6f5dd88a93a5f445862cc3dcd8797ed2.ppt
- Количество слайдов: 39
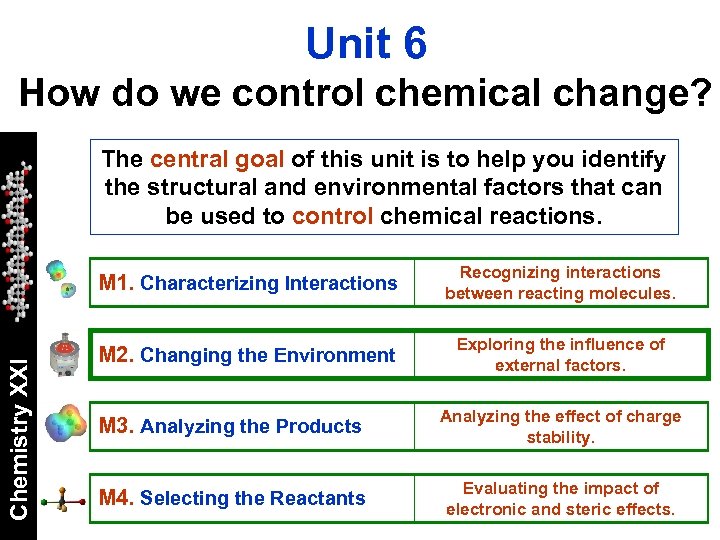 Unit 6 How do we control chemical change? The central goal of this unit is to help you identify the structural and environmental factors that can be used to control chemical reactions. Chemistry XXI M 1. Characterizing Interactions Recognizing interactions between reacting molecules. M 2. Changing the Environment Exploring the influence of. external factors. M 3. Analyzing the Products Analyzing the effect of charge stability. M 4. Selecting the Reactants Evaluating the impact of electronic and steric effects.
Unit 6 How do we control chemical change? The central goal of this unit is to help you identify the structural and environmental factors that can be used to control chemical reactions. Chemistry XXI M 1. Characterizing Interactions Recognizing interactions between reacting molecules. M 2. Changing the Environment Exploring the influence of. external factors. M 3. Analyzing the Products Analyzing the effect of charge stability. M 4. Selecting the Reactants Evaluating the impact of electronic and steric effects.
 Unit 6 How do we control chemical change? Chemistry XXI Module 2: Changing the Environment Central goal: To analyze the effect concentration, temperature, and nature of the solvent on reaction extent.
Unit 6 How do we control chemical change? Chemistry XXI Module 2: Changing the Environment Central goal: To analyze the effect concentration, temperature, and nature of the solvent on reaction extent.
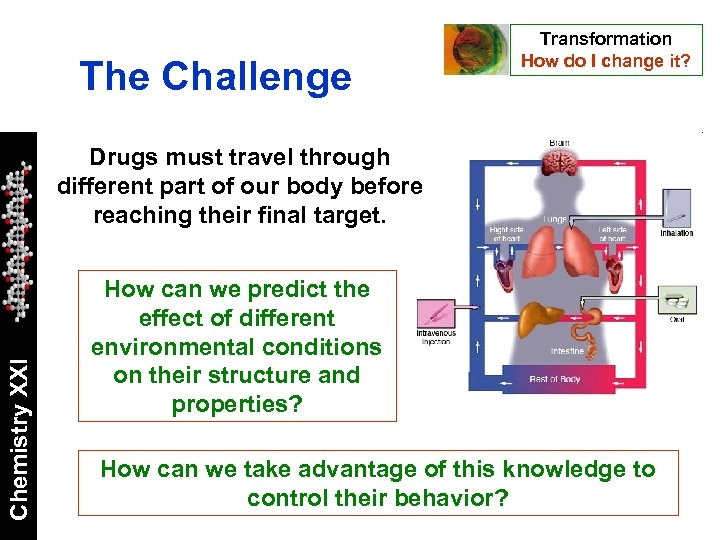 The Challenge Transformation How do I change it? Chemistry XXI Drugs must travel through different part of our body before reaching their final target. How can we predict the effect of different environmental conditions on their structure and properties? How can we take advantage of this knowledge to control their behavior?
The Challenge Transformation How do I change it? Chemistry XXI Drugs must travel through different part of our body before reaching their final target. How can we predict the effect of different environmental conditions on their structure and properties? How can we take advantage of this knowledge to control their behavior?
 Reaction Control Chemistry XXI The extent (Thermodynamics) and rate (Kinetics) to which a substance, like an drug, reacts with another, like water, depends on the environmental conditions. We can affect and control chemical reactions by changing the concentration of reactants and products, the temperature and pressure of the surroundings, or the nature of the solvent in which the process takes place.
Reaction Control Chemistry XXI The extent (Thermodynamics) and rate (Kinetics) to which a substance, like an drug, reacts with another, like water, depends on the environmental conditions. We can affect and control chemical reactions by changing the concentration of reactants and products, the temperature and pressure of the surroundings, or the nature of the solvent in which the process takes place.
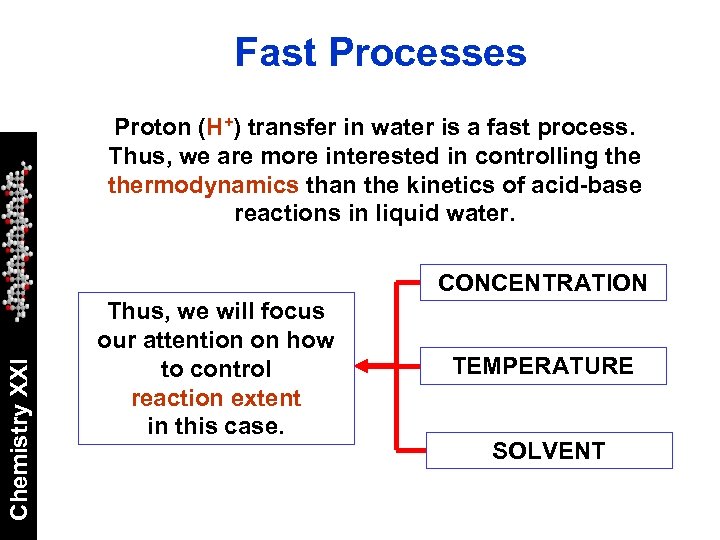 Fast Processes Proton (H+) transfer in water is a fast process. Thus, we are more interested in controlling thermodynamics than the kinetics of acid-base reactions in liquid water. Chemistry XXI CONCENTRATION Thus, we will focus our attention on how to control reaction extent in this case. TEMPERATURE SOLVENT
Fast Processes Proton (H+) transfer in water is a fast process. Thus, we are more interested in controlling thermodynamics than the kinetics of acid-base reactions in liquid water. Chemistry XXI CONCENTRATION Thus, we will focus our attention on how to control reaction extent in this case. TEMPERATURE SOLVENT
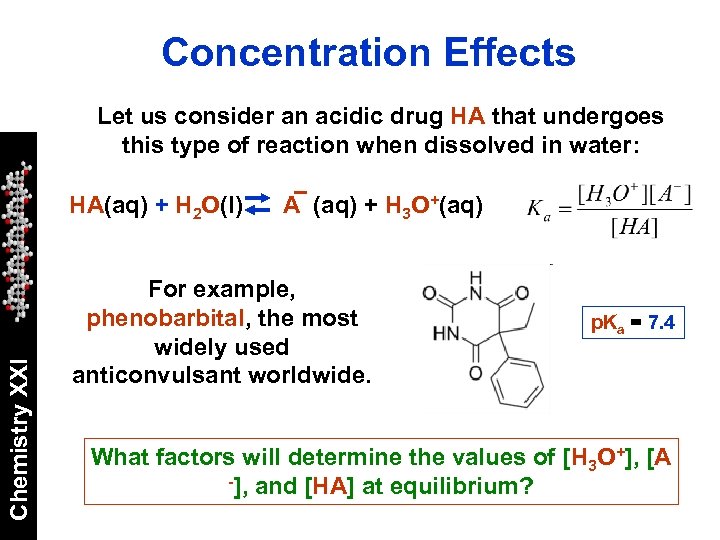 Concentration Effects Let us consider an acidic drug HA that undergoes this type of reaction when dissolved in water: Chemistry XXI HA(aq) + H 2 O(l) A (aq) + H 3 O+(aq) For example, phenobarbital, the most widely used anticonvulsant worldwide. p. Ka = 7. 4 What factors will determine the values of [H 3 O+], [A -], and [HA] at equilibrium?
