113a978d1be284f56f9c134633272fcb.ppt
- Количество слайдов: 19

UNIT 1 MATERIALS Vocabulary • • Names of materials Characteristics of materials Adjectives and dimensions Word formation: SUFFIXES to form ADJECTIVES Grammar and functions • Giving definitions & describing • Articles • Expressing measurements

NAMES OF MATERIALS p 9 1. Aluminium b Light to carry and silvery to look at. 2. Brass p Mixture of copper and zinc. 3. Bronze o Mixture of copper and tin. 4. Carbon dioxide f Gas produced in the combustion of fossil fuels 5. Chromium l Hard, shiny metal used to coat other metals to prevent rust. 6. Concrete k Building material made by mixing cement and gravel. 7. Copper n Soft, reddish-brown metal, used in wires 8. Gold g Valuable yellow metal which is a very good conductor. 9. Hydrogen j The lightest gas and the simplest element in nature 10. Iron e With symbol Fe, it is the main component of steel. 11. Lead r Soft, grey, heavy metal used in pipes, whose symbol is Pb 12. Mercury d Heavy, silvery metal, usually a liquid at room temperature. 13. Nitrogen m 80% of the air. 14. Oxygen q Colourless and tasteless gas supporting life 15. Tin i A can is made of it and its symbol is Sn 16. Uranium c Heavy, white metal whose atoms can be fissioned 17. Zinc a Hard, bluish-white metal used in alloys and in roofing. 18. Steel h Iron plus carbon.

VIDEO p 10 SECTION 1 : elements found in all stars. Profile of the abundance of elements found in stars: -immense amounts of HYDROGEN and HELIUM. -LOWER amounts (2%) of the heavier elements • Peaks CARBON • OXYGEN • MAGNESIUM • SILICON • SULPHUR • IRON

VIDEO Process: nuclear fusion • 2 HYDROGEN atoms= HELIUM + ENERGY-> SUNLIGHT • HELIUM + HELIUM = CARBON • HELIUM + CARBON = OXYGEN • OXYGEN + HELIUM = MAGNESIUM • Right up to IRON • For each of these fusion reactions to occur, INCREASING TEMPERATURE and PRESSURE are needed

DESCRIPTIONS p 11 • Silver is a very ductile malleable shiny greyish-white element having the highest electrical and thermal conductivity of any metal. (0) Silver has long been valued as a precious metal, and it is used as an investment, to make (0) jewelry, and (0) currency coins. Today, (0) silver is also used in (0) electrical contacts and (0) conductors, and for making (0) mirrors, (0) photographic chemicals, etc. • Glass is an amorphous inorganic solid; it is a hard, brittle, noncrystalline, more or less transparent substance produced by (0) fusion, usually consisting of a mixture of (0) dissolved silicates, as in the ordinary variety used for (0) window panes and (0) bottles. • Graphite: a blackish soft allotropic form of (0) carbon, with (0) metallic luster and (0) greasy feel. It consists of (0) layers of (0) carbon atoms. Unlike (0) diamond, (0) graphite is an electrical conductor. It is used in (0) pencils, (0) coatings and (0) electrodes, as a lubricant, as a moderator in (0) nuclear reactors, and, in a carbon fibre form, as a tough light material for (0) sporting equipment

LISTENING 1: what is a metal? 12 • • • • We are so familiar with metals that it might be quite a surprise to be asked the question: What is a metal? . Take magnesium for example. It burns easily. Why then do we regard magnesium as a metal similar to say iron which will not burn? The most important properties that distinguish metals from non-metals are: they reflect light and thus are shiny They are good conductors of heat and electricity They combine with fluorine and chlorine Most react with acids and with oxygen Apart from these similarities, metals show a great deal of variation. Gold, lead and sodium are very soft materials, much softer for example than silicon and graphite, both non-metals. Many metals corrode easily. Gold, chromium and platinum, however, do not. Some metals are very active: sodium, calcium and potassium combine easily with oxygen, chlorine and fluorine. Gold, silver and mercury, on the other hand, do not form compounds so easily. Metals far outnumber non-metals: only 20 of the 103 elements known today are non-metallic

