-dilate the Device • Select the appropriate balloon size for the endoprosthesis implanted. • Dilate the entire length of the graftlined region per the balloon manufacturer’s Instructions for Use. • Do not use a balloon larger than the device implanted. • Ballooning device to full labeled diameter is not mandatory. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 9 Post-dilate the Device • Select the appropriate balloon size for the endoprosthesis implanted. • Dilate the entire length of the graftlined region per the balloon manufacturer’s Instructions for Use. • Do not use a balloon larger than the device implanted. • Ballooning device to full labeled diameter is not mandatory. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Implantation Tips for Success: Step 10 Completion Imaging • Confirm positioning and patency of device. • NOTE: If placing a second endoprosthesis to provide adequate length coverage, ensure ≥ 2 cm of lined graft-to-lined graft overlap of the telescoping devices. • Take final PSG measurement and re-balloon as necessary. • Use caution to not displace a deployed GORE® VIATORR® TIPS Endoprosthesis by re-introduction of an introducer sheath or working catheter back through the endoprosthesis. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Implantation Tips for Success: Step 10 Completion Imaging • Confirm positioning and patency of device. • NOTE: If placing a second endoprosthesis to provide adequate length coverage, ensure ≥ 2 cm of lined graft-to-lined graft overlap of the telescoping devices. • Take final PSG measurement and re-balloon as necessary. • Use caution to not displace a deployed GORE® VIATORR® TIPS Endoprosthesis by re-introduction of an introducer sheath or working catheter back through the endoprosthesis. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Conclusion • A large body of published data demonstrate numerous clinical advantages of GORE® VIATORR® TIPS Endoprosthesis in treatment of patients with refractory ascites and variceal bleeding. • The improvement of TIPS patency by using e. PTFE-covered stents compared to bare metal stents is maintained over time with a decreased risk of hepatic encephalopathy and a decreased risk of death. • The data demonstrate a clinical advantage of GORE® VIATORR® TIPS Endoprosthesis in treatment of patients with variceal bleeding and refractory ascites. • Considering these results, the role of GORE® VIATORR® TIPS Endoprosthesis in the management of portal hypertension should be considered. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Conclusion • A large body of published data demonstrate numerous clinical advantages of GORE® VIATORR® TIPS Endoprosthesis in treatment of patients with refractory ascites and variceal bleeding. • The improvement of TIPS patency by using e. PTFE-covered stents compared to bare metal stents is maintained over time with a decreased risk of hepatic encephalopathy and a decreased risk of death. • The data demonstrate a clinical advantage of GORE® VIATORR® TIPS Endoprosthesis in treatment of patients with variceal bleeding and refractory ascites. • Considering these results, the role of GORE® VIATORR® TIPS Endoprosthesis in the management of portal hypertension should be considered. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Animation and Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Animation and Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Deployment Animation Click to view or download animation at goremedical. com Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Deployment Animation Click to view or download animation at goremedical. com Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
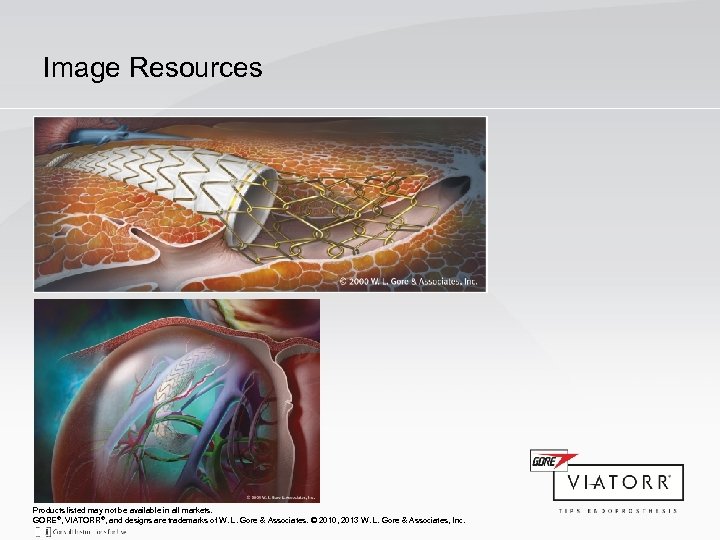 Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
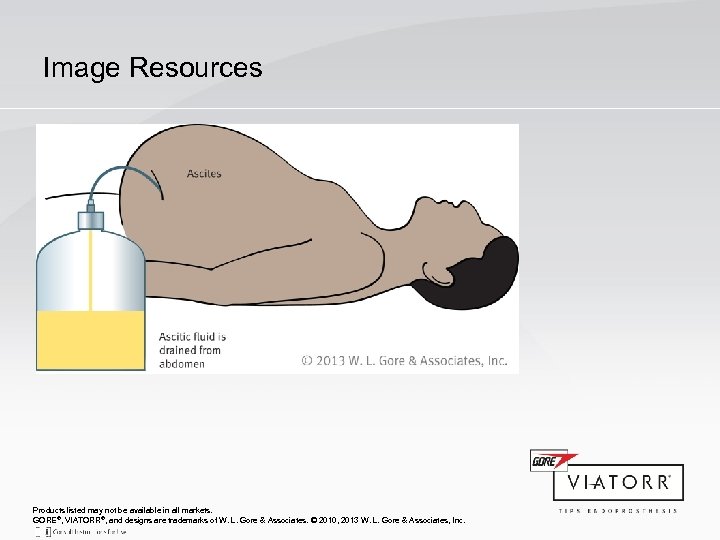 Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
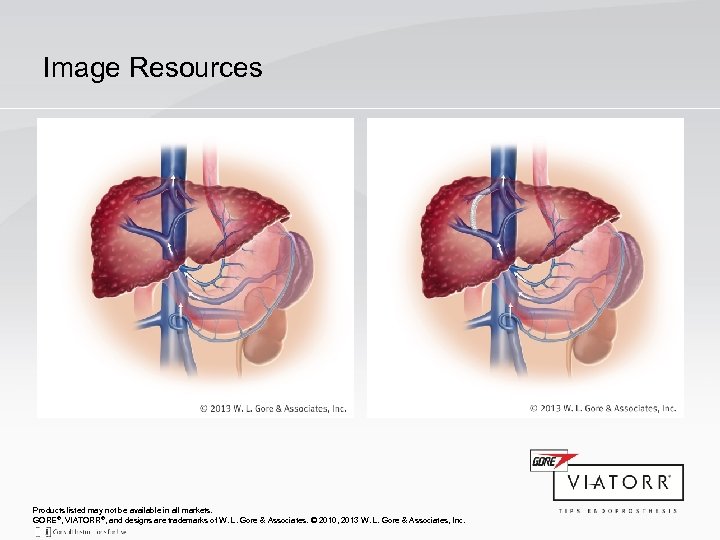 Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
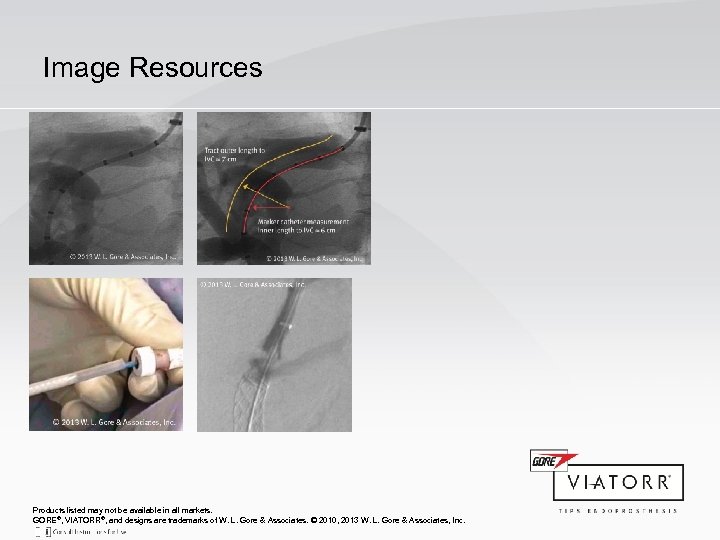 Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
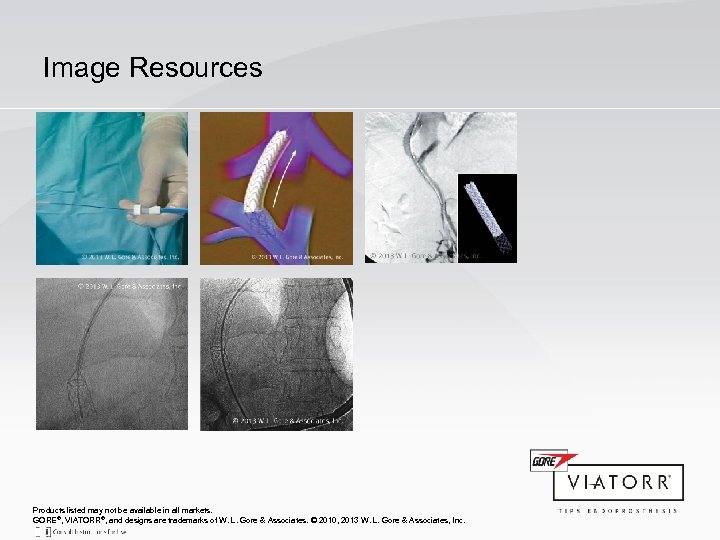 Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Image Resources Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Suggested Reading 1. Bureau C, Pagan JCG, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long term results of a randomized multicentre study. Liver International 2007; 27(6): 742 -747 2. Amarapurkar DN, Punamiya S, Patel ND. An experience with covered transjugular intrahepatic portosystemic shunt for refractory ascites from western India. Annals of Hepatology 2006; 5(2): 103 -108. 3. Rössle M, Siegerstetter V, Euringer W, et al. The use of a polytetrafluoroethylene-covered stent graft for transjugular intrahepatic portosystemic shunt (TIPS): long-term follow-up of 100 patients. Acta Radiologica 2006; 47(7): 660 -666. 4. Tripathi D, Ferguston J, Barkell H, et al. Improved clinical outcome with transjugular intrahepatic portosystemic stent-shunt utilizing polytetrafluoroethylenecovered stents. European Journal of Gastroenterology & Hepatology 2006; 18(3): 225 -232. 5. José Barrio, Cristina Ripoll, Rafael Bañares, et al. Comparison of transjugular intrahepatic portosystemic shunt dysfunction in PTFE-covered stent-grafts versus bare stents. European Journal of Radiology 2005; 55(1): 120 -124. 