Concentration Effects Let us consider an acidic drug HA that undergoes this type of reaction when dissolved in water: Chemistry XXI HA(aq) + H 2 O(l) A (aq) + H 3 O+(aq) For example, phenobarbital, the most widely used anticonvulsant worldwide. p. Ka = 7. 4 What factors will determine the values of [H 3 O+], [A -], and [HA] at equilibrium?
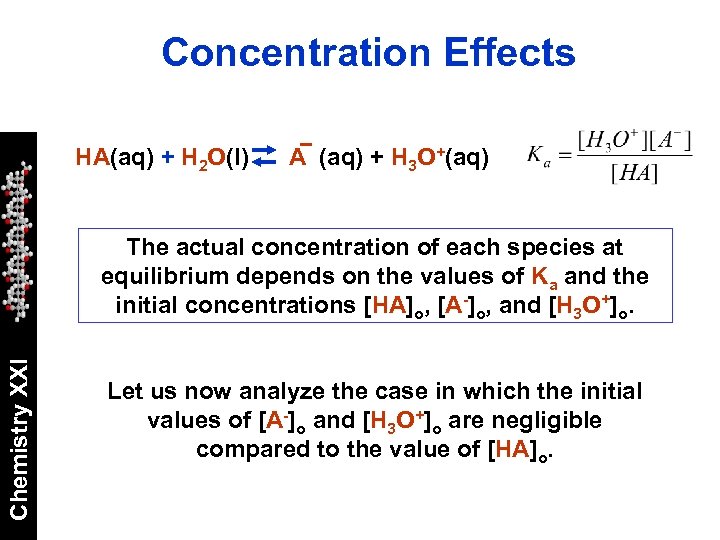 Concentration Effects HA(aq) + H 2 O(l) A (aq) + H 3 O+(aq) Chemistry XXI The actual concentration of each species at equilibrium depends on the values of Ka and the initial concentrations [HA]o, [A-]o, and [H 3 O+]o. Let us now analyze the case in which the initial values of [A-]o and [H 3 O+]o are negligible compared to the value of [HA]o.
Concentration Effects HA(aq) + H 2 O(l) A (aq) + H 3 O+(aq) Chemistry XXI The actual concentration of each species at equilibrium depends on the values of Ka and the initial concentrations [HA]o, [A-]o, and [H 3 O+]o. Let us now analyze the case in which the initial values of [A-]o and [H 3 O+]o are negligible compared to the value of [HA]o.
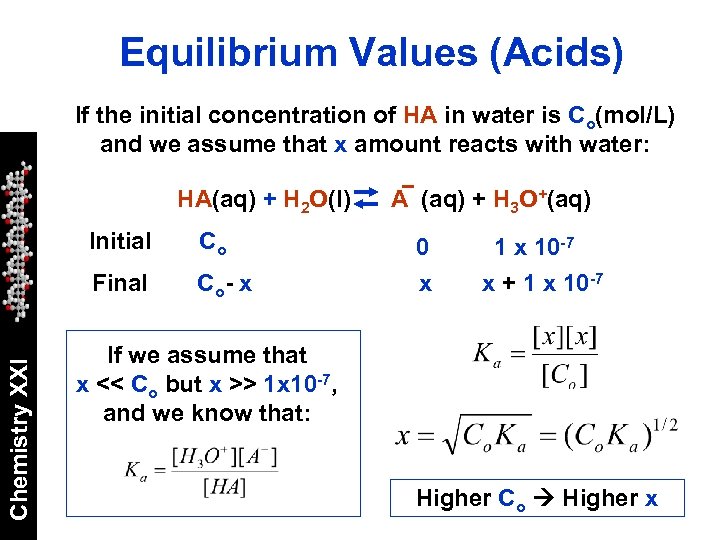 Equilibrium Values (Acids) If the initial concentration of HA in water is Co(mol/L) and we assume that x amount reacts with water: HA(aq) + H 2 O(l) Co Final Chemistry XXI Initial Co - x A (aq) + H 3 O+(aq) 0 x 10 -7 x + 1 x 10 -7 If we assume that x << Co but x >> 1 x 10 -7, and we know that: Higher Co Higher x
Equilibrium Values (Acids) If the initial concentration of HA in water is Co(mol/L) and we assume that x amount reacts with water: HA(aq) + H 2 O(l) Co Final Chemistry XXI Initial Co - x A (aq) + H 3 O+(aq) 0 x 10 -7 x + 1 x 10 -7 If we assume that x << Co but x >> 1 x 10 -7, and we know that: Higher Co Higher x
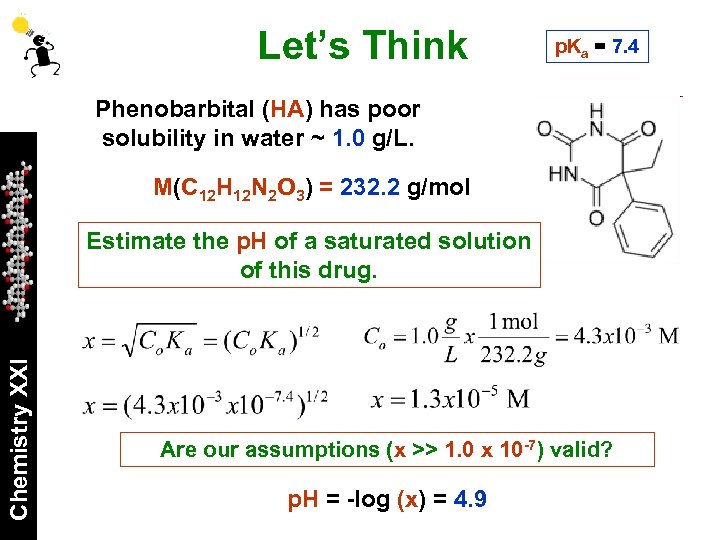 Let’s Think p. Ka = 7. 4 Phenobarbital (HA) has poor solubility in water ~ 1. 0 g/L. M(C 12 H 12 N 2 O 3) = 232. 2 g/mol Chemistry XXI Estimate the p. H of a saturated solution of this drug. Are our assumptions (x >> 1. 0 x 10 -7) valid? p. H = -log (x) = 4. 9
Let’s Think p. Ka = 7. 4 Phenobarbital (HA) has poor solubility in water ~ 1. 0 g/L. M(C 12 H 12 N 2 O 3) = 232. 2 g/mol Chemistry XXI Estimate the p. H of a saturated solution of this drug. Are our assumptions (x >> 1. 0 x 10 -7) valid? p. H = -log (x) = 4. 9