DESCRIBING p 13 • Definition: • Composition: X is It is made (up) of It consists of It has It is composed of It is formed by GENERAL CLASS Materials Substances Components Parts Pieces • Characteristics: WHAT IS IT LIKE? To be To look To seem To become To have + ADJ +ADJ + ADJ Shape (circular, elliptical) Properties (flexible, tough) Colour (blue, greenish) Texture (hard, smooth) Temperature (warm, cold) Dimensions (long, thin, small) + NOUN • Applications: X is used for (+GER) / to (+INF) X is used in/as (+NOUN) X serves to (+INF)
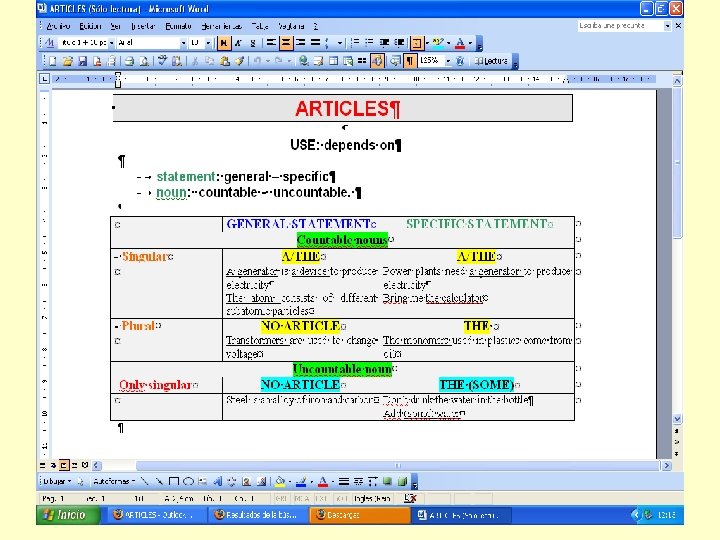
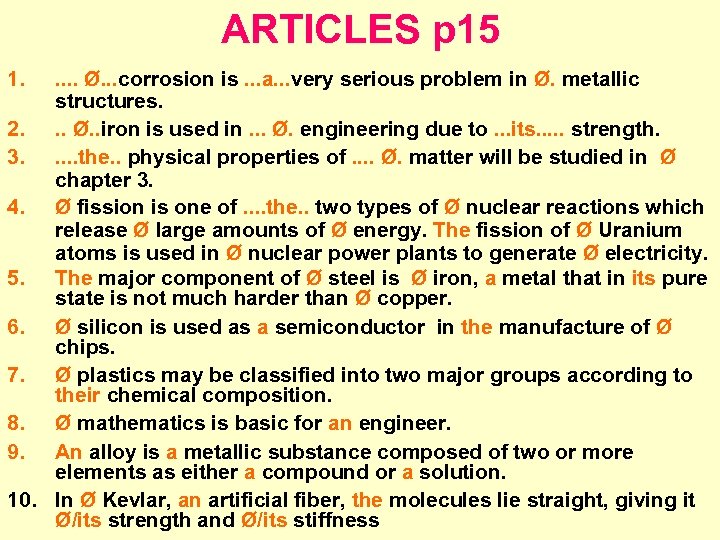
ARTICLES p 15 1. . . Ø. . . corrosion is. . . a. . . very serious problem in Ø. metallic structures. 2. . . Ø. . iron is used in. . . Ø. engineering due to. . . its. . . strength. 3. . . the. . physical properties of. . Ø. matter will be studied in Ø chapter 3. 4. Ø fission is one of. . the. . two types of Ø nuclear reactions which release Ø large amounts of Ø energy. The fission of Ø Uranium atoms is used in Ø nuclear power plants to generate Ø electricity. 5. The major component of Ø steel is Ø iron, a metal that in its pure state is not much harder than Ø copper. 6. Ø silicon is used as a semiconductor in the manufacture of Ø chips. 7. Ø plastics may be classified into two major groups according to their chemical composition. 8. Ø mathematics is basic for an engineer. 9. An alloy is a metallic substance composed of two or more elements as either a compound or a solution. 10. In Ø Kevlar, an artificial fiber, the molecules lie straight, giving it Ø/its strength and Ø/its stiffness

ARTICLES: MERCURY p 15 Ø mercury is a/the chemical element whose symbol is Hg. It is a silvery-white, heavy, liquid metal. Compared with other metals, it is a poor conductor of Ø heat and a fair conductor of Ø electricity. Ø mercury is the only common metal that is a liquid at Ø ordinary temperatures. It easily forms Ø alloys with many other metals. When it combines with certain metals (such as Ø silver, Ø zinc or Ø tin), the resulting alloy is called an amalgam. Both the element and Ø most of its compounds are poisonous. Ø mercury and its compounds are used in Ø electrolytic cells, Ø dentistry, Ø thermometers, Ø batteries, and in Ø medicine.

