555e3d95dd28e405daa56c4e05f7baff.ppt
- Количество слайдов: 30
 Unit 1: Chemistry and Measurement What is Chemistry?
Unit 1: Chemistry and Measurement What is Chemistry?
 What is chemistry? https: //www. youtube. com/ watch? v=bhof. N 1 x. X 6 u 0
What is chemistry? https: //www. youtube. com/ watch? v=bhof. N 1 x. X 6 u 0
 6 3 1 2 7 CHEMISTRY IS EVERYWHERE!! 4 9 10 5 8
6 3 1 2 7 CHEMISTRY IS EVERYWHERE!! 4 9 10 5 8
 What is measurement? • Chemistry is about observation! Therefore, all lab experiments involve data collection. There are two types of data we can collect in any lab: qualitative and quantitative. What is the difference between them? Qualitative: Descriptive, non-numerical data (ex) color, phase, texture Quantitative: Numbers with units **We need both types of measurements in chemistry.
What is measurement? • Chemistry is about observation! Therefore, all lab experiments involve data collection. There are two types of data we can collect in any lab: qualitative and quantitative. What is the difference between them? Qualitative: Descriptive, non-numerical data (ex) color, phase, texture Quantitative: Numbers with units **We need both types of measurements in chemistry.
 What is a GOOD meas? • Most data is collected at STP. Why do you think STP is important when trying to obtain data? • Any good data that is collected needs to be both precise and accurate. What is the difference between precision and accuracy? Accuracy: Precision: getting the same results for multiple trials proximity of your results to the actual answer
What is a GOOD meas? • Most data is collected at STP. Why do you think STP is important when trying to obtain data? • Any good data that is collected needs to be both precise and accurate. What is the difference between precision and accuracy? Accuracy: Precision: getting the same results for multiple trials proximity of your results to the actual answer
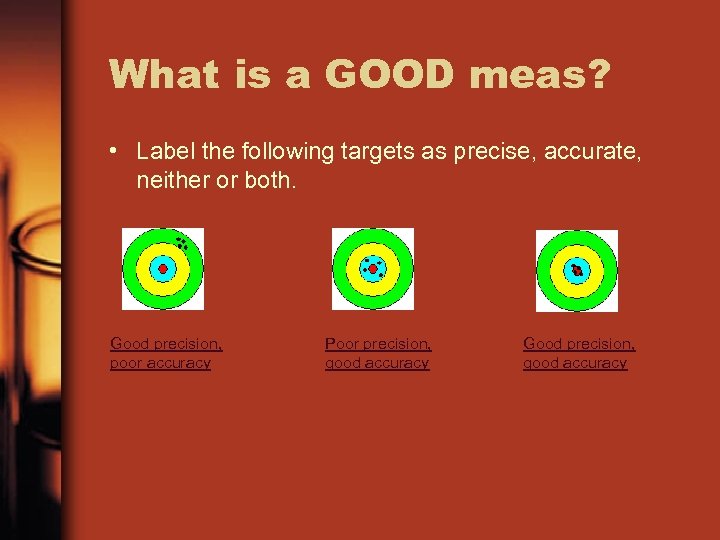 What is a GOOD meas? • Label the following targets as precise, accurate, neither or both. Good precision, poor accuracy Poor precision, good accuracy Good precision, good accuracy
What is a GOOD meas? • Label the following targets as precise, accurate, neither or both. Good precision, poor accuracy Poor precision, good accuracy Good precision, good accuracy
 • Error Measured values are never absolutely accurate, they always have error. There is a certain amount of error that is allowable. What determines the allowable error? The measuring tool For example, a balance that reads to the nearest 0. 001 g is more accurate than one that reads to the nearest 0. 01 g. Ex) A graduated cylinder that measures to the nearest hundredths place contains exactly 32. 70 m. L of liquid. One of your classmates reads the volume in the graduated cylinder to be 32. 2 m. L. Is this amount of error acceptable? What if another classmate reads the volume of the liquid to be 33. 2 m. L? Is this an acceptable amount of error? Why?
• Error Measured values are never absolutely accurate, they always have error. There is a certain amount of error that is allowable. What determines the allowable error? The measuring tool For example, a balance that reads to the nearest 0. 001 g is more accurate than one that reads to the nearest 0. 01 g. Ex) A graduated cylinder that measures to the nearest hundredths place contains exactly 32. 70 m. L of liquid. One of your classmates reads the volume in the graduated cylinder to be 32. 2 m. L. Is this amount of error acceptable? What if another classmate reads the volume of the liquid to be 33. 2 m. L? Is this an acceptable amount of error? Why?
 Percent Error • What would the percent error be for the first student? Percent error % error = measured value – accepted value x 100 accepted value ** % error can be either positive or negative. Positive error means that your value was larger than the real value and negative means your value was smaller.
Percent Error • What would the percent error be for the first student? Percent error % error = measured value – accepted value x 100 accepted value ** % error can be either positive or negative. Positive error means that your value was larger than the real value and negative means your value was smaller.
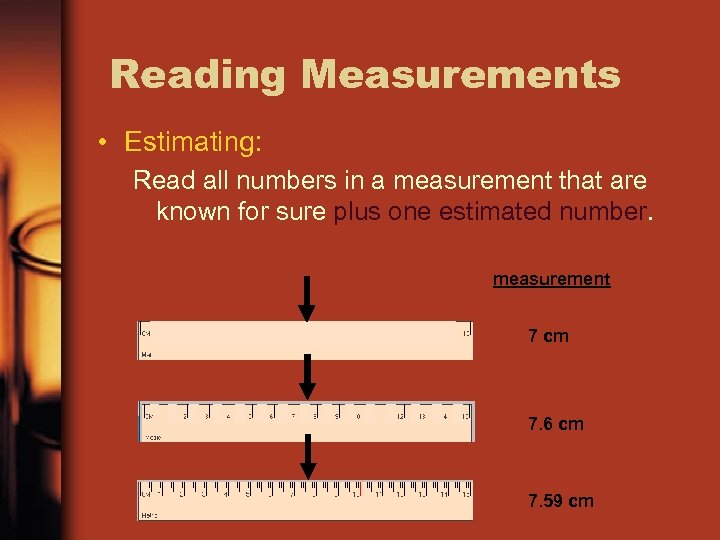 Reading Measurements • Estimating: Read all numbers in a measurement that are known for sure plus one estimated number. measurement 7 cm 7. 6 cm 7. 59 cm
Reading Measurements • Estimating: Read all numbers in a measurement that are known for sure plus one estimated number. measurement 7 cm 7. 6 cm 7. 59 cm
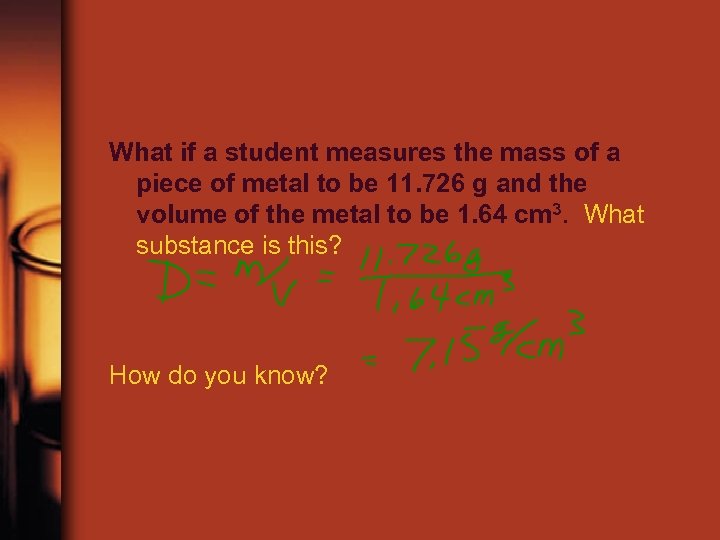 What if a student measures the mass of a piece of metal to be 11. 726 g and the volume of the metal to be 1. 64 cm 3. What substance is this? How do you know?