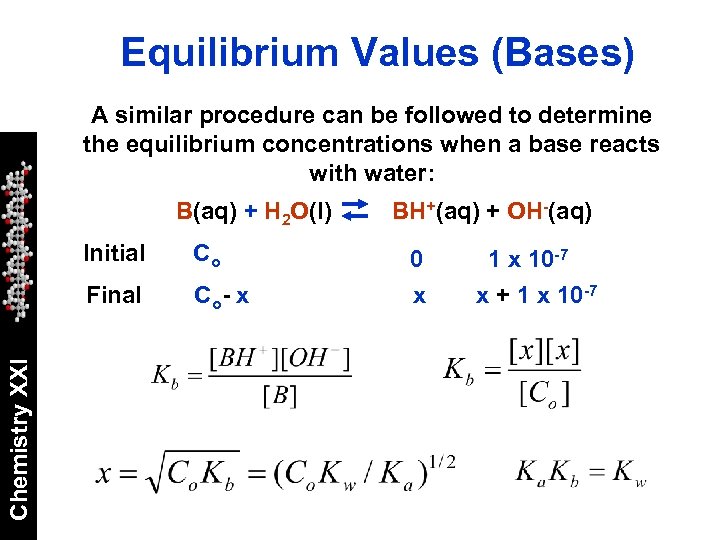 Equilibrium Values (Bases) A similar procedure can be followed to determine the equilibrium concentrations when a base reacts with water: B(aq) + H 2 O(l) Co Final Chemistry XXI Initial Co - x BH+(aq) + OH-(aq) 0 x 10 -7 x + 1 x 10 -7
Equilibrium Values (Bases) A similar procedure can be followed to determine the equilibrium concentrations when a base reacts with water: B(aq) + H 2 O(l) Co Final Chemistry XXI Initial Co - x BH+(aq) + OH-(aq) 0 x 10 -7 x + 1 x 10 -7
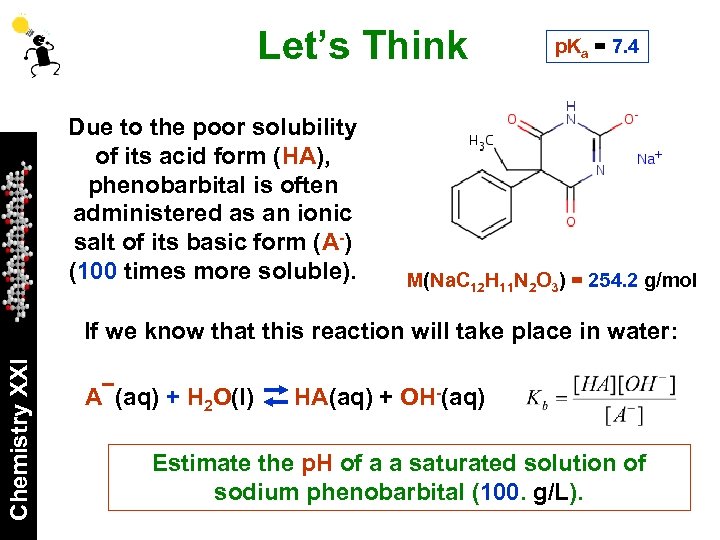 Let’s Think Due to the poor solubility of its acid form (HA), phenobarbital is often administered as an ionic salt of its basic form (A-) (100 times more soluble). p. Ka = 7. 4 M(Na. C 12 H 11 N 2 O 3) = 254. 2 g/mol Chemistry XXI If we know that this reaction will take place in water: A (aq) + H 2 O(l) HA(aq) + OH-(aq) Estimate the p. H of a a saturated solution of sodium phenobarbital (100. g/L).
Let’s Think Due to the poor solubility of its acid form (HA), phenobarbital is often administered as an ionic salt of its basic form (A-) (100 times more soluble). p. Ka = 7. 4 M(Na. C 12 H 11 N 2 O 3) = 254. 2 g/mol Chemistry XXI If we know that this reaction will take place in water: A (aq) + H 2 O(l) HA(aq) + OH-(aq) Estimate the p. H of a a saturated solution of sodium phenobarbital (100. g/L).
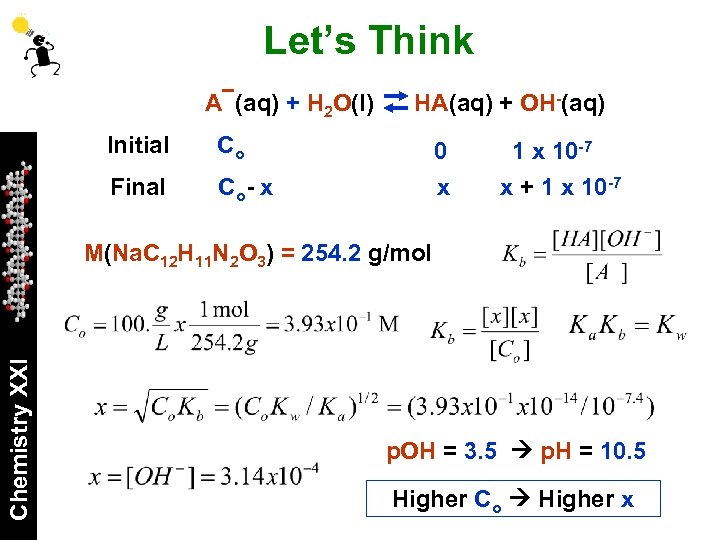 Let’s Think A (aq) + H 2 O(l) Initial Co Final HA(aq) + OH-(aq) Co - x 0 x 10 -7 x + 1 x 10 -7 Chemistry XXI M(Na. C 12 H 11 N 2 O 3) = 254. 2 g/mol p. OH = 3. 5 p. H = 10. 5 Higher Co Higher x
Let’s Think A (aq) + H 2 O(l) Initial Co Final HA(aq) + OH-(aq) Co - x 0 x 10 -7 x + 1 x 10 -7 Chemistry XXI M(Na. C 12 H 11 N 2 O 3) = 254. 2 g/mol p. OH = 3. 5 p. H = 10. 5 Higher Co Higher x
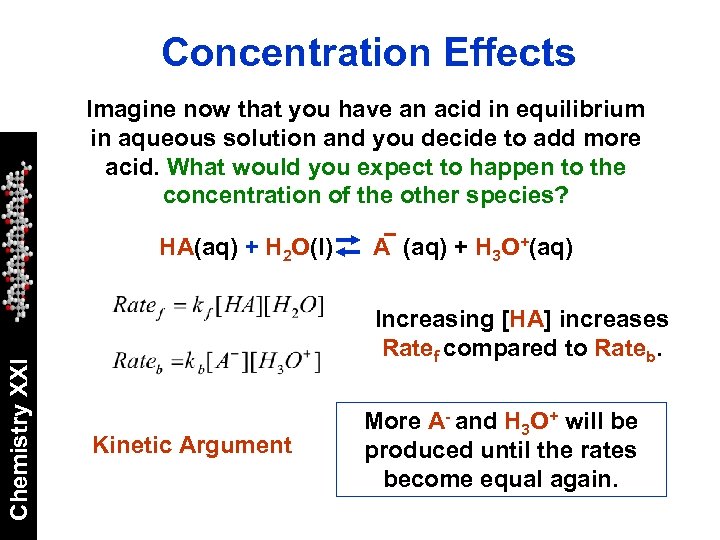 Concentration Effects Imagine now that you have an acid in equilibrium in aqueous solution and you decide to add more acid. What would you expect to happen to the concentration of the other species? Chemistry XXI HA(aq) + H 2 O(l) A (aq) + H 3 O+(aq) Increasing [HA] increases Ratef compared to Rateb. Kinetic Argument More A- and H 3 O+ will be produced until the rates become equal again.