VOCABULARY: Adjectives p 16 TEMPERATURE: Boiling, Molten, Cool, warm, Burning, Icy TEXTURE: rough, hard, smooth, soft, sticky SHAPE: rectangular, straight, cylindrical, square, rounded, sharp, curved COMPOSITION: metallic, plastic, ferrous, synthetic, organic, golden SIZE: huge, tiny, minute, large, broad, short COLOUR: light, bright, dark, opaque, reddish, golden

WORD FORMATION: Adjectives p 17 ADJECTIVES FROM NOUNS LATIN ORIGIN OR ROOT ENGLISH ORIGIN -IC atomic -FUL beautiful -AL/AR usual/linear -LESS harmless -OUS poisonous/ lustrous -(L)Ysunny -IVE destructive -ISH yellowish -ENT/-ANT different/important ADJECTIVES FROM VERBS -ING boring -ED concentrated IBLE/ -ABLE responsible/drinkable

WORD FORMATION p 18 STAINLESS STEELS • • Stain. LESS steels contain chromium, nickel and other alloy. ING elements that keep them rust resist. ANT in spite of the action of moisture or corros. IVE acids and gases. • Some steels have unus. UAL strength. • Because of their shin. Y surfaces • architects wide. LY use them • for decorat. IVE purposes.

VIDEO: METEORITES p 10 -11 • • • • • The Ballwell meteorite fell in 1965. BROWN METALLIC skin due to ATMOSPHERIC HEATING it consists of : SILICATES. . . . (. . GREEN. . olivine) some. . METAL (BROWN DISCOLORATION DUE TO OXIDATION OF IRON) chondrules Section of a chondritic meteorite: chondrules SILICATES. . . (OLIVINE. . . . ) DARK. . . patches( IRON METAL. . ) Iron meteorites: Blades OF IRON-NICKEL ALLOY (IRON WITH A LITTLE NICKEL) INCLUSIONS OF IRON SULFIDE. . . Meteorites contain three principal phases: 1 - OLIVINE (MAGNESIUM SILICATES) 2 - IRON OFTEN WITH A LITTLE NICKEL 3 - IRON SULFIDE. . . Chondritic meteorites appear to have changed chemically the least since their condensation from the primitive solar nebula. They contain HYDROGEN and HELIUM but otherwise their element abundance should be similar to the abundance in the solar spectrum.

LISTENING: Temperatures 10 -11 • The most commonly used metal in industry is IRON. Its symbol is Fe, its atomic weight 55. 19 and its specific weight is 7. 86 GR/CM 3. Its melting point is 1, 528ºC; this is a metal which is magnetized quite strongly but above 768ºC it cannot be magnetized. • Another metal of a great importance in engineering is ALUMINIUM, with an atomic weight of 26. 97, a specific weight of 2. 7 GR/CM 3 and its melting point is 658ºC • Among metals, LEAD is the metal which possesses the highest density, with an atomic weight of 207. 22 and a specific weight of 11. 34 GR/CM 3; contrarily to other metals, however, its melting point is relatively low as it melts at 327ºC.