6. Echenagusia M, Rodriguez-Rosales G, Simo G, Camuñez F, Bañares R, Echenagusia A. Expanded PTFE-covered stent-grafts in the treatment of transjugular portosystemic shunt (TIPS) stenosis and occlusions. Abdominal Imaging 2005; 30(6): 750 -754. 7. Vignali C, Bargellini I, Grosso M, et al. TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. AJR. American Journal of Roentgenology 2005; 185(2): 472 -480. 8. Angeloni S, Merli M, Salvatori M, et al. Polytetrafluoroethylene-covered stent grafts for TIPS procedure: 1 year-patency and clinical results. American Journal of Gastroenterology 2004; 99(2): 280 -285. 9. Charon J-P M, Alaeddin FH, Pimpalwar SA, et al. Results of a retrospective multicenter trial of the Viatorr expanded polytetrafluoroethylene-covered stentgraft for transjugular intrahepatic portosystemic shunt creation. Journal of Vascular & Interventional Radiology 2004; 15(11): 1219 -1230. 10. Hausegger KA, Karnel F, Georgieva B, et al. Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylenecovered stent-graft. Journal of Vascular & Interventional Radiology 2004; 15(3): 239 -248. 11. Maleux G, Nevens F, Wilmer A, et al. Early and long-term clinical and radiological follow-up results of expanded-polytetrafluoroethylene-covered stentgrafts for transjugular intrahepatic portosystemic shunt procedures. European Radiology 2004; 14(10): 1842 -1850. 12. Rossi P, Salvatori FM, Fanelli F, et al. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3 -year experience. Radiology 2004; 231(3): 820 -830. 13. Otal P, Smayra T, Bureau C, et al. Preliminary results of a new expanded-polytetrafluoroethylene-covered stent-graft for transjugular intrahepatic portosystemic shunt procedures. American Journal of Roentgenology 2002; 178(1): 141 -147. 14. Cejna M, Peck-Radosavljevic M, Thurnher SA, Hittmair K, Schoder M, Lammer J. Creation of transjugular intrahepatic portosystemic shunts with stent-grafts: initial experiences with a polytetrafluoroethylene-covered nitinol endoprosthesis. Radiology 2001; 221(2): 437 -446. 15. Barange K, Péron JM, Imani K, et al. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology 1999; 30(5): 1139 -1143. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Suggested Reading 1. Bureau C, Pagan JCG, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long term results of a randomized multicentre study. Liver International 2007; 27(6): 742 -747 2. Amarapurkar DN, Punamiya S, Patel ND. An experience with covered transjugular intrahepatic portosystemic shunt for refractory ascites from western India. Annals of Hepatology 2006; 5(2): 103 -108. 3. Rössle M, Siegerstetter V, Euringer W, et al. The use of a polytetrafluoroethylene-covered stent graft for transjugular intrahepatic portosystemic shunt (TIPS): long-term follow-up of 100 patients. Acta Radiologica 2006; 47(7): 660 -666. 