Concentration Effects Imagine now that you have an acid in equilibrium in aqueous solution and you decide to add more acid. What would you expect to happen to the concentration of the other species? Chemistry XXI HA(aq) + H 2 O(l) A (aq) + H 3 O+(aq) Increasing [HA] increases Ratef compared to Rateb. Kinetic Argument More A- and H 3 O+ will be produced until the rates become equal again.
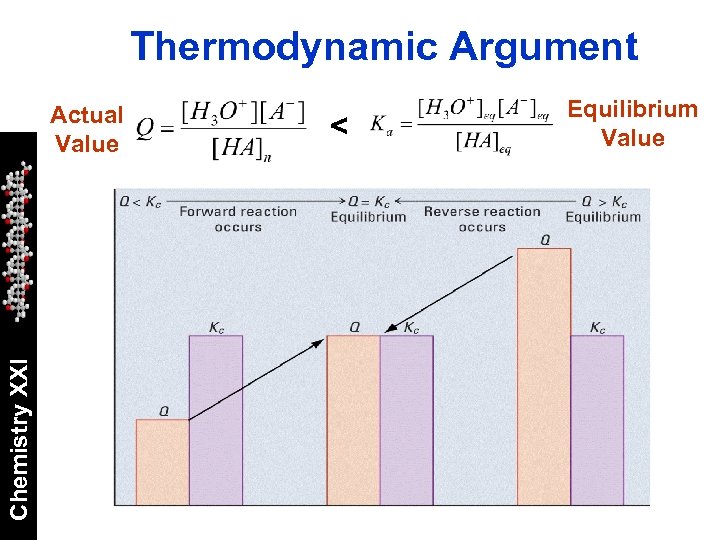 Thermodynamic Argument Chemistry XXI Actual Value < Equilibrium Value
Thermodynamic Argument Chemistry XXI Actual Value < Equilibrium Value
 Let’s Think Imagine you have a 0. 125 M aqueous solution of aspirin, an acid drug with p. Ka = 3. 5, in equilibrium. a) Estimate the p. H of the solution. Chemistry XXI b) Predict what would happen to the p. H when: v you add more A-; v you add more HA; v you add OHv you add more H O; 2 Use both, kinetic and thermodynamic arguments.
Let’s Think Imagine you have a 0. 125 M aqueous solution of aspirin, an acid drug with p. Ka = 3. 5, in equilibrium. a) Estimate the p. H of the solution. Chemistry XXI b) Predict what would happen to the p. H when: v you add more A-; v you add more HA; v you add OHv you add more H O; 2 Use both, kinetic and thermodynamic arguments.
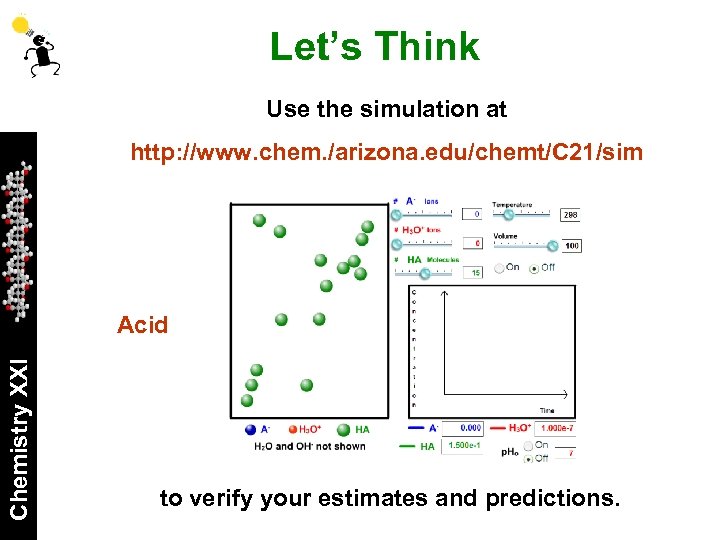 Let’s Think Use the simulation at http: //www. chem. /arizona. edu/chemt/C 21/sim Chemistry XXI Acid to verify your estimates and predictions.
Let’s Think Use the simulation at http: //www. chem. /arizona. edu/chemt/C 21/sim Chemistry XXI Acid to verify your estimates and predictions.
 Chemistry XXI Let’s Think
Chemistry XXI Let’s Think
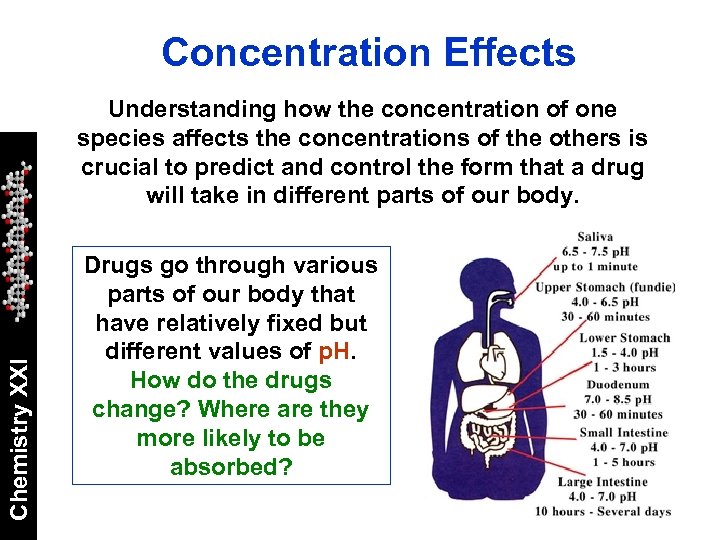 Concentration Effects Chemistry XXI Understanding how the concentration of one species affects the concentrations of the others is crucial to predict and control the form that a drug will take in different parts of our body. Drugs go through various parts of our body that have relatively fixed but different values of p. H. How do the drugs change? Where are they more likely to be absorbed?
Concentration Effects Chemistry XXI Understanding how the concentration of one species affects the concentrations of the others is crucial to predict and control the form that a drug will take in different parts of our body. Drugs go through various parts of our body that have relatively fixed but different values of p. H. How do the drugs change? Where are they more likely to be absorbed?
