afb5b0cfdf3fa4ce32c34a4e7848b05d.ppt
- Количество слайдов: 17

Unit Processes of Air Pollution Control Benno Rahardyan

References • Calvert, S. , Englund, H. M, Handbook of Air Pollution Technology, Willey-Interscience Publication, (Chapter 9) • Peavy, H. S. , Rowe, D. R, Tchobanoglous, G. , 1985, Environmental Engineering. International Edition, Mc. Graw. Hill, Singapore (Chapter 9)

• Control of Particulate Contaminants • Control of Gaseous Contaminant

Control of Particulate Contaminants • • • Gravitation Settling Chamber Centrifugal Collector Wet Collectors Fabric Filters (Baghouse filters) Electrostatic Precipitation (ESP)
Control of Gaseous Contaminant • • Adsorption Absorption Condensation Combustion
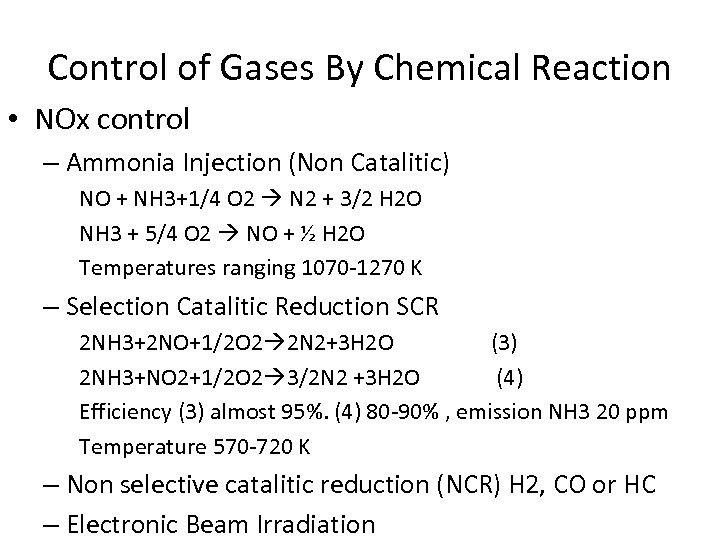
Control of Gases By Chemical Reaction • NOx control – Ammonia Injection (Non Catalitic) NO + NH 3+1/4 O 2 N 2 + 3/2 H 2 O NH 3 + 5/4 O 2 NO + ½ H 2 O Temperatures ranging 1070 -1270 K – Selection Catalitic Reduction SCR 2 NH 3+2 NO+1/2 O 2 2 N 2+3 H 2 O (3) 2 NH 3+NO 2+1/2 O 2 3/2 N 2 +3 H 2 O (4) Efficiency (3) almost 95%. (4) 80 -90% , emission NH 3 20 ppm Temperature 570 -720 K – Non selective catalitic reduction (NCR) H 2, CO or HC – Electronic Beam Irradiation
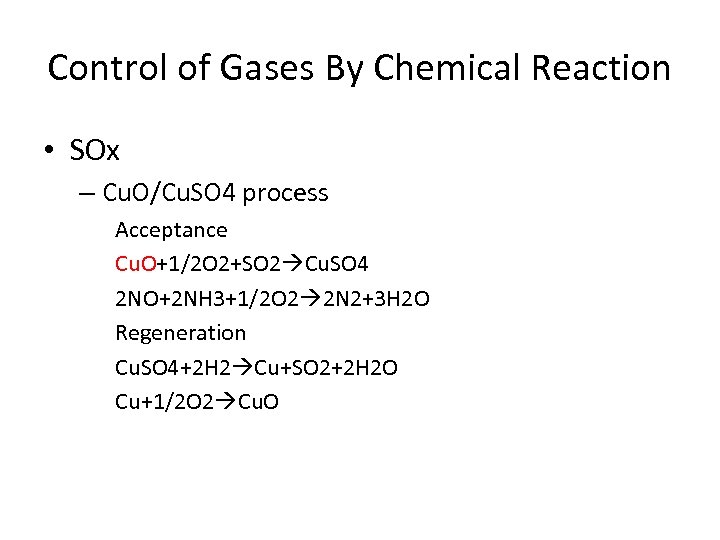
Control of Gases By Chemical Reaction • SOx – Cu. O/Cu. SO 4 process Acceptance Cu. O+1/2 O 2+SO 2 Cu. SO 4 2 NO+2 NH 3+1/2 O 2 2 N 2+3 H 2 O Regeneration Cu. SO 4+2 H 2 Cu+SO 2+2 H 2 O Cu+1/2 O 2 Cu. O
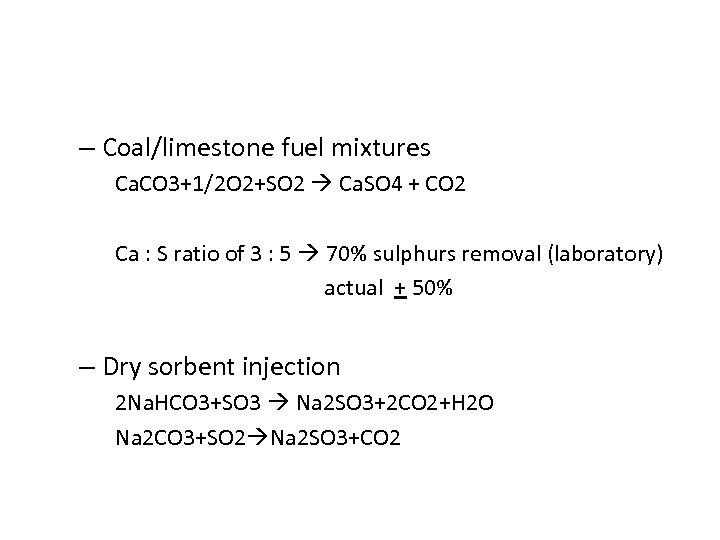
– Coal/limestone fuel mixtures Ca. CO 3+1/2 O 2+SO 2 Ca. SO 4 + CO 2 Ca : S ratio of 3 : 5 70% sulphurs removal (laboratory) actual + 50% – Dry sorbent injection 2 Na. HCO 3+SO 3 Na 2 SO 3+2 CO 2+H 2 O Na 2 CO 3+SO 2 Na 2 SO 3+CO 2


