8ec8bbe4a3443ef2528cb7ada4f07fc8.ppt
- Количество слайдов: 56

• ULTRAVIOLET-VISIBLE-NEAR INFRARED (UV-VIS-NIR) SPECTROSCOPY • ELECTRON PARAMAGNETIC RESONANCE (EPR) or ELECTRON SPIN RESONANCE (ESR) OF ZEOLITES Robert A. SCHOONHEYDT Center for Surface Chemistry and Catalysis K. U. Leuven Kasteelpark Arenberg 23, 3001 Leuven Belgium Robert. schoonheydt@biw. kuleuven. ac. be
OUTLINE 1. Principles of UV-VIS-NIR - physical basis - methodology 2. In-situ UV-VIS 3. Optical and fluorescence microscopies 4. Principles of EPR - physical basis - methodology 5. In-situ EPR 6. Pulse EPR 7. Coordination of transition metal ions (TMI) 8. Conclusions
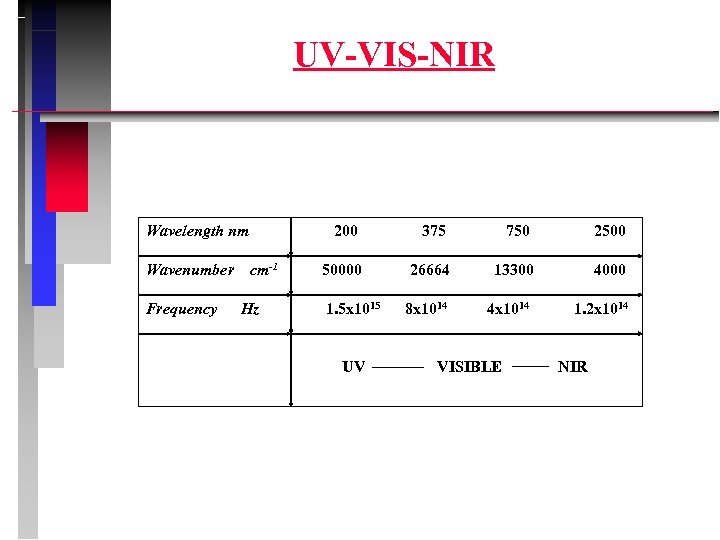
UV-VIS-NIR Wavelength nm Wavenumber Frequency 200 cm-1 Hz 375 750 2500 50000 26664 13300 4000 8 x 1014 4 x 1014 1. 2 x 1014 1. 5 x 1015 UV VISIBLE NIR
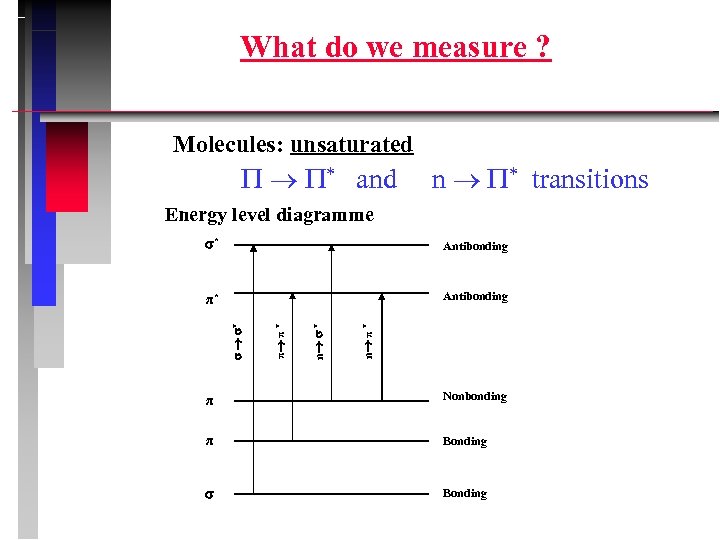
What do we measure ? Molecules: unsaturated * and n * transitions Energy level diagramme n π * Antibonding n * π* π π * Antibonding * * π Nonbonding π Bonding
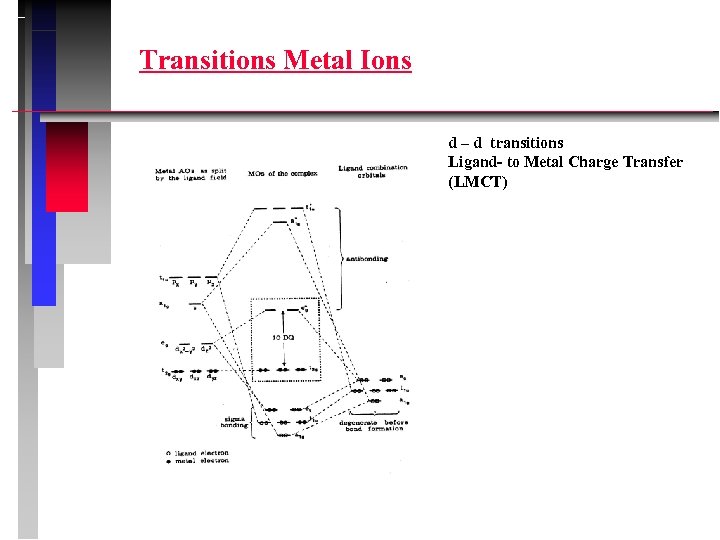