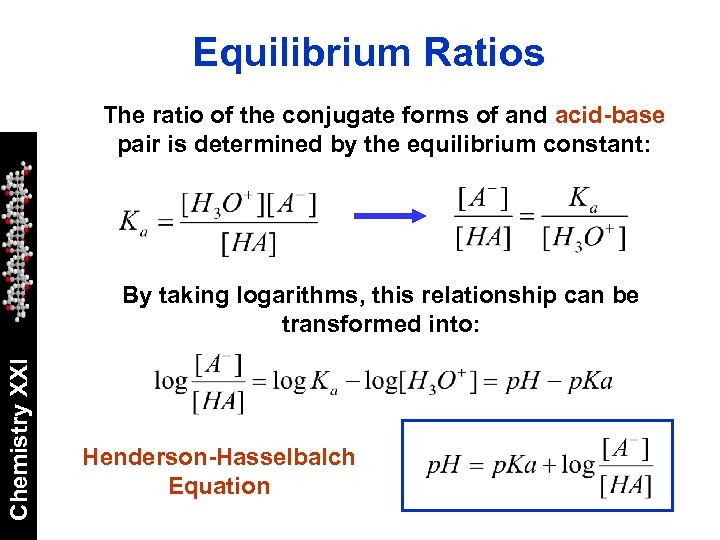 Equilibrium Ratios The ratio of the conjugate forms of and acid-base pair is determined by the equilibrium constant: Chemistry XXI By taking logarithms, this relationship can be transformed into: Henderson-Hasselbalch Equation
Equilibrium Ratios The ratio of the conjugate forms of and acid-base pair is determined by the equilibrium constant: Chemistry XXI By taking logarithms, this relationship can be transformed into: Henderson-Hasselbalch Equation
 Henderson-Hasselbalch How much of a an acid is in A- or HA form depends on the p. H of the medium where we put it. Chemistry XXI For example, [HA] = [A-] when p. H = p. Ka When analyzing drugs, it is useful to calculate the percentage of the drug that exist in acid or basic form in different parts of the body:
Henderson-Hasselbalch How much of a an acid is in A- or HA form depends on the p. H of the medium where we put it. Chemistry XXI For example, [HA] = [A-] when p. H = p. Ka When analyzing drugs, it is useful to calculate the percentage of the drug that exist in acid or basic form in different parts of the body:
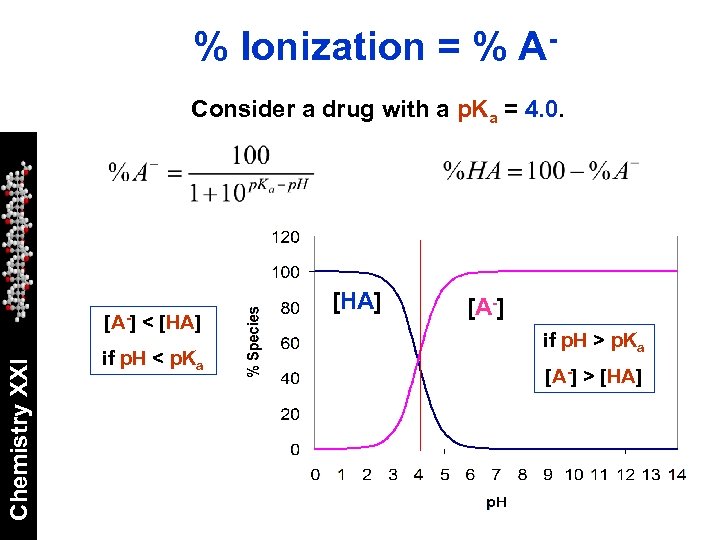 % Ionization = % AConsider a drug with a p. Ka = 4. 0. Chemistry XXI [A-] < [HA] if p. H < p. Ka [HA] [A-] if p. H > p. Ka [A-] > [HA]
% Ionization = % AConsider a drug with a p. Ka = 4. 0. Chemistry XXI [A-] < [HA] if p. H < p. Ka [HA] [A-] if p. H > p. Ka [A-] > [HA]
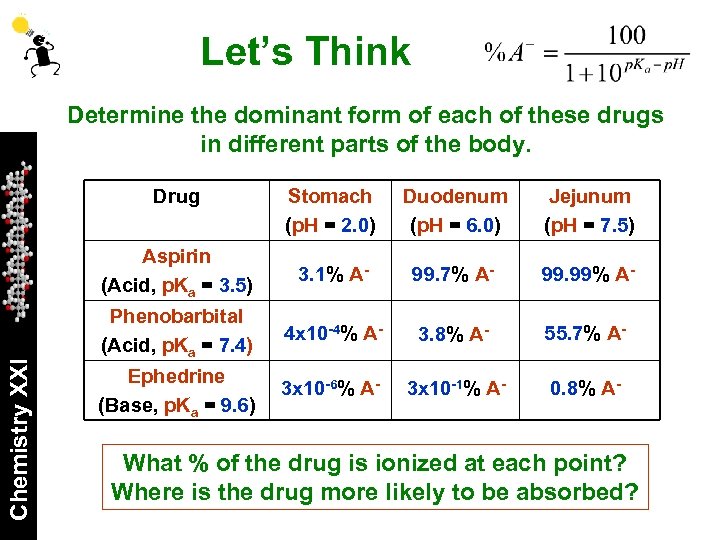 Let’s Think Determine the dominant form of each of these drugs in different parts of the body. Stomach (p. H = 2. 0) Duodenum (p. H = 6. 0) Jejunum (p. H = 7. 5) Aspirin (Acid, p. Ka = 3. 5) Chemistry XXI Drug 3. 1% A- 99. 7% A- 99. 99% A- Phenobarbital (Acid, p. Ka = 7. 4) 4 x 10 -4% A- 3. 8% A- 55. 7% A- Ephedrine (Base, p. Ka = 9. 6) 3 x 10 -6% A- 3 x 10 -1% A- 0. 8% A- What % of the drug is ionized at each point? Where is the drug more likely to be absorbed?
Let’s Think Determine the dominant form of each of these drugs in different parts of the body. Stomach (p. H = 2. 0) Duodenum (p. H = 6. 0) Jejunum (p. H = 7. 5) Aspirin (Acid, p. Ka = 3. 5) Chemistry XXI Drug 3. 1% A- 99. 7% A- 99. 99% A- Phenobarbital (Acid, p. Ka = 7. 4) 4 x 10 -4% A- 3. 8% A- 55. 7% A- Ephedrine (Base, p. Ka = 9. 6) 3 x 10 -6% A- 3 x 10 -1% A- 0. 8% A- What % of the drug is ionized at each point? Where is the drug more likely to be absorbed?
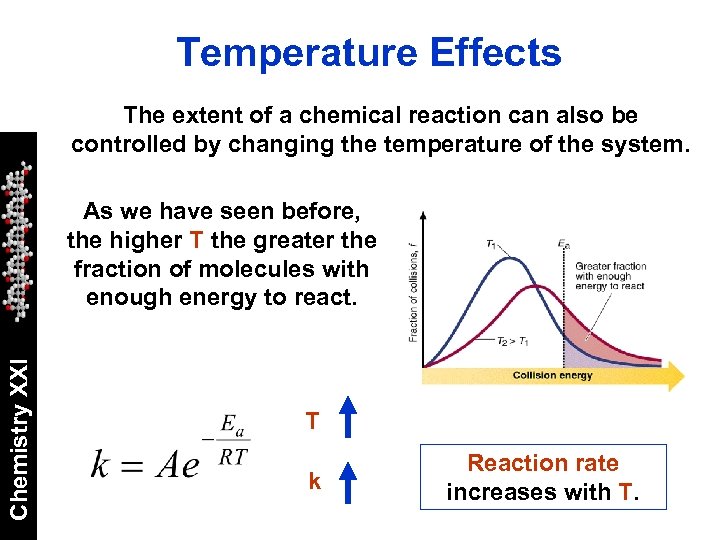 Temperature Effects The extent of a chemical reaction can also be controlled by changing the temperature of the system. Chemistry XXI As we have seen before, the higher T the greater the fraction of molecules with enough energy to react. T k Reaction rate increases with T.
Temperature Effects The extent of a chemical reaction can also be controlled by changing the temperature of the system. Chemistry XXI As we have seen before, the higher T the greater the fraction of molecules with enough energy to react. T k Reaction rate increases with T.