4. Tripathi D, Ferguston J, Barkell H, et al. Improved clinical outcome with transjugular intrahepatic portosystemic stent-shunt utilizing polytetrafluoroethylenecovered stents. European Journal of Gastroenterology & Hepatology 2006; 18(3): 225 -232. 5. José Barrio, Cristina Ripoll, Rafael Bañares, et al. Comparison of transjugular intrahepatic portosystemic shunt dysfunction in PTFE-covered stent-grafts versus bare stents. European Journal of Radiology 2005; 55(1): 120 -124. 6. Echenagusia M, Rodriguez-Rosales G, Simo G, Camuñez F, Bañares R, Echenagusia A. Expanded PTFE-covered stent-grafts in the treatment of transjugular portosystemic shunt (TIPS) stenosis and occlusions. Abdominal Imaging 2005; 30(6): 750 -754. 7. Vignali C, Bargellini I, Grosso M, et al. TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. AJR. American Journal of Roentgenology 2005; 185(2): 472 -480. 8. Angeloni S, Merli M, Salvatori M, et al. Polytetrafluoroethylene-covered stent grafts for TIPS procedure: 1 year-patency and clinical results. American Journal of Gastroenterology 2004; 99(2): 280 -285. 9. Charon J-P M, Alaeddin FH, Pimpalwar SA, et al. Results of a retrospective multicenter trial of the Viatorr expanded polytetrafluoroethylene-covered stentgraft for transjugular intrahepatic portosystemic shunt creation. Journal of Vascular & Interventional Radiology 2004; 15(11): 1219 -1230. 10. Hausegger KA, Karnel F, Georgieva B, et al. Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylenecovered stent-graft. Journal of Vascular & Interventional Radiology 2004; 15(3): 239 -248. 11. Maleux G, Nevens F, Wilmer A, et al. Early and long-term clinical and radiological follow-up results of expanded-polytetrafluoroethylene-covered stentgrafts for transjugular intrahepatic portosystemic shunt procedures. European Radiology 2004; 14(10): 1842 -1850. 12. Rossi P, Salvatori FM, Fanelli F, et al. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3 -year experience. Radiology 2004; 231(3): 820 -830. 13. Otal P, Smayra T, Bureau C, et al. Preliminary results of a new expanded-polytetrafluoroethylene-covered stent-graft for transjugular intrahepatic portosystemic shunt procedures. American Journal of Roentgenology 2002; 178(1): 141 -147. 14. Cejna M, Peck-Radosavljevic M, Thurnher SA, Hittmair K, Schoder M, Lammer J. Creation of transjugular intrahepatic portosystemic shunts with stent-grafts: initial experiences with a polytetrafluoroethylene-covered nitinol endoprosthesis. Radiology 2001; 221(2): 437 -446. 15. Barange K, Péron JM, Imani K, et al. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology 1999; 30(5): 1139 -1143. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