• Determining the solvent requirement in an absorption tower. • An absorption tower is to be used to remove SO 2 from the stack gas of a coal-fired furnace. The flow rate of the stack gas, measured at 1 atm pressure and 25 o. C, is 10 m 3/s and the SO 2 content is 3 percent by volume. Removal of 90% of the SO 2 is required and water initially pure with the respect of SO 2 is to be used as the liquid solvent. The equilibrium line for SO 2 and water can be estimated by y = 30 x. Determine the flow rate of water that represents 150 percent of the minimum liquid requirement.

• The gas flow (G’ + ammonia) is 50 kg-mol/h and is 10 percent by volume ammonia. Water that is initally pure with respect to ammonia is used as the solvent. Determine the water flow rate that is 1. 3 times the minimum required to remove 96 percent of ammonia.

• 450 kg/hour of a mixture of ethyl-methyl – ketone (butanone) in air (1. 5 mol percent) is to be passed through a countercurrent absorber. The equilbirium equation for butanone in water is y = 2, 5 x. Assume that the gas and water do not react chemically. What is the minimum flow of water-free butanone that must be used to absorb 95% percent of butanone? .


• A well water is to be softened by split-flow ion exchange with the exchanger on the sodium cycle. The flow is 3. 2 l/s, the hardness is 225 mg/L as Ca. CO 3, the desired hardness is 50 mg/L Ca. CO 3, and the moisture content of the resin is 45 percent. A test column has been used in thelaboratory to obtain a breakthrough curve. The computed hardness removed by the resin at the allowable breakthrough concentration Ca=0, 05 Co was 282 meq/100 g resin on a dry weight basis. Determine the kilograms of resin required if the allowable breakthrough is 7 days.

• A well water is to be softened by split flow ion exange with the exchanger resin on the sodium cycle. The flow is 2. 56 l/s. The hardness is 195 mg/l as Ca. CO 3. The desired hardness is 50 mg/l as Ca. CO 3, the resin removes 296 meq/100 gram on a dry basis at an allowable breaktrough of 5% Co, and moisture content is 45 %. The allowable breakthrough time is seven days. Determine the weight of resin required on both moist and dry basis

• An industrial wastewater with 107 mg/L of Cu 2+ (3. 37 meq/L) is to be treated by an exchange column. The allowable effluent concentration is 5 percent Co. A bereakthrough curve has been obtained from anexpereimental laboratory column on the sodium cycle. Determine the k value of the column.

• Data concerning the column are : inside diameter 1, 25 cm, length = 45 cm masss of resin = 41, 5 g on moist basis (23. 24 g on dry basis), moisture = 44%, bulk density 20 kg/ft 3 and liquid flow rate = 1, 0428 l/day. • The design will have flowrate of 100, 000 gal/day, the allowable breakthrough time is seven days of flow, and the resin depth is approximately two timesthe column diameter. Using the kinetic approach to column design determine: 1. resin required 2. the diameter and depth 3. the height of sorption zone
afb5b0cfdf3fa4ce32c34a4e7848b05d.ppt