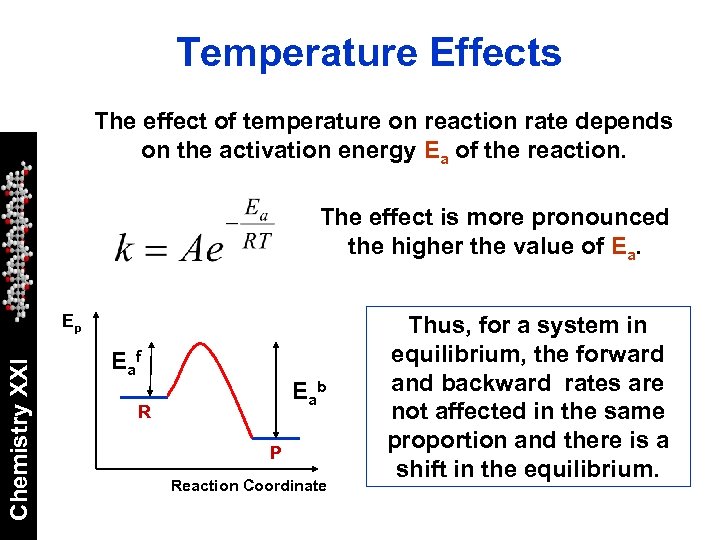 Temperature Effects The effect of temperature on reaction rate depends on the activation energy Ea of the reaction. The effect is more pronounced the higher the value of Ea. Chemistry XXI Ep E af E ab R P Reaction Coordinate Thus, for a system in equilibrium, the forward and backward rates are not affected in the same proportion and there is a shift in the equilibrium.
Temperature Effects The effect of temperature on reaction rate depends on the activation energy Ea of the reaction. The effect is more pronounced the higher the value of Ea. Chemistry XXI Ep E af E ab R P Reaction Coordinate Thus, for a system in equilibrium, the forward and backward rates are not affected in the same proportion and there is a shift in the equilibrium.
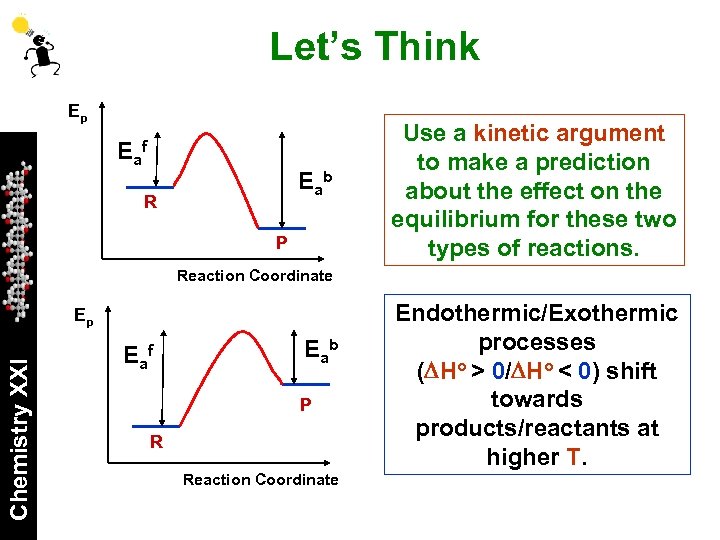 Let’s Think Ep Ea f E ab R P Use a kinetic argument to make a prediction about the effect on the equilibrium for these two types of reactions. Reaction Coordinate Chemistry XXI Ep E af E ab P R Reaction Coordinate Endothermic/Exothermic processes (DHo > 0/DHo < 0) shift towards products/reactants at higher T.
Let’s Think Ep Ea f E ab R P Use a kinetic argument to make a prediction about the effect on the equilibrium for these two types of reactions. Reaction Coordinate Chemistry XXI Ep E af E ab P R Reaction Coordinate Endothermic/Exothermic processes (DHo > 0/DHo < 0) shift towards products/reactants at higher T.
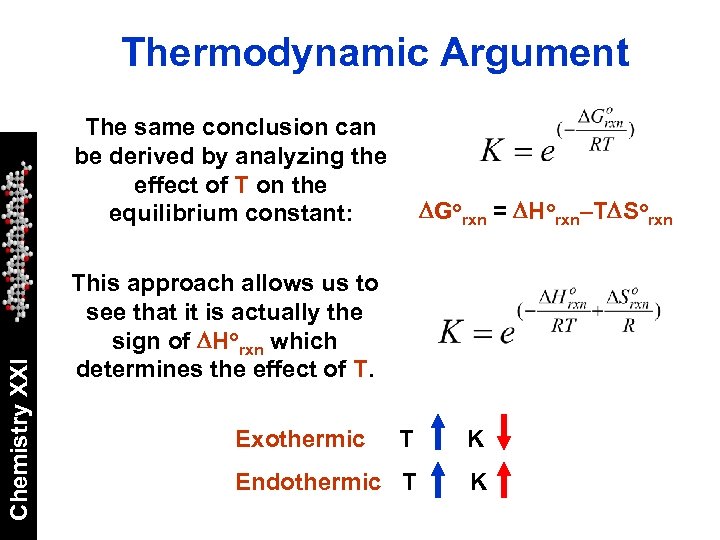 Thermodynamic Argument Chemistry XXI The same conclusion can be derived by analyzing the effect of T on the equilibrium constant: DGorxn = DHorxn–TDSorxn This approach allows us to see that it is actually the sign of DHorxn which determines the effect of T. Exothermic T K Endothermic T K
Thermodynamic Argument Chemistry XXI The same conclusion can be derived by analyzing the effect of T on the equilibrium constant: DGorxn = DHorxn–TDSorxn This approach allows us to see that it is actually the sign of DHorxn which determines the effect of T. Exothermic T K Endothermic T K
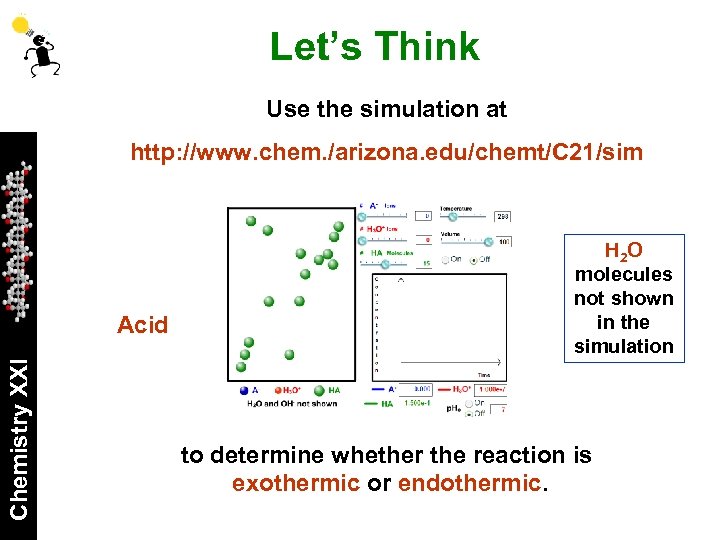 Let’s Think Use the simulation at http: //www. chem. /arizona. edu/chemt/C 21/sim Chemistry XXI Acid H 2 O molecules not shown in the simulation to determine whether the reaction is exothermic or endothermic.
Let’s Think Use the simulation at http: //www. chem. /arizona. edu/chemt/C 21/sim Chemistry XXI Acid H 2 O molecules not shown in the simulation to determine whether the reaction is exothermic or endothermic.