What if a student measures the mass of a piece of metal to be 11. 726 g and the volume of the metal to be 1. 64 cm 3. What substance is this? How do you know?
 Density formula: Units: g/cm 3 or g/m. L 1 m. L=1 cm 3 Is density an intrinsic or extrinsic property…in other words does the density of a substance change if I have more of the substance? (ex) 10 g vs. 20 g
Density formula: Units: g/cm 3 or g/m. L 1 m. L=1 cm 3 Is density an intrinsic or extrinsic property…in other words does the density of a substance change if I have more of the substance? (ex) 10 g vs. 20 g
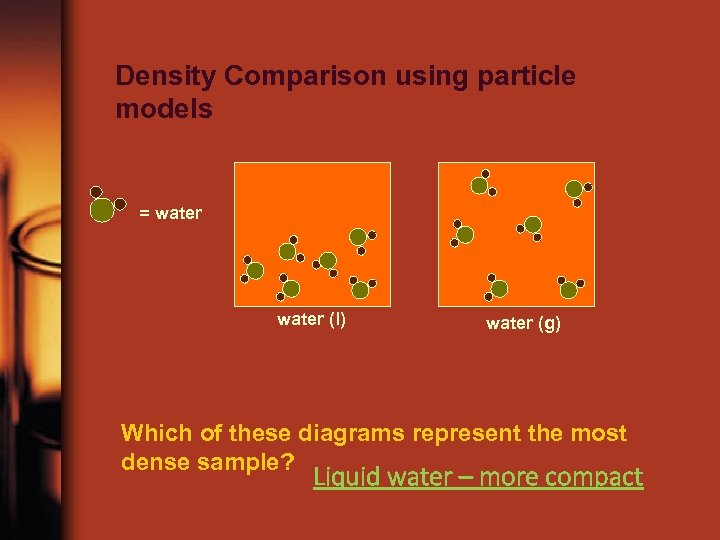 Density Comparison using particle models = water (l) water (g) Which of these diagrams represent the most dense sample? Liquid water – more compact
Density Comparison using particle models = water (l) water (g) Which of these diagrams represent the most dense sample? Liquid water – more compact
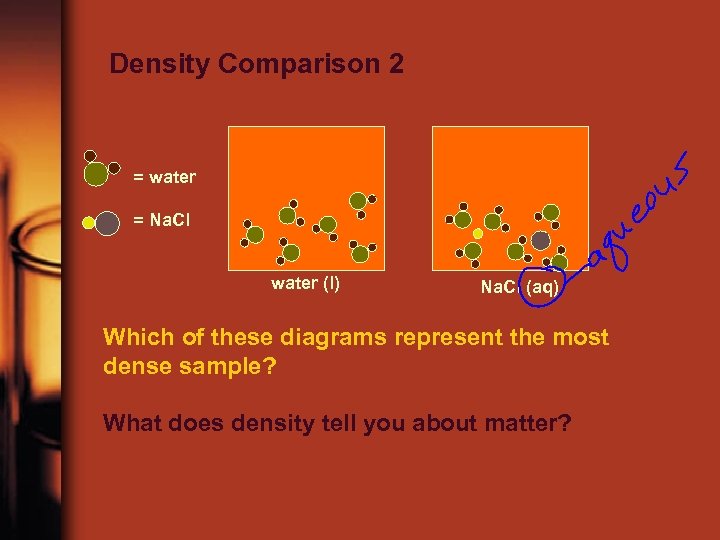 Density Comparison 2 = water = Na. Cl water (l) Na. Cl (aq) Which of these diagrams represent the most dense sample? What does density tell you about matter?
Density Comparison 2 = water = Na. Cl water (l) Na. Cl (aq) Which of these diagrams represent the most dense sample? What does density tell you about matter?