Transitions Metal Ions d – d transitions Ligand- to Metal Charge Transfer (LMCT)
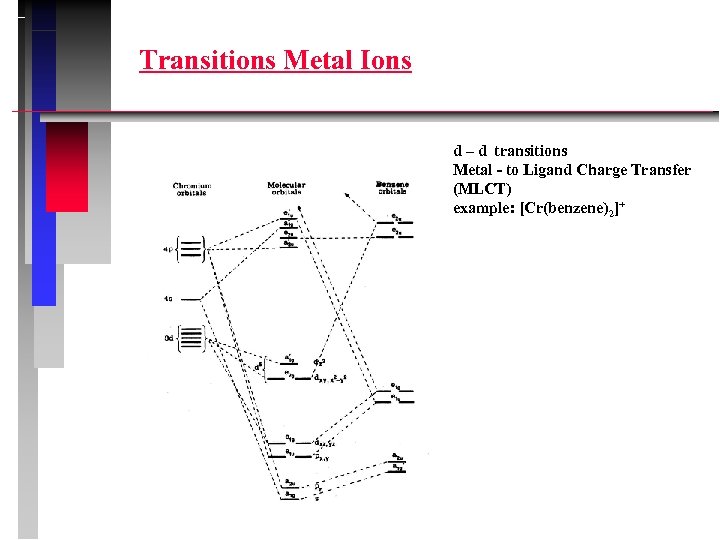
Transitions Metal Ions d – d transitions Metal - to Ligand Charge Transfer (MLCT) example: [Cr(benzene)2]+
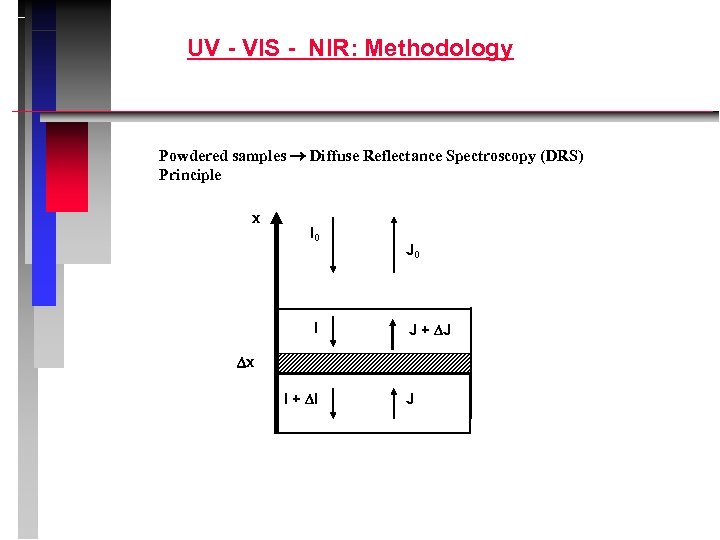
UV - VIS - NIR: Methodology Powdered samples Diffuse Reflectance Spectroscopy (DRS) Principle x I 0 I J 0 J + J x I + I J
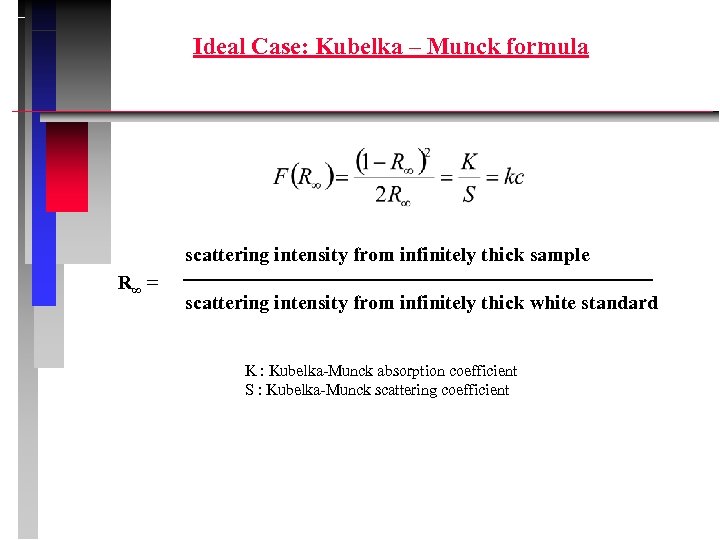
Ideal Case: Kubelka – Munck formula scattering intensity from infinitely thick sample R∞ = scattering intensity from infinitely thick white standard K : Kubelka-Munck absorption coefficient S : Kubelka-Munck scattering coefficient

Conditions for use of K M-formula • diffuse monochromatic irradiation • isotropic scattering • infinite sample thickness • low concentration of absorbing centers • uniform distribution of absorbing centers • absence of fluorescence

UV – VIS – NIR: instrumentation • Every compagny has a UV-VIS-NIR spectrophotometer with two sources ( Nerst glower, D 2 lamp) and two detectors (Pb. S, PM). • Integration sphere for DRS • White standards: Mg. O, Ba. SO 4, HALON.