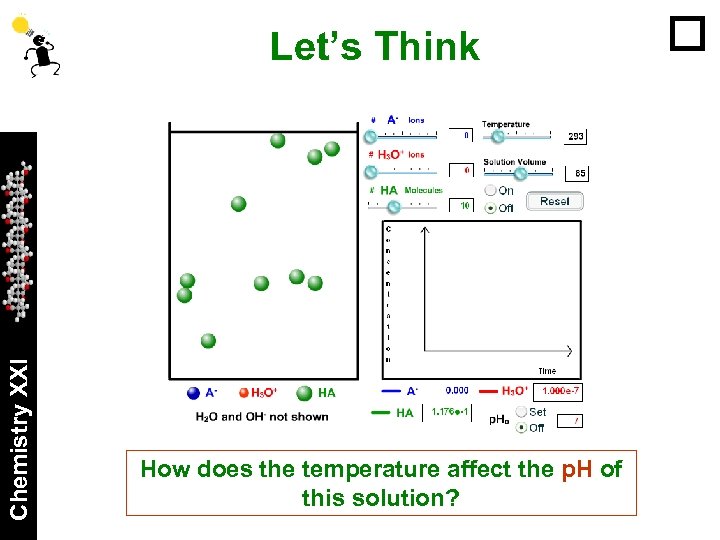 Chemistry XXI Let’s Think How does the temperature affect the p. H of this solution?
Chemistry XXI Let’s Think How does the temperature affect the p. H of this solution?
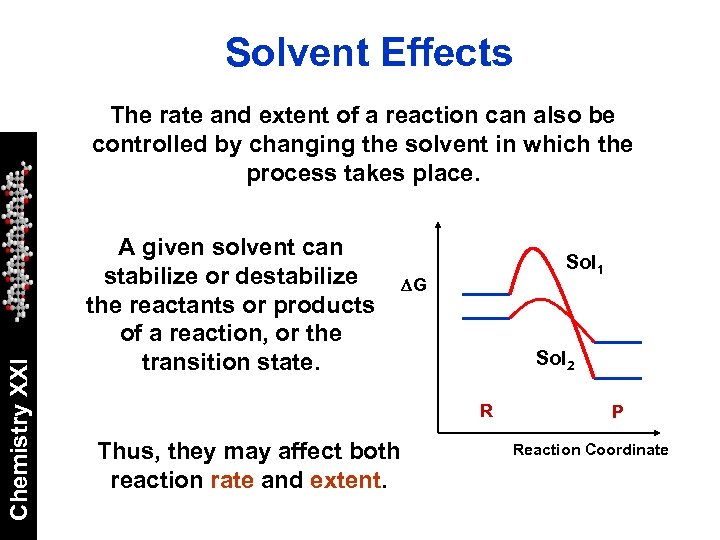 Solvent Effects Chemistry XXI The rate and extent of a reaction can also be controlled by changing the solvent in which the process takes place. A given solvent can stabilize or destabilize the reactants or products of a reaction, or the transition state. Sol 1 DG Sol 2 R Thus, they may affect both reaction rate and extent. P Reaction Coordinate
Solvent Effects Chemistry XXI The rate and extent of a reaction can also be controlled by changing the solvent in which the process takes place. A given solvent can stabilize or destabilize the reactants or products of a reaction, or the transition state. Sol 1 DG Sol 2 R Thus, they may affect both reaction rate and extent. P Reaction Coordinate
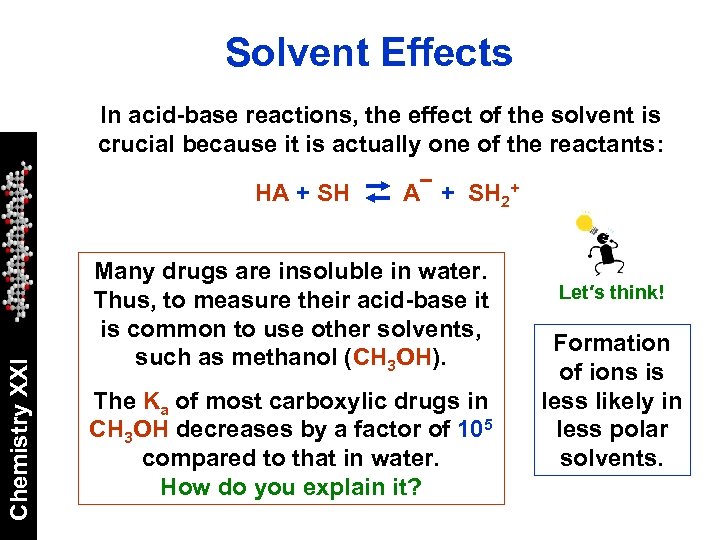 Solvent Effects In acid-base reactions, the effect of the solvent is crucial because it is actually one of the reactants: Chemistry XXI HA + SH 2+ Many drugs are insoluble in water. Thus, to measure their acid-base it is common to use other solvents, such as methanol (CH 3 OH). The Ka of most carboxylic drugs in CH 3 OH decreases by a factor of 105 compared to that in water. How do you explain it? Let′s think! Formation of ions is less likely in less polar solvents.
Solvent Effects In acid-base reactions, the effect of the solvent is crucial because it is actually one of the reactants: Chemistry XXI HA + SH 2+ Many drugs are insoluble in water. Thus, to measure their acid-base it is common to use other solvents, such as methanol (CH 3 OH). The Ka of most carboxylic drugs in CH 3 OH decreases by a factor of 105 compared to that in water. How do you explain it? Let′s think! Formation of ions is less likely in less polar solvents.
 Reaction Control Chemistry XXI Our analysis reveals the central role that environmental factors play in the extent and rate of chemical processes: v We can control the extent and rate of chemical reactions by altering the concentration of reactants and products, modifying the temperature and pressure of the system, or changing the solvent in which the reaction takes place. v The effect of these factors is better understood by considering both kinetic and thermodynamic arguments.
Reaction Control Chemistry XXI Our analysis reveals the central role that environmental factors play in the extent and rate of chemical processes: v We can control the extent and rate of chemical reactions by altering the concentration of reactants and products, modifying the temperature and pressure of the system, or changing the solvent in which the reaction takes place. v The effect of these factors is better understood by considering both kinetic and thermodynamic arguments.