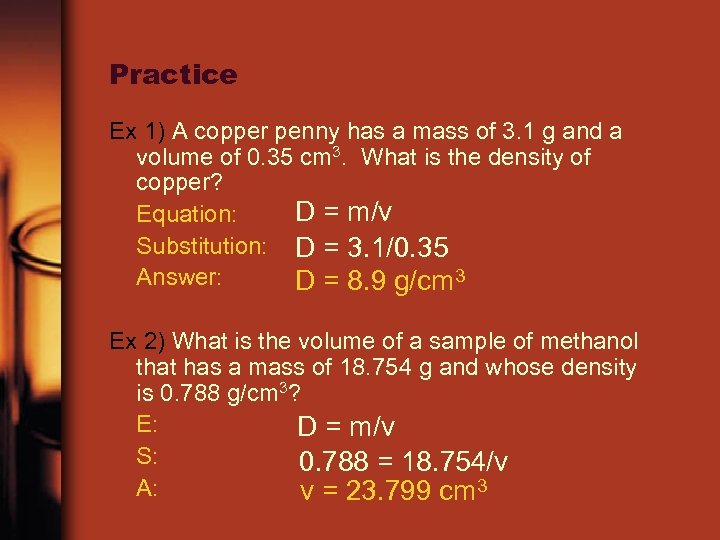 Practice Ex 1) A copper penny has a mass of 3. 1 g and a volume of 0. 35 cm 3. What is the density of copper? D = m/v Equation: Substitution: D = 3. 1/0. 35 Answer: D = 8. 9 g/cm 3 Ex 2) What is the volume of a sample of methanol that has a mass of 18. 754 g and whose density is 0. 788 g/cm 3? E: D = m/v S: 0. 788 = 18. 754/v A: v = 23. 799 cm 3
Practice Ex 1) A copper penny has a mass of 3. 1 g and a volume of 0. 35 cm 3. What is the density of copper? D = m/v Equation: Substitution: D = 3. 1/0. 35 Answer: D = 8. 9 g/cm 3 Ex 2) What is the volume of a sample of methanol that has a mass of 18. 754 g and whose density is 0. 788 g/cm 3? E: D = m/v S: 0. 788 = 18. 754/v A: v = 23. 799 cm 3
 Ex 3) What is the density of a board whose dimensions are 5. 54 cm x 10. 6 cm x 199 cm and whose mass is 28, 600 g? D = 2. 4 g/cm 3 Ex 4) A prospector finds a nugget that looks like gold. To be sure the substance is gold, he finds that the mass of the nugget is 1. 83 g. When put into water, the water level changes from 1. 3290 m. L to 1. 4237 m. L. Is this substance gold? d = 19. 32 g/cm 3 Bonus!! Yes it is gold
Ex 3) What is the density of a board whose dimensions are 5. 54 cm x 10. 6 cm x 199 cm and whose mass is 28, 600 g? D = 2. 4 g/cm 3 Ex 4) A prospector finds a nugget that looks like gold. To be sure the substance is gold, he finds that the mass of the nugget is 1. 83 g. When put into water, the water level changes from 1. 3290 m. L to 1. 4237 m. L. Is this substance gold? d = 19. 32 g/cm 3 Bonus!! Yes it is gold
 Properties of Matter • Physical Property: A quality of matter that can be observed or measured without chemically changing the matter. • Chemical Property: The ability of a substance to undergo bond breaking and bond making (chemical reactions) and form NEW substances REACTIVITY. (Ex) H 2 O is clear (Ex) The density of water (Ex) wood burns is 1. 0 g/m. L (Ex) sodium explodes in water
Properties of Matter • Physical Property: A quality of matter that can be observed or measured without chemically changing the matter. • Chemical Property: The ability of a substance to undergo bond breaking and bond making (chemical reactions) and form NEW substances REACTIVITY. (Ex) H 2 O is clear (Ex) The density of water (Ex) wood burns is 1. 0 g/m. L (Ex) sodium explodes in water
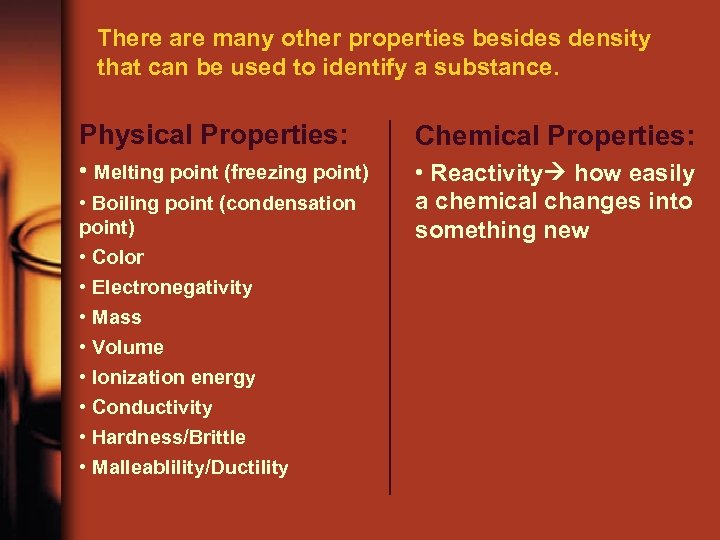 There are many other properties besides density that can be used to identify a substance. Physical Properties: Chemical Properties: • Melting point (freezing point) • Reactivity how easily a chemical changes into something new • Boiling point (condensation point) • Color • Electronegativity • Mass • Volume • Ionization energy • Conductivity • Hardness/Brittle • Malleablility/Ductility
There are many other properties besides density that can be used to identify a substance. Physical Properties: Chemical Properties: • Melting point (freezing point) • Reactivity how easily a chemical changes into something new • Boiling point (condensation point) • Color • Electronegativity • Mass • Volume • Ionization energy • Conductivity • Hardness/Brittle • Malleablility/Ductility
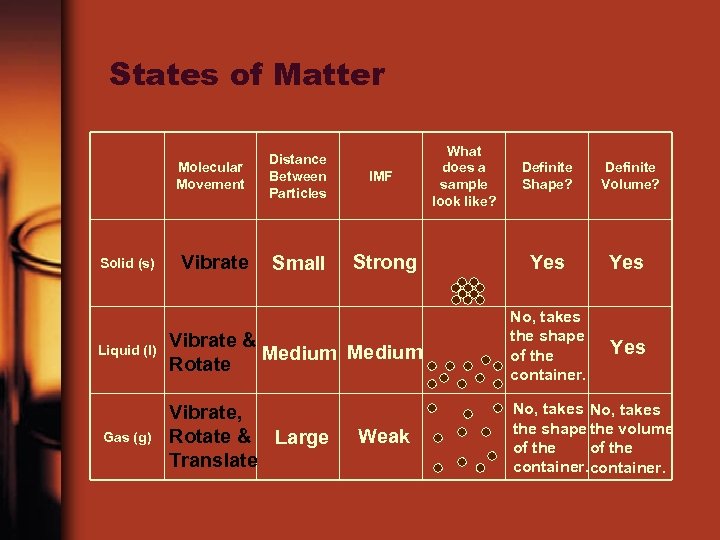 States of Matter Molecular Movement Solid (s) Liquid (l) Gas (g) Distance Between Particles IMF Vibrate Small Strong What does a sample look like? Definite Shape? Definite Volume? Yes Vibrate & Medium Rotate No, takes the shape of the container. Yes Vibrate, Rotate & Large Translate No, takes the shape the volume of the container. Weak
States of Matter Molecular Movement Solid (s) Liquid (l) Gas (g) Distance Between Particles IMF Vibrate Small Strong What does a sample look like? Definite Shape? Definite Volume? Yes Vibrate & Medium Rotate No, takes the shape of the container. Yes Vibrate, Rotate & Large Translate No, takes the shape the volume of the container. Weak
 Physical Change: Chemical Change: A change that does not alter the chemical composition of the matter. It only changes the position of the particles. One or more substances are made into one or more NEW substances (bonds are broken and/or bonds are made). • • • Burning (combustion or flammability) • Rusting • Decomposing • Cooking • Explosions Melting (phase ∆) Boiling (phase ∆) Cutting Grinding into powder Bending Evaporation (phase ∆) Crystallization (phase ∆) Freezing (phase ∆) Subliming (phase ∆) Dissolving Mixing
Physical Change: Chemical Change: A change that does not alter the chemical composition of the matter. It only changes the position of the particles. One or more substances are made into one or more NEW substances (bonds are broken and/or bonds are made). • • • Burning (combustion or flammability) • Rusting • Decomposing • Cooking • Explosions Melting (phase ∆) Boiling (phase ∆) Cutting Grinding into powder Bending Evaporation (phase ∆) Crystallization (phase ∆) Freezing (phase ∆) Subliming (phase ∆) Dissolving Mixing