 Suggested Reading 16. Borsa JJ, Fontaine AB, Hoffer EK, et al. Retrospective comparison of the patency of Wallstents and Palmaz long-medium stents used for TIPS. Transjugular intrahepatic portosystemic shunts. Cardiovascular & Interventional Radiology 2000; 23(5): 332 -339. 17. Clark TWI, Agarwal R, Haskal ZJ, Stavropoulos SW. The effect of initial shunt outflow position on patency of transjugular intrahepatic portosystemic shunts. Journal of Vascular & Interventional Radiology 2004; 15(2)Part 1: 147 -152. 18. Escorsell A, Bañares R, García-Pagán JC, et al. TIPS versus drug therapy in preventing variceal rebleeding in advanced cirrhosis: a randomized controlled trial. Hepatology 2002; 35(2): 385 -392. 19. Patel NH, Sasadeusz KJ, Seshadri R, et al. Increase in hepatic arterial blood flow after transjugular intrahepatic portosystemic shunt creation and its potential predictive value of postprocedural encephalopathy and mortality. Journal of Vascular & Interventional Radiology 2001; 12(11): 1279 -1284. 20. Péron JM, Barange K, Otal P, et al. Transjugular intrahepatic portosystemic shunts in the treatment of refractory ascites: results in 48 consecutive patients. Journal of Vascular & Interventional Radiology 2000; 11(9): 1211 -1216. 21. Shibata D, Brophy DP, Gordon FD, Anastopoulos HT, Sentovich SM, Bleday R. Transjugular intrahepatic portosystemic shunt for treatment of bleeding ectopic varices with portal hypertension. Diseases of the Colon & Rectum 1999; 42(12): 1581 -1585. 22. ter Borg PC, Hollemans M, Van Buuren HR, et al. Transjugular intrahepatic portosystemic shunts: long-term patency and clinical results in a patient cohort observed for 3 -9 years. Radiology 2004; 231(2): 537 -545. 23. Tesdal IK, Filser T, Weiss C, Holm E, Dueber C, Jaschke W. Transjugular intrahepatic portosystemic shunts: adjunctive embolotherapy of gastroesophageal collateral vessels in the prevention of variceal rebleeding. Radiology 2005; 236(1): 360 -367. 24. Tripathi D, Helmy A, Macbeth K, et al. Ten years’ follow-up of 472 patients following transjugular intrahepatic portosystemic stent-shunt insertion at a single centre. European Journal of Gastroenterology & Hepatology 2004; 16(1): 9 -18. 25. Zhuang ZW, Teng GJ, Jeffery RF, Gemery JM, Janne d’Othee B, Bettmann MA. Long-term results and quality of life in patients treated with transjugular intrahepatic portosystemic shunts. AJR. American Journal of Roentgenology 2002; 179(6): 1597 -1603. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Suggested Reading 16. Borsa JJ, Fontaine AB, Hoffer EK, et al. Retrospective comparison of the patency of Wallstents and Palmaz long-medium stents used for TIPS. Transjugular intrahepatic portosystemic shunts. Cardiovascular & Interventional Radiology 2000; 23(5): 332 -339. 17. Clark TWI, Agarwal R, Haskal ZJ, Stavropoulos SW. The effect of initial shunt outflow position on patency of transjugular intrahepatic portosystemic shunts. Journal of Vascular & Interventional Radiology 2004; 15(2)Part 1: 147 -152. 18. Escorsell A, Bañares R, García-Pagán JC, et al. TIPS versus drug therapy in preventing variceal rebleeding in advanced cirrhosis: a randomized controlled trial. Hepatology 2002; 35(2): 385 -392. 19. Patel NH, Sasadeusz KJ, Seshadri R, et al. Increase in hepatic arterial blood flow after transjugular intrahepatic portosystemic shunt creation and its potential predictive value of postprocedural encephalopathy and mortality. Journal of Vascular & Interventional Radiology 2001; 12(11): 1279 -1284. 20. Péron JM, Barange K, Otal P, et al. Transjugular intrahepatic portosystemic shunts in the treatment of refractory ascites: results in 48 consecutive patients. Journal of Vascular & Interventional Radiology 2000; 11(9): 1211 -1216. 21. Shibata D, Brophy DP, Gordon FD, Anastopoulos HT, Sentovich SM, Bleday R. Transjugular intrahepatic portosystemic shunt for treatment of bleeding ectopic varices with portal hypertension. Diseases of the Colon & Rectum 1999; 42(12): 1581 -1585. 22. ter Borg PC, Hollemans M, Van Buuren HR, et al. Transjugular intrahepatic portosystemic shunts: long-term patency and clinical results in a patient cohort observed for 3 -9 years. Radiology 2004; 231(2): 537 -545. 23. Tesdal IK, Filser T, Weiss C, Holm E, Dueber C, Jaschke W. Transjugular intrahepatic portosystemic shunts: adjunctive embolotherapy of gastroesophageal collateral vessels in the prevention of variceal rebleeding. Radiology 2005; 236(1): 360 -367. 24. Tripathi D, Helmy A, Macbeth K, et al. Ten years’ follow-up of 472 patients following transjugular intrahepatic portosystemic stent-shunt insertion at a single centre. European Journal of Gastroenterology & Hepatology 2004; 16(1): 9 -18. 25. Zhuang ZW, Teng GJ, Jeffery RF, Gemery JM, Janne d’Othee B, Bettmann MA. Long-term results and quality of life in patients treated with transjugular intrahepatic portosystemic shunts. AJR. American Journal of Roentgenology 2002; 179(6): 1597 -1603. Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
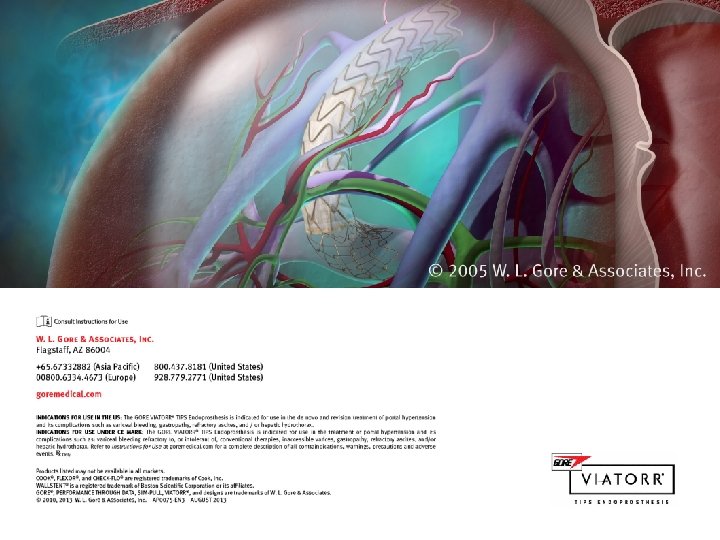 Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.
Products listed may not be available in all markets. GORE®, VIATORR®, and designs are trademarks of W. L. Gore & Associates. © 2010, 2013 W. L. Gore & Associates, Inc.