 Chemistry XXI Let′s apply! Assess what you know
Chemistry XXI Let′s apply! Assess what you know
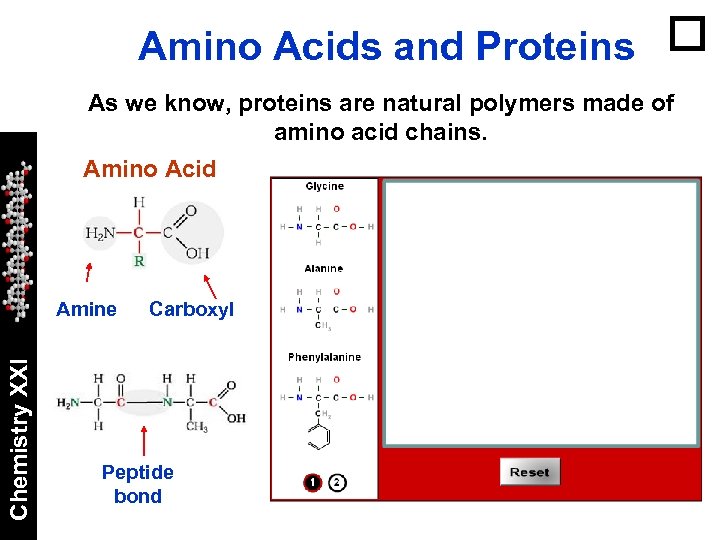 Amino Acids and Proteins As we know, proteins are natural polymers made of amino acid chains. Amino Acid Chemistry XXI Amine Carboxyl Peptide bond
Amino Acids and Proteins As we know, proteins are natural polymers made of amino acid chains. Amino Acid Chemistry XXI Amine Carboxyl Peptide bond
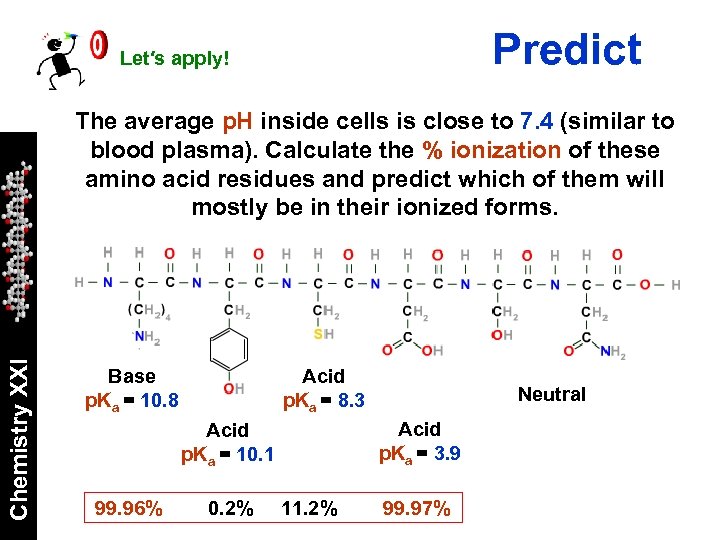 Predict Let′s apply! Chemistry XXI The average p. H inside cells is close to 7. 4 (similar to blood plasma). Calculate the % ionization of these amino acid residues and predict which of them will mostly be in their ionized forms. Base p. Ka = 10. 8 Acid p. Ka = 8. 3 Acid p. Ka = 3. 9 Acid p. Ka = 10. 1 99. 96% 0. 2% Neutral 11. 2% 99. 97%
Predict Let′s apply! Chemistry XXI The average p. H inside cells is close to 7. 4 (similar to blood plasma). Calculate the % ionization of these amino acid residues and predict which of them will mostly be in their ionized forms. Base p. Ka = 10. 8 Acid p. Ka = 8. 3 Acid p. Ka = 3. 9 Acid p. Ka = 10. 1 99. 96% 0. 2% Neutral 11. 2% 99. 97%
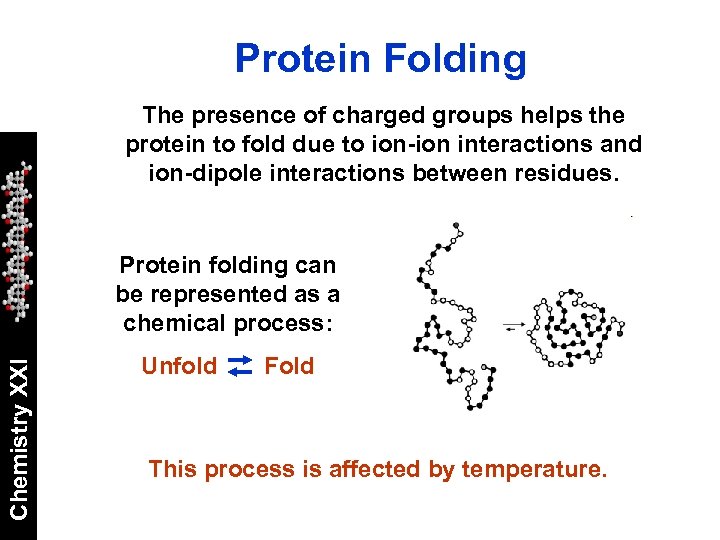 Protein Folding The presence of charged groups helps the protein to fold due to ion-ion interactions and ion-dipole interactions between residues. Chemistry XXI Protein folding can be represented as a chemical process: Unfold Fold This process is affected by temperature.
Protein Folding The presence of charged groups helps the protein to fold due to ion-ion interactions and ion-dipole interactions between residues. Chemistry XXI Protein folding can be represented as a chemical process: Unfold Fold This process is affected by temperature.
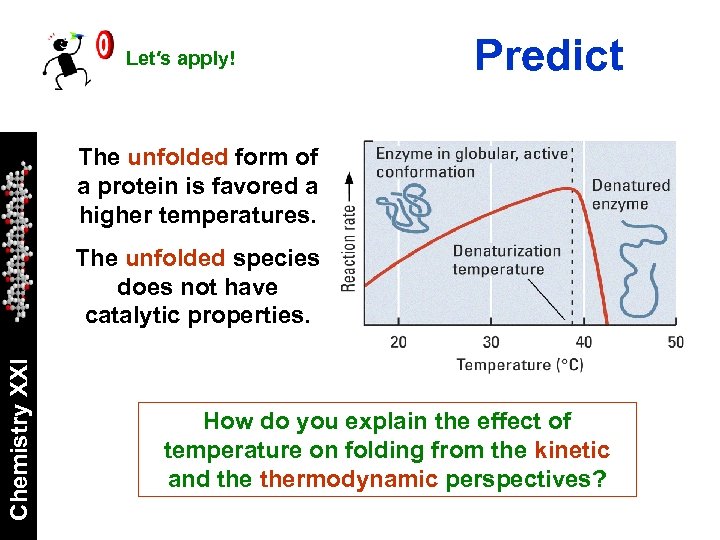 Let′s apply! Predict The unfolded form of a protein is favored a higher temperatures. Chemistry XXI The unfolded species does not have catalytic properties. How do you explain the effect of temperature on folding from the kinetic and thermodynamic perspectives?
Let′s apply! Predict The unfolded form of a protein is favored a higher temperatures. Chemistry XXI The unfolded species does not have catalytic properties. How do you explain the effect of temperature on folding from the kinetic and thermodynamic perspectives?
 Chemistry XXI Imagine someone gives you the p. Ka of a drug. Work with a partner making a list of the things you could tell that person about the properties of the drug outside and inside your body.
Chemistry XXI Imagine someone gives you the p. Ka of a drug. Work with a partner making a list of the things you could tell that person about the properties of the drug outside and inside your body.
 Changing the Environment Summary Chemistry XXI We can control the extent of a reaction by altering the concentration of reactants and products, modifying the temperature, or changing the solvent in which the reactions takes place. Given the expression and value of the dissociation constant for and acid or base in water (p. Ka, p. Kb), we can evaluate things such as: p. H of solution; Degree of dissociation as function of p. H; Effect on p. H of changes in C and T.
Changing the Environment Summary Chemistry XXI We can control the extent of a reaction by altering the concentration of reactants and products, modifying the temperature, or changing the solvent in which the reactions takes place. Given the expression and value of the dissociation constant for and acid or base in water (p. Ka, p. Kb), we can evaluate things such as: p. H of solution; Degree of dissociation as function of p. H; Effect on p. H of changes in C and T.
 Chemistry XXI For next class, Investigate what structural features of substances can be used to predict their relative acid strength. How can we predict whether one substance will be a stronger acid than another by analyzing their molecular structure?
Chemistry XXI For next class, Investigate what structural features of substances can be used to predict their relative acid strength. How can we predict whether one substance will be a stronger acid than another by analyzing their molecular structure?