 Chemical Changes What are some indicators that a chemical change has occurred? – A bold color change can indicate that the reaction (change) is chemical. – The formation of a new solid, liquid or gas is a second indication that there is a chemical reaction. (something is rearranged) **Heat is released or absorbed in every chemical and physical reaction
Chemical Changes What are some indicators that a chemical change has occurred? – A bold color change can indicate that the reaction (change) is chemical. – The formation of a new solid, liquid or gas is a second indication that there is a chemical reaction. (something is rearranged) **Heat is released or absorbed in every chemical and physical reaction
 Practice: Ex) Ca. Cl 2(s) + H 2 O Ca. Cl 2(aq) + heat Ex) Mg. SO 4(s) + H 2 O(l) + heat Mg. SO 4(aq) Ex) CH 2 CH 3 OH(l) CH 2 CH 3 OH(s) + heat Ex) H 2 SO 4(aq) + Li. OH(aq) Li 2 SO 4(aq) + heat Ex) CH 3 OH(g) + O 2(g) CO 2(g) + H 2 O(g) + heat Ex) H 2 O(l) + heat H 2 O(g) Ex) cooking rare steak to well done Ex) sugar dissolving in coffee Ex) apple juice goes bad Ex) wood burning
Practice: Ex) Ca. Cl 2(s) + H 2 O Ca. Cl 2(aq) + heat Ex) Mg. SO 4(s) + H 2 O(l) + heat Mg. SO 4(aq) Ex) CH 2 CH 3 OH(l) CH 2 CH 3 OH(s) + heat Ex) H 2 SO 4(aq) + Li. OH(aq) Li 2 SO 4(aq) + heat Ex) CH 3 OH(g) + O 2(g) CO 2(g) + H 2 O(g) + heat Ex) H 2 O(l) + heat H 2 O(g) Ex) cooking rare steak to well done Ex) sugar dissolving in coffee Ex) apple juice goes bad Ex) wood burning
 Law of Conservation of Mass cannot be created or destroyed in a chemical reaction. Ex) If 27. 5 g of CH 3 OH reacts with 55. 0 g of O 2 to produce 37. 8 g of CO 2, how many grams of H 2 O will be produced according to the reaction below? 2 CH 3 OH(g) + 4 O 2(g) 2 CO 2(g) + 4 H 2 O(g)
Law of Conservation of Mass cannot be created or destroyed in a chemical reaction. Ex) If 27. 5 g of CH 3 OH reacts with 55. 0 g of O 2 to produce 37. 8 g of CO 2, how many grams of H 2 O will be produced according to the reaction below? 2 CH 3 OH(g) + 4 O 2(g) 2 CO 2(g) + 4 H 2 O(g)
 What if a student measures the mass and volume of a liquid in the lab and after calculating the density of the substance, the density does not match any of the densities on Table S? What are some possible reasons why the density of your liquid is not listed?
What if a student measures the mass and volume of a liquid in the lab and after calculating the density of the substance, the density does not match any of the densities on Table S? What are some possible reasons why the density of your liquid is not listed?
 For example, you are given a clear liquid that you suspect to be a mixture of salt and water and you are asked to determine if it is a pure substance or a mixture. What do you have to do?
For example, you are given a clear liquid that you suspect to be a mixture of salt and water and you are asked to determine if it is a pure substance or a mixture. What do you have to do?
 The Scientific Method 1. 2. 3. 4. 5. 6. Identify the problem: What do you want to know? What question are we trying to answer? Hypothesis: Make an educated guess on what you think the answer will be. Experiment: Design an experiment to test if your guess is correct. (Control, variables, procedure) Data: Record observations from your experiment. Conclusion: Interpret the data you’ve collected to determine if your answer was correct. Evaluate (reflect): the validity of your results and REPEAT.
The Scientific Method 1. 2. 3. 4. 5. 6. Identify the problem: What do you want to know? What question are we trying to answer? Hypothesis: Make an educated guess on what you think the answer will be. Experiment: Design an experiment to test if your guess is correct. (Control, variables, procedure) Data: Record observations from your experiment. Conclusion: Interpret the data you’ve collected to determine if your answer was correct. Evaluate (reflect): the validity of your results and REPEAT.
 Conversions Sometimes when using data collected in experiments you will need to convert from one unit to another. Conversions: In chemistry we use the International System of Units (SI). These units are the same units that scientists all over the world use so that scientific studies from different countries will be easily readable. It is based on the metric system with a few extras. Let’s take a D look at Table _____. These are all base units. Converting using the same base units: K H D B d c m Kilo Hecto Deka base deci centi milli King Henry Died by drinking chocolate milk
Conversions Sometimes when using data collected in experiments you will need to convert from one unit to another. Conversions: In chemistry we use the International System of Units (SI). These units are the same units that scientists all over the world use so that scientific studies from different countries will be easily readable. It is based on the metric system with a few extras. Let’s take a D look at Table _____. These are all base units. Converting using the same base units: K H D B d c m Kilo Hecto Deka base deci centi milli King Henry Died by drinking chocolate milk
 Ex) How many decigrams are in 32. 1 milligrams? mg dg **We’re going from ______ to ______. K H D B d c m 0. 321 32. 1 mg = _____ dg When moving left, the decimal also moves left. kg Ex) How many grams are in 4. 9 kg? We are going from _____ to g ____. K H D B d c m 4, 900 4. 9 kg = ______ g When moving right, the decimal also moves right.
Ex) How many decigrams are in 32. 1 milligrams? mg dg **We’re going from ______ to ______. K H D B d c m 0. 321 32. 1 mg = _____ dg When moving left, the decimal also moves left. kg Ex) How many grams are in 4. 9 kg? We are going from _____ to g ____. K H D B d c m 4, 900 4. 9 kg = ______ g When moving right, the decimal also moves right.
 There are some conversions you can just use a formula for. Ex) Convert 24 o. C to Kelvin. How is the Kelvin temperature scale different from the Celsius scale? Why do you think the Kelvin scale is called the absolute temperature scale? Ex 3) Which represents the highest temperature? 5 K 105 K 5 °C 105 °C
There are some conversions you can just use a formula for. Ex) Convert 24 o. C to Kelvin. How is the Kelvin temperature scale different from the Celsius scale? Why do you think the Kelvin scale is called the absolute temperature scale? Ex 3) Which represents the highest temperature? 5 K 105 K 5 °C 105 °C
 Scientific Notation: Ex) 5. 2 x 10 -2 means that the original # was smaller than 1 because the exponent is negative. 5. 2 x 10 -2 0. 052 Original # __________ Ex 2) 9. 8 x 103 means that the original # was larger than 1 because the exponent is positive. 9. 8 x 103 9, 800 Original # __________ It works the same way when changing #’s into scientific notation. Ex 3) 523000 This is a # larger than 1 so it will have a positive exponent 523000 5. 23 x 105 Scientific Notation __________ Ex 4) 0. 000425 This is a # smaller than 1 so it will have a negative exponent. 0. 000425 4. 25 x 10 -4 Scientific Notation __________ http: //science. widener. edu/svb/tutorial/scinotcsn 7. html
Scientific Notation: Ex) 5. 2 x 10 -2 means that the original # was smaller than 1 because the exponent is negative. 5. 2 x 10 -2 0. 052 Original # __________ Ex 2) 9. 8 x 103 means that the original # was larger than 1 because the exponent is positive. 9. 8 x 103 9, 800 Original # __________ It works the same way when changing #’s into scientific notation. Ex 3) 523000 This is a # larger than 1 so it will have a positive exponent 523000 5. 23 x 105 Scientific Notation __________ Ex 4) 0. 000425 This is a # smaller than 1 so it will have a negative exponent. 0. 000425 4. 25 x 10 -4 Scientific Notation __________ http: //science. widener. edu/svb/tutorial/scinotcsn 7. html
 Works Cited 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. http: //www. 220 -electronics. com/plasma/sonymultisystemplasma 42 v 1. JPG http: //yhspatriot. net/~abeland/images/darkroom 2. jpg http: //www. house-maids. co. uk/cleaning%20 products%20 for%20 website. jpg http: //images. google. com/imgres? imgurl=http: //www. happynews. com/living/haircare/perms-spiralhairstyles. jpg&imgrefurl=http: //www. happynews. com/living/haircare/perms-spiralhairstyles. htm&h=168&w=175&sz=13&hl=en&start=4&tbnid=xn. Pc 1 l. K_Xy 4 UNM: &tbnh=96&tbnw=100&prev=/ima ges%3 Fq%3 Dperms%26 gbv%3 D 2%26 svnum%3 D 10%26 hl%3 Den%26 ie%3 DUTF-8 http: //www. cartoonstock. com/lowres/amc 0432 l. jpg http: //www. cookies-in-motion. com/images/tools. jpg http: //www. webelements. com/webelements/media/kossel-pics/Mn. jpg http: //www. uncp. edu/home/mcclurem/ptable/mn_1. jpg http: //chandra. harvard. edu/photo/2005/neon_hand. jpg http: //gwydir. demon. co. uk/jo/minerals/pix/copper 1. jpg http: //www. cofc. edu/~martine/111 Lect. Week 1_files/image 008. jpg http: //mynasadata. larc. nasa. gov/images/Kelvin_scale. jpg http: //www. chateaucountry. org/images/portraits/du. Pont-E. I. -RGB. jpg
Works Cited 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. http: //www. 220 -electronics. com/plasma/sonymultisystemplasma 42 v 1. JPG http: //yhspatriot. net/~abeland/images/darkroom 2. jpg http: //www. house-maids. co. uk/cleaning%20 products%20 for%20 website. jpg http: //images. google. com/imgres? imgurl=http: //www. happynews. com/living/haircare/perms-spiralhairstyles. jpg&imgrefurl=http: //www. happynews. com/living/haircare/perms-spiralhairstyles. htm&h=168&w=175&sz=13&hl=en&start=4&tbnid=xn. Pc 1 l. K_Xy 4 UNM: &tbnh=96&tbnw=100&prev=/ima ges%3 Fq%3 Dperms%26 gbv%3 D 2%26 svnum%3 D 10%26 hl%3 Den%26 ie%3 DUTF-8 http: //www. cartoonstock. com/lowres/amc 0432 l. jpg http: //www. cookies-in-motion. com/images/tools. jpg http: //www. webelements. com/webelements/media/kossel-pics/Mn. jpg http: //www. uncp. edu/home/mcclurem/ptable/mn_1. jpg http: //chandra. harvard. edu/photo/2005/neon_hand. jpg http: //gwydir. demon. co. uk/jo/minerals/pix/copper 1. jpg http: //www. cofc. edu/~martine/111 Lect. Week 1_files/image 008. jpg http: //mynasadata. larc. nasa. gov/images/Kelvin_scale. jpg http: //www. chateaucountry. org/images/portraits/du. Pont-E. I. -RGB. jpg