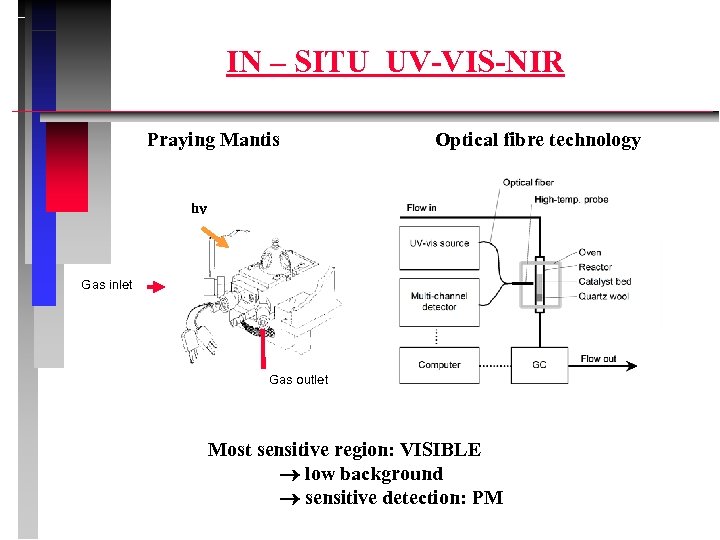
IN – SITU UV-VIS-NIR Praying Mantis Optical fibre technology hn Gas inlet Gas outlet Most sensitive region: VISIBLE low background sensitive detection: PM

IN – SITU UV-VIS-NIR Examples: d d (pseudo)tetrahedral Co 2+ O Cr 6+ charge transfer (chromate, dichromate) O Cu 2+ bis(µ-oxo)dicopper
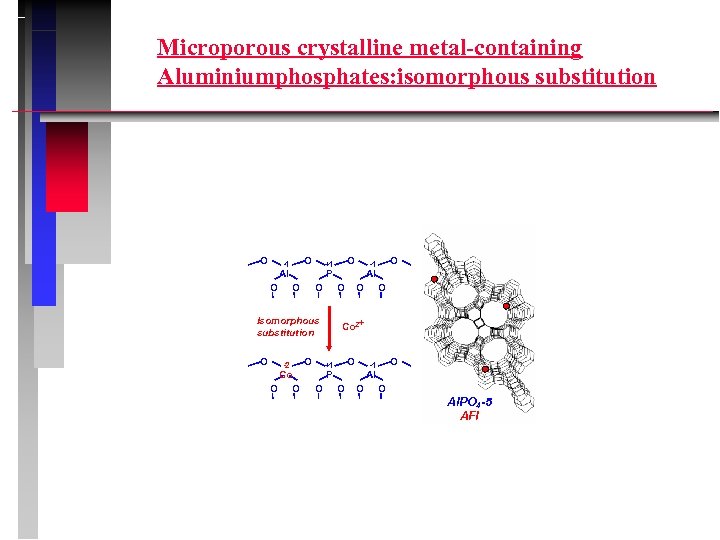
Microporous crystalline metal-containing Aluminiumphosphates: isomorphous substitution O O -1 Al O O -2 Co O O O Al O O O Co 2+ O +1 O -1 P O Isomorphous substitution O O +1 Al O O O Al. PO 4 -5 AFI
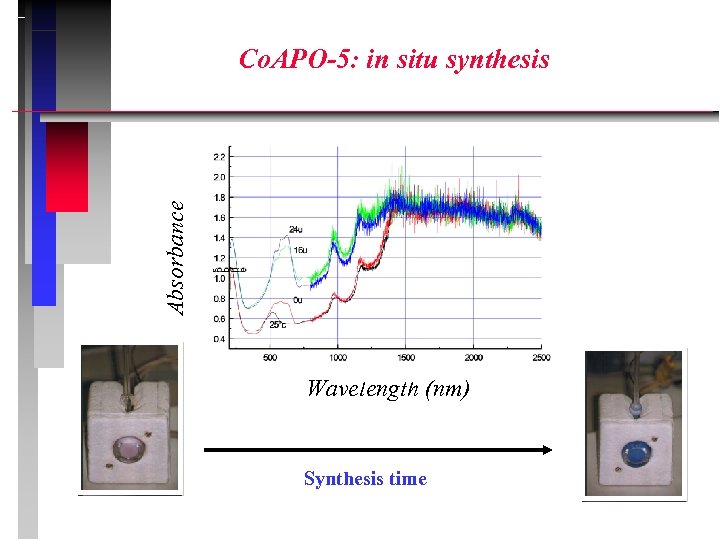
Absorbance Co. APO-5: in situ synthesis Wavelength (nm) Synthesis time
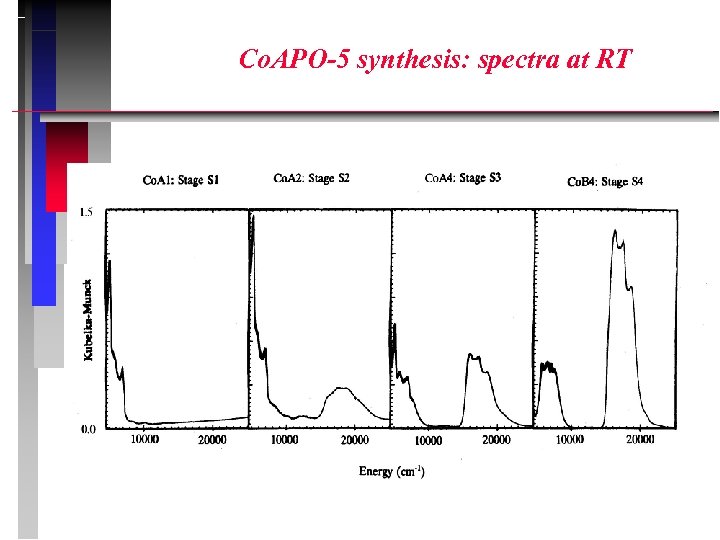
Co. APO-5 synthesis: spectra at RT
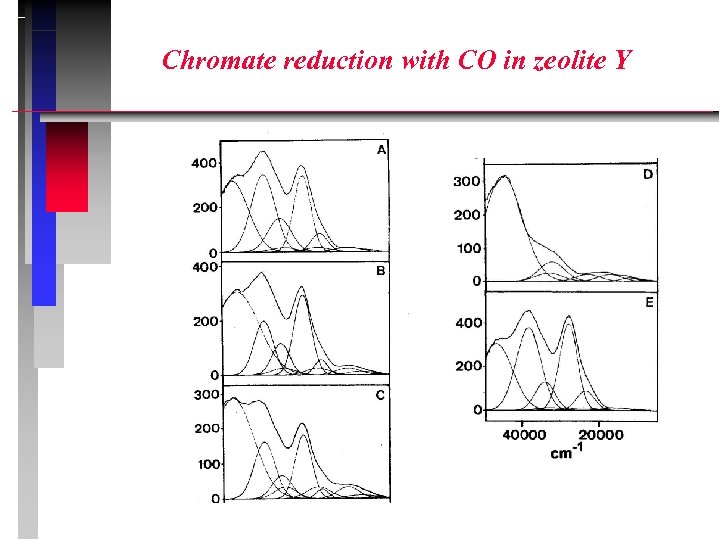
Chromate reduction with CO in zeolite Y
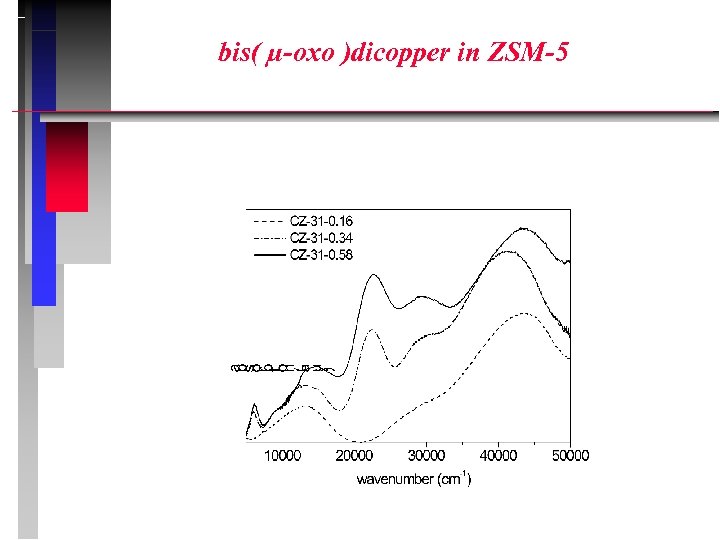
bis( µ-oxo )dicopper in ZSM-5
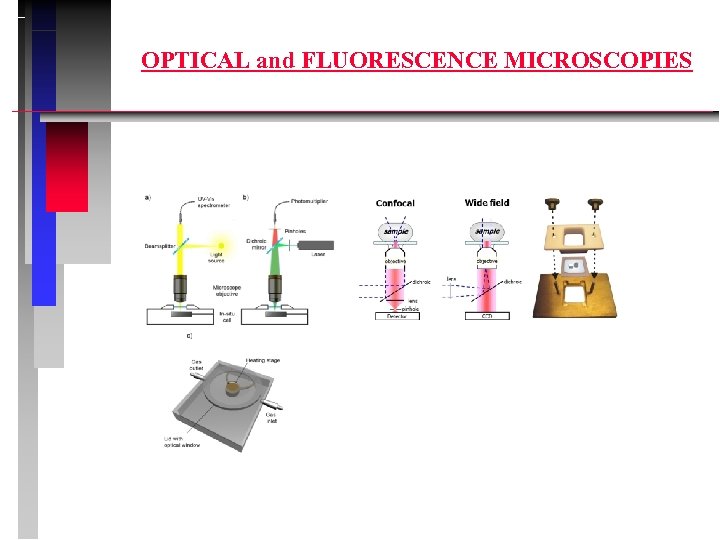
OPTICAL and FLUORESCENCE MICROSCOPIES
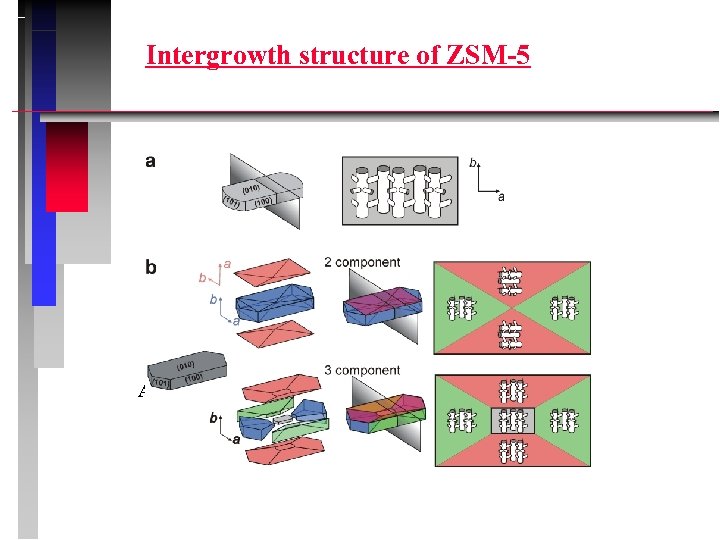
Intergrowth structure of ZSM-5 Accessibility?
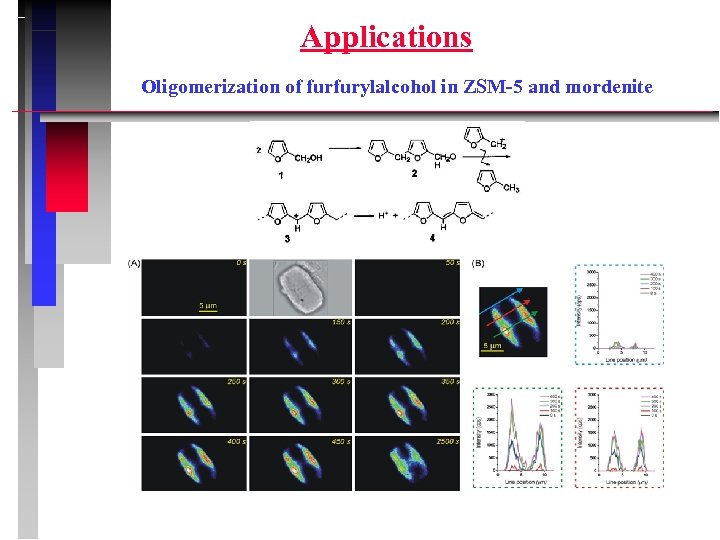
Applications Oligomerization of furfurylalcohol in ZSM-5 and mordenite
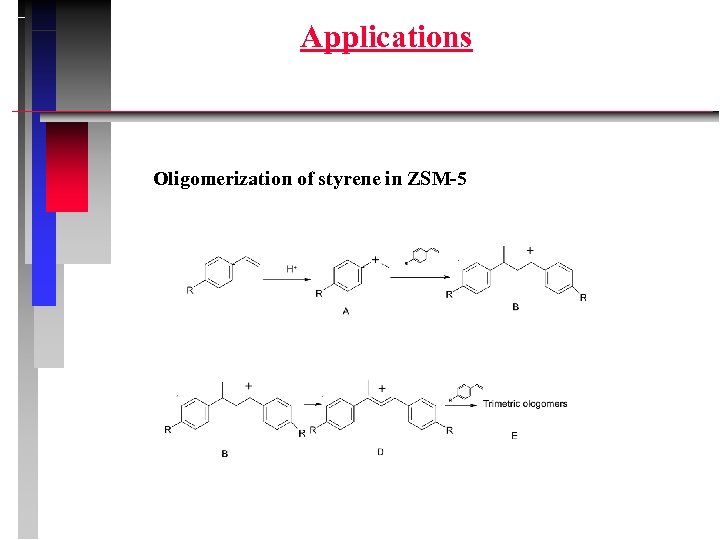
Applications Oligomerization of styrene in ZSM-5
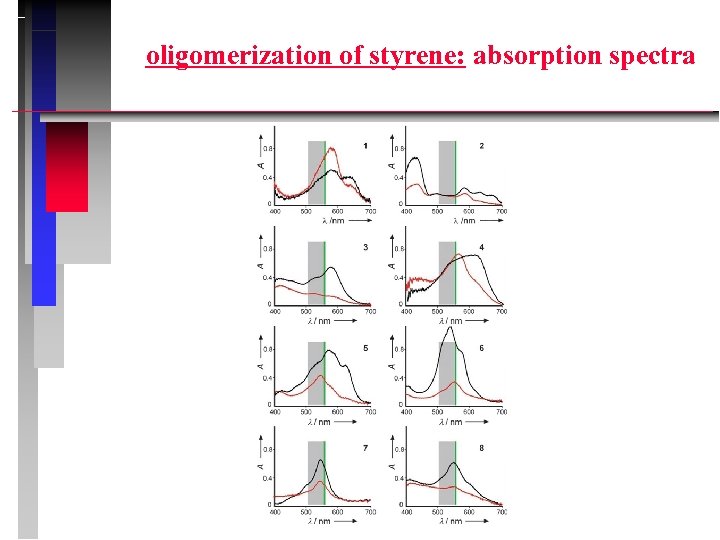
oligomerization of styrene: absorption spectra

Decomposition of template molecules in Cr. APO-5
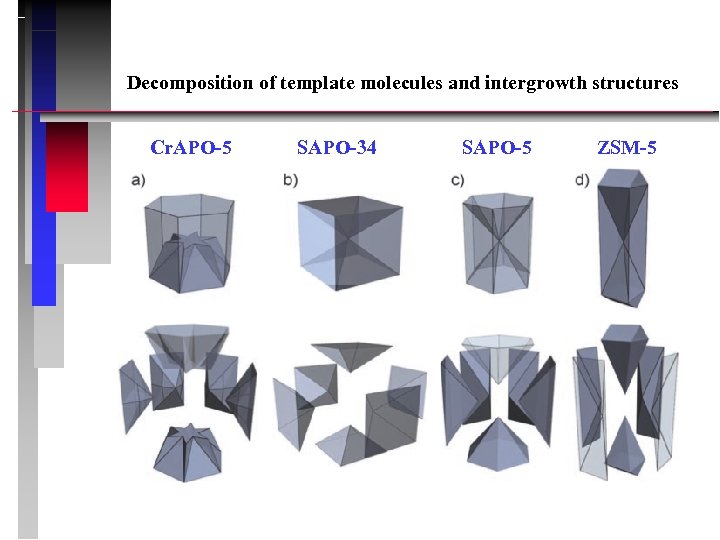
Decomposition of template molecules and intergrowth structures Cr. APO-5 SAPO-34 SAPO-5 ZSM-5
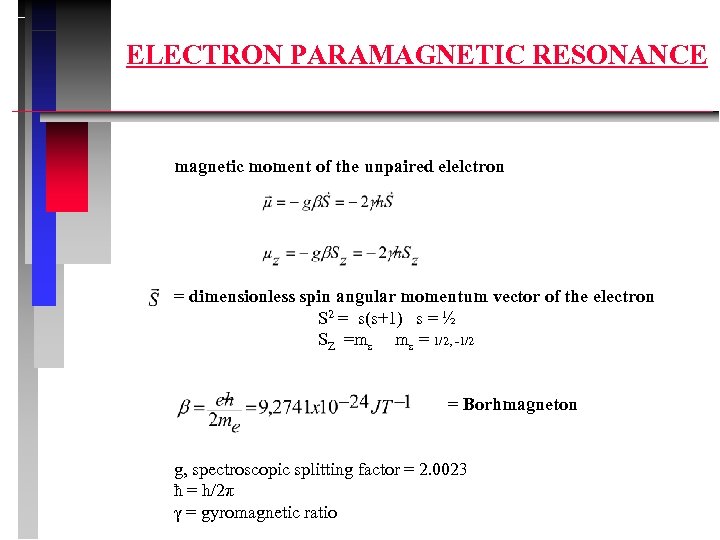
ELECTRON PARAMAGNETIC RESONANCE magnetic moment of the unpaired elelctron = dimensionless spin angular momentum vector of the electron S 2 = s(s+1) s = ½ SZ =ms ms = 1/2, -1/2 = Borhmagneton g, spectroscopic splitting factor = 2. 0023 ħ = h/2π γ = gyromagnetic ratio
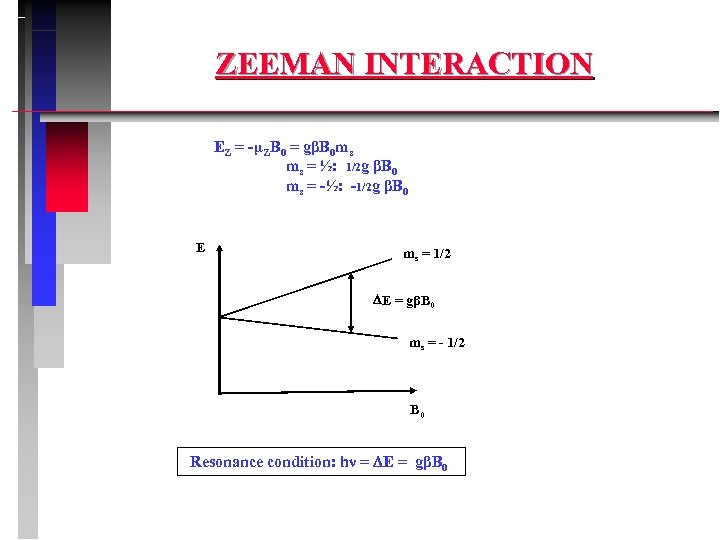
ZEEMAN INTERACTION EZ = -µZB 0 = gβB 0 ms ms = ½: 1/2 g βB 0 ms = -½: -1/2 g βB 0 E ms = 1/2 E = gβB 0 ms = - 1/2 B 0 Resonance condition: hν = E = gβB 0
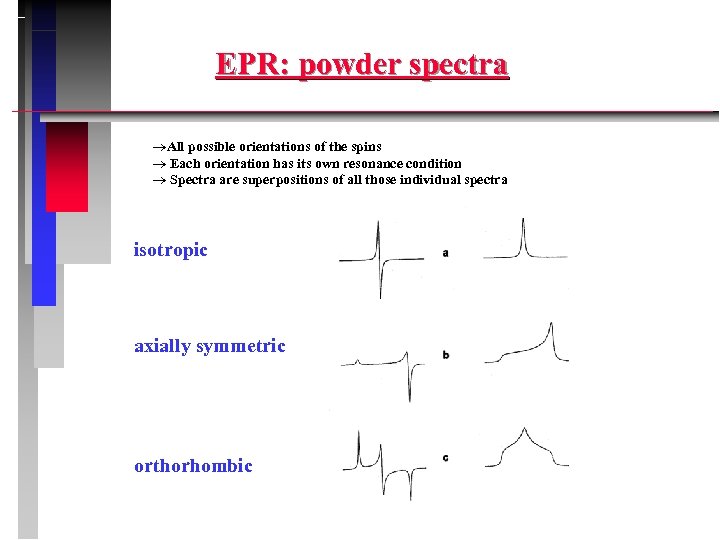
EPR: powder spectra All possible orientations of the spins Each orientation has its own resonance condition Spectra are superpositions of all those individual spectra isotropic axially symmetric orthorhombic
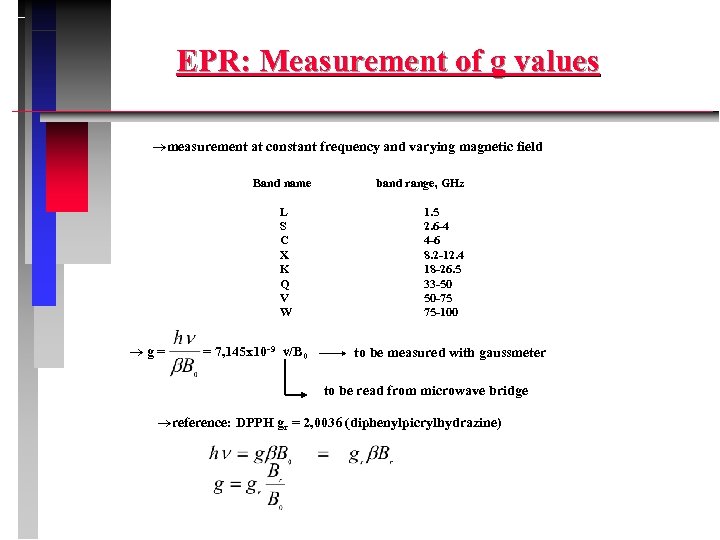
EPR: Measurement of g values measurement at constant frequency and varying magnetic field Band name L S C X K Q V W g= = 7, 145 x 10 -9 ν/B 0 band range, GHz 1. 5 2. 6 -4 4 -6 8. 2 -12. 4 18 -26. 5 33 -50 50 -75 75 -100 to be measured with gaussmeter to be read from microwave bridge reference: DPPH gr = 2, 0036 (diphenylpicrylhydrazine)

EPR: METHODOLOGIES Resonance cavities

EPR: Spin Hamiltonian -Hyperfine interaction: unpaired electron-nuclear spin: I m. I = I, I - 1, …. . , - I each energy level of the electron is split according to m I selection rule for EPR: ms = 1: m. I = 0 - S > ½ more than one unpaired electron: ZERO FIELD SPLITTING - QUADRUPOLAR INTERACTION: nuclear spins with I > 1/2 -SPIN HAMILTONIAN

EPR: Quantitative
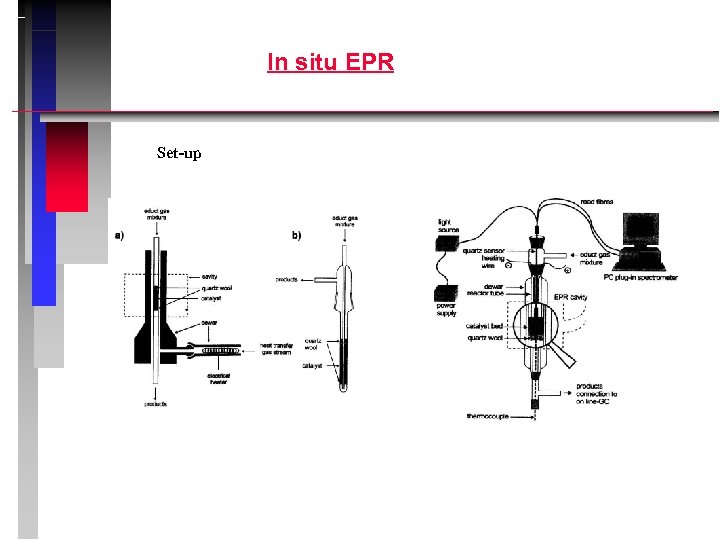
In situ EPR Set-up
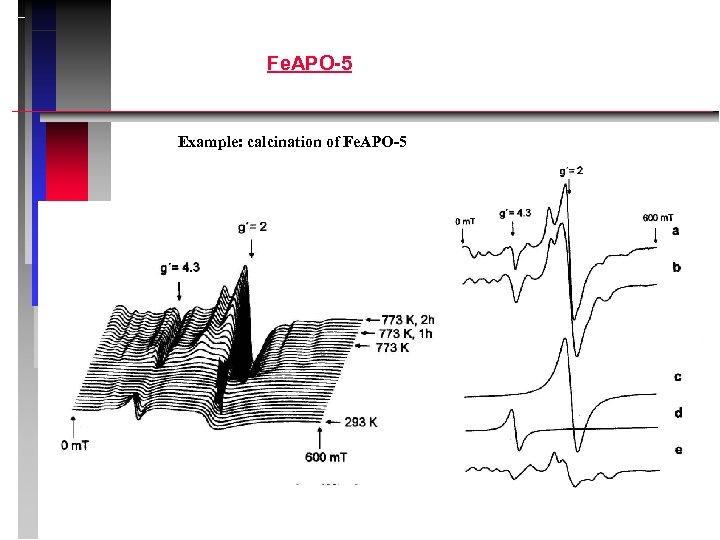
Fe. APO-5 Example: calcination of Fe. APO-5

PULSE EPR D. Goldfarb, Weizmann Institute, Israel ESEEM: electron spin echo envelope modulation ENDOR: electron nuclear double resonance Examples: 1. Interaction of Cu 2+ with Al nuclei in the zeolite lattice 2. Copper –histidine complexes in supercages of zeolite Y.
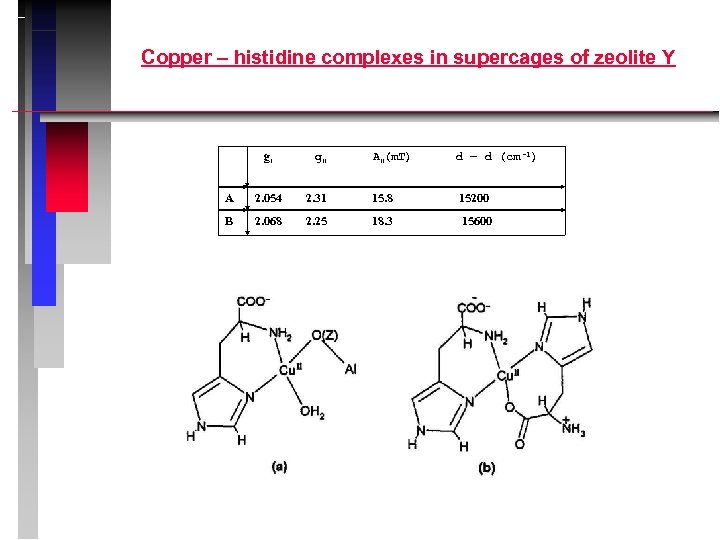
Copper – histidine complexes in supercages of zeolite Y g ﺍﺍ A (ﺍﺍ m. T) d – d (cm -1) A 2. 054 2. 31 15. 8 15200 B 2. 068 2. 25 18. 3 15600

TRANSITION METAL IONS IN ZEOLITES
Coordination to lattice oxygens Characteristics • Low coordination number • Free coordination sites • Low symmetry Examples: Cu 2+, Co 2+
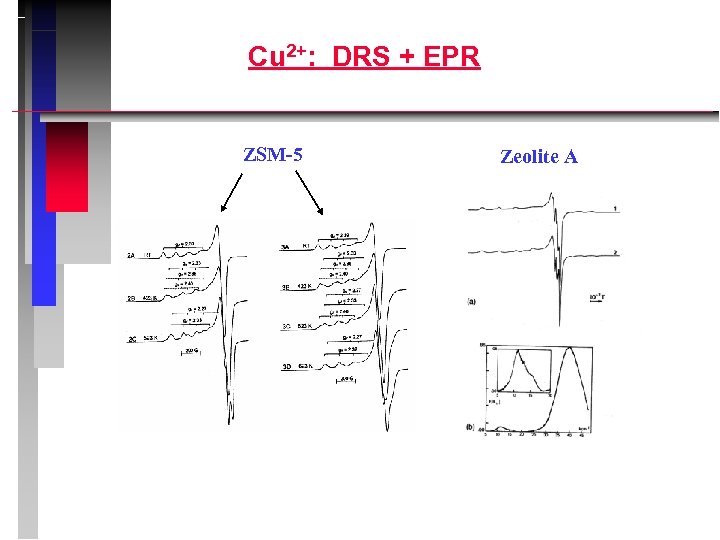
Cu 2+: DRS + EPR ZSM-5 Zeolite A
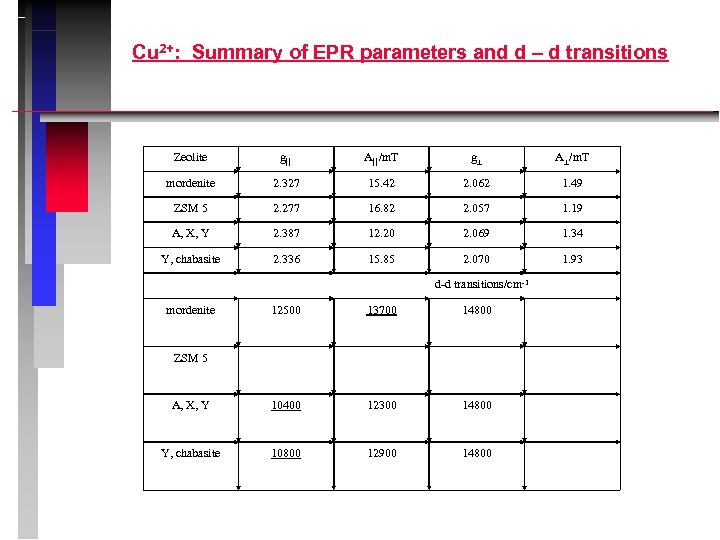
Cu 2+: Summary of EPR parameters and d – d transitions Zeolite g A /m. T mordenite 2. 327 15. 42 2. 062 1. 49 ZSM 5 2. 277 16. 82 2. 057 1. 19 A, X, Y 2. 387 12. 20 2. 069 1. 34 Y, chabasite 2. 336 15. 85 2. 070 1. 93 d-d transitions/cm-1 mordenite 12500 13700 14800 A, X, Y 10400 12300 14800 Y, chabasite 10800 12900 14800 ZSM 5
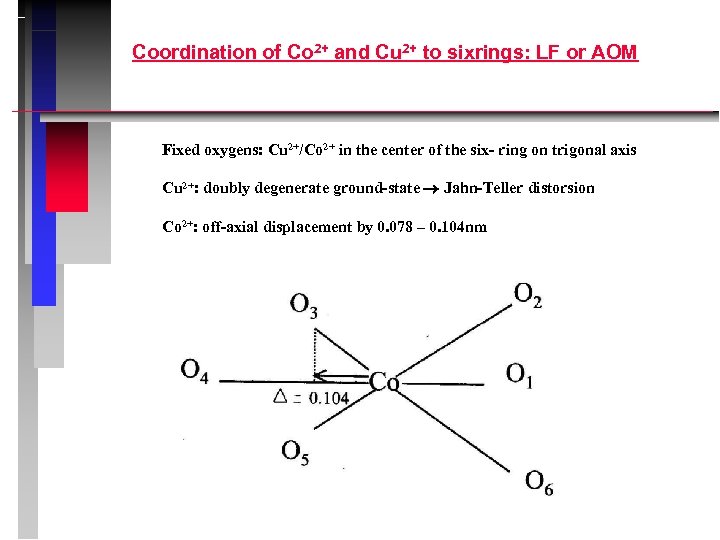
Coordination of Co 2+ and Cu 2+ to sixrings: LF or AOM Fixed oxygens: Cu 2+/Co 2+ in the center of the six- ring on trigonal axis Cu 2+: doubly degenerate ground-state Jahn-Teller distorsion Co 2+: off-axial displacement by 0. 078 – 0. 104 nm
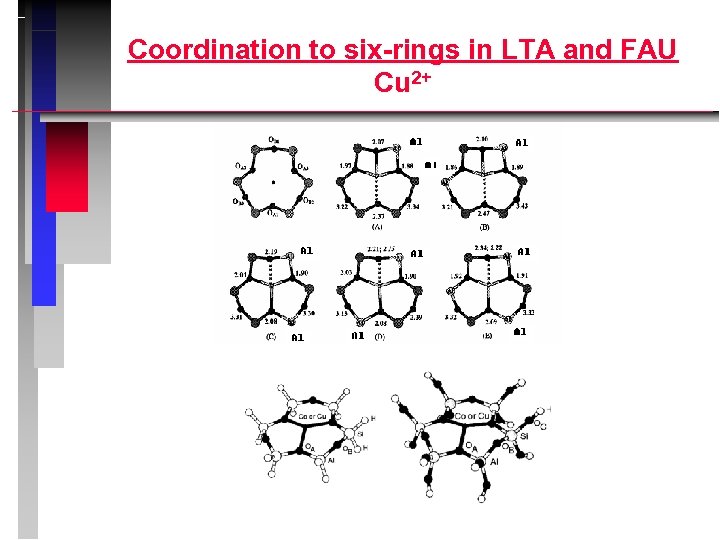
Coordination to six-rings in LTA and FAU Cu 2+
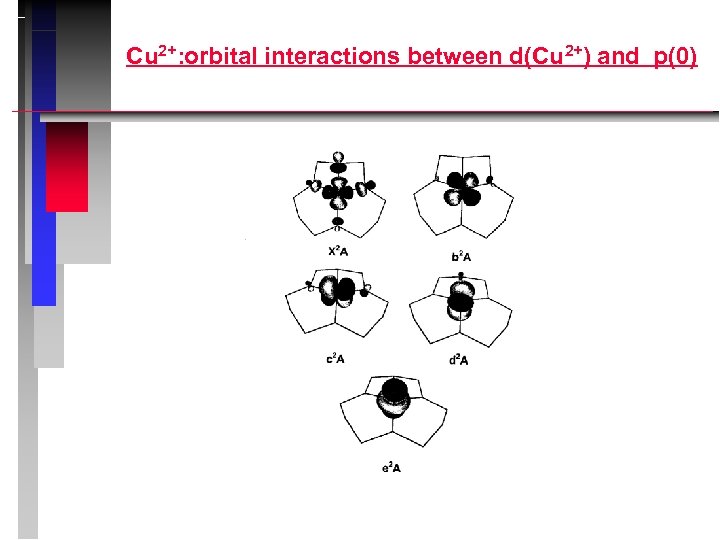
Cu 2+: orbital interactions between d(Cu 2+) and p(0)
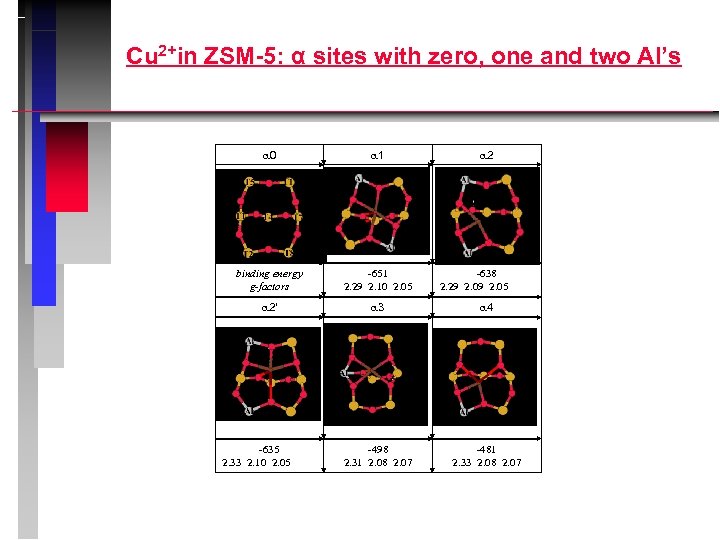
Cu 2+in ZSM-5: α sites with zero, one and two Al’s 0 1 binding energy g-factors -651 2. 29 2. 10 2. 05 2' 3 4 -498 2. 31 2. 08 2. 07 -481 2. 33 2. 08 2. 07 -635 2. 33 2. 10 2. 05 2 -638 2. 29 2. 05
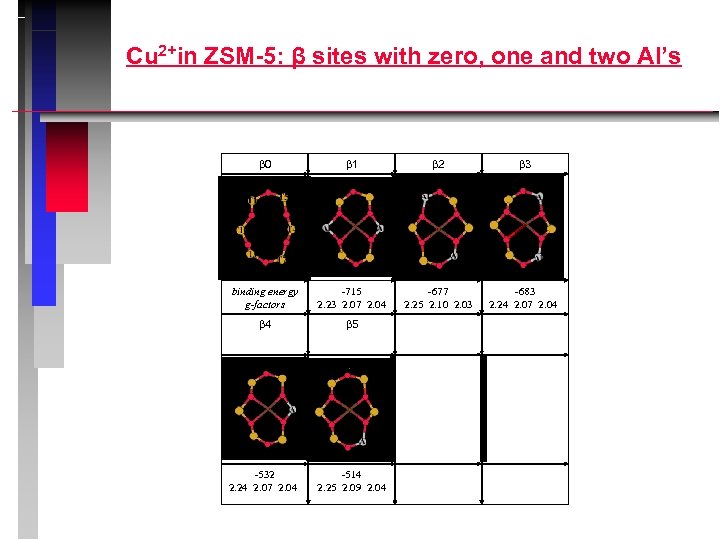
Cu 2+in ZSM-5: β sites with zero, one and two Al’s 0 1 2 3 binding energy g-factors -715 2. 23 2. 07 2. 04 -677 2. 25 2. 10 2. 03 -683 2. 24 2. 07 2. 04 4 5 -532 2. 24 2. 07 2. 04 -514 2. 25 2. 09 2. 04
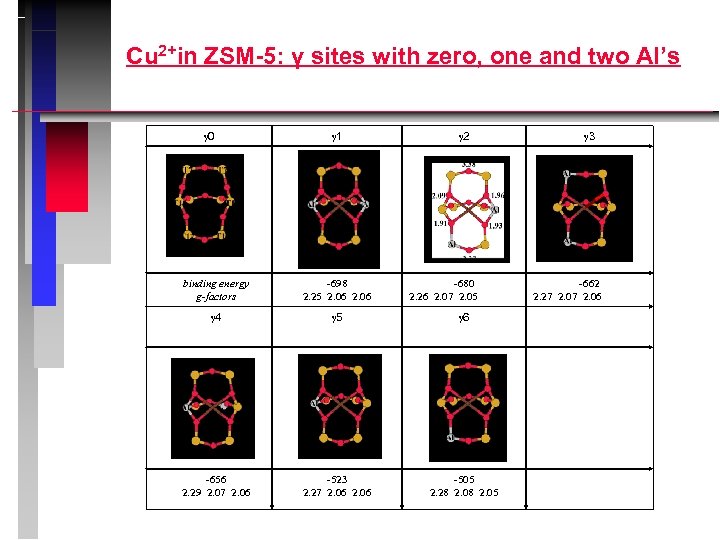
Cu 2+in ZSM-5: γ sites with zero, one and two Al’s 0 1 2 binding energy g-factors -698 2. 25 2. 06 -680 2. 26 2. 07 2. 05 4 5 6 -656 2. 29 2. 07 2. 06 -523 2. 27 2. 06 -505 2. 28 2. 05 3 -662 2. 27 2. 06
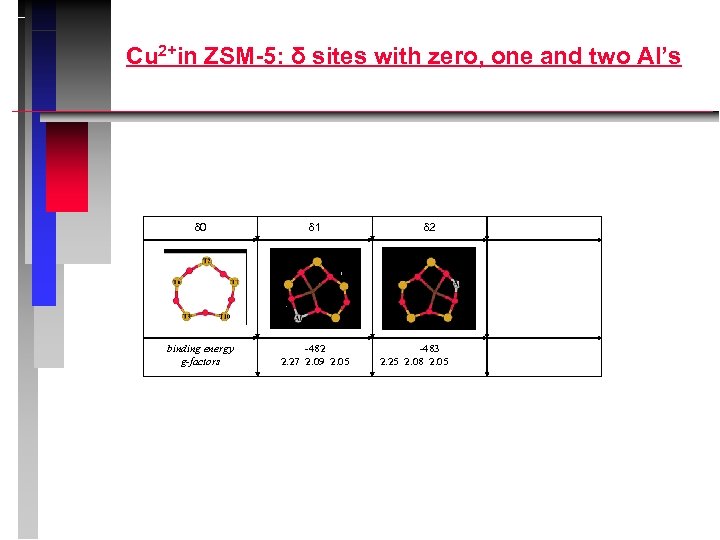
Cu 2+in ZSM-5: δ sites with zero, one and two Al’s 0 1 binding energy g-factors -482 2. 27 2. 09 2. 05 2 -483 2. 25 2. 08 2. 05
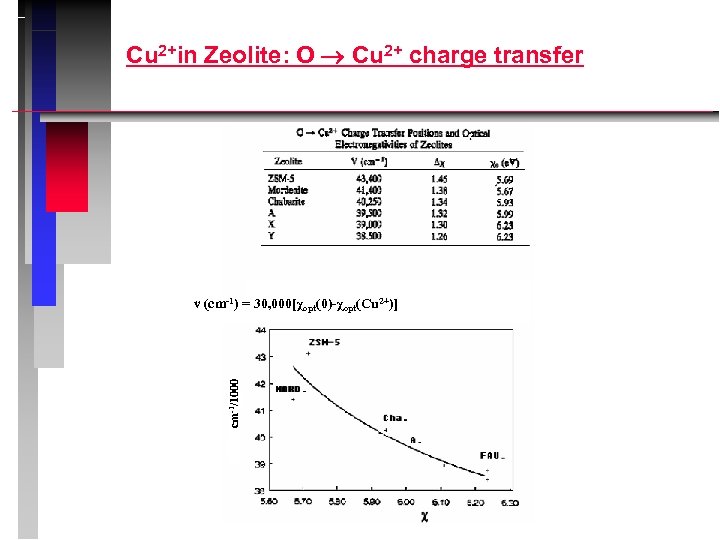
Cu 2+in Zeolite: O Cu 2+ charge transfer cm-1/1000 ν (cm-1) = 30, 000[χopt(0)-χopt(Cu 2+)]
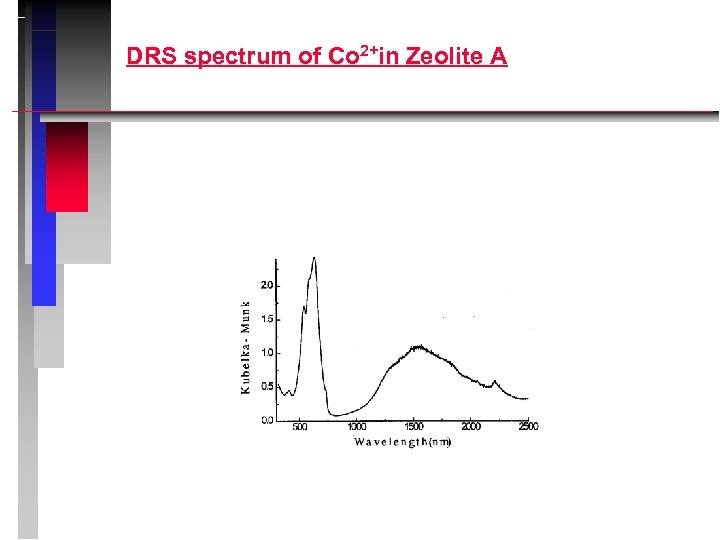
DRS spectrum of Co 2+in Zeolite A