LISTENING: Temperatures • However not all metals have the same characteristics; as an example we have MERCURY which is A LIQUID at room temperature; thus, the temperature at which this metal changes from liquid to solid is – 38. 9ºC and its boiling point is 357. 2ºC. • Non-metals, on the other hand, vary greatly with regard to their characteristics. For example, CHLORINE has a specific weight of 0. 0032 GR/CM 3 and its boiling point is – 33. 7ºC. However, SILICON, whose specific weight is 2. 33, melts at 1, 310ºC and boils at 2, 355ºC.
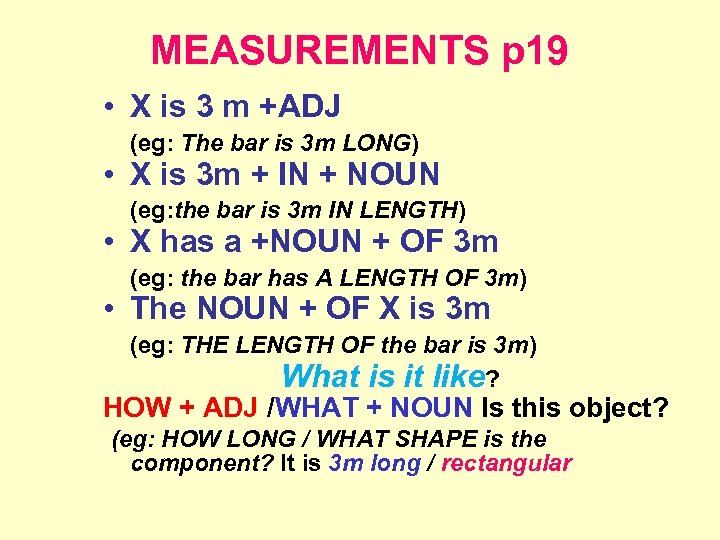
MEASUREMENTS p 19 • X is 3 m +ADJ (eg: The bar is 3 m LONG) • X is 3 m + IN + NOUN (eg: the bar is 3 m IN LENGTH) • X has a +NOUN + OF 3 m (eg: the bar has A LENGTH OF 3 m) • The NOUN + OF X is 3 m (eg: THE LENGTH OF the bar is 3 m) What is it like? HOW + ADJ /WHAT + NOUN Is this object? (eg: HOW LONG / WHAT SHAPE is the component? It is 3 m long / rectangular

1. EXERCISE: Measurements 20 The film coating the piece must be very THIN. It should be 0. 05 mm THICK 2. The light travels along THE LENGTH of the optical fibre 3. How HIGH is the new tower? It is 10 ft HIGH 4. To make chips, a single crystal in the shape of a long bar of about 10 cm IN diameter is cut into circular slices 1/2 mm THICK 5. The beams needed for the structure must be 3 m LONG and 25 cm THICK/WIDE 6. To take the recordings, thermometer was placed at a HEIGHT of 1. 5 m above ground level. 7. The piece of the machine is too long to fit in the slot, so we must make it SHORTER 8. 3 -D objects have three main dimensions: HEIGHT, WIDTH and LENGTH 9. The reaction produces a layer on top of the solution, with THICKNESSES ranging from 0. 2 to 0. 6 mm 10. WHAT IS THE PIECE LIKE? /WHAT SHAPE is it? . Well, it is cylindrical
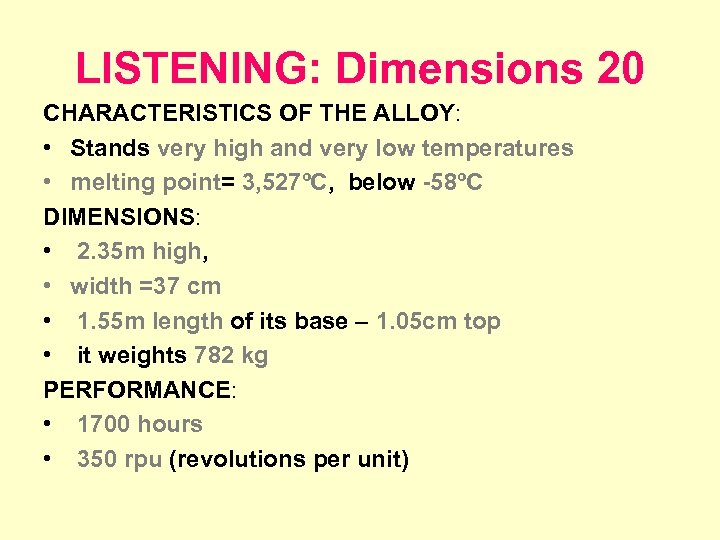
LISTENING: Dimensions 20 CHARACTERISTICS OF THE ALLOY: • Stands very high and very low temperatures • melting point= 3, 527ºC, below -58ºC DIMENSIONS: • 2. 35 m high, • width =37 cm • 1. 55 m length of its base – 1. 05 cm top • it weights 782 kg PERFORMANCE: • 1700 hours • 350 rpu (revolutions per unit)
113a978d1be284f56f9c134633272fcb.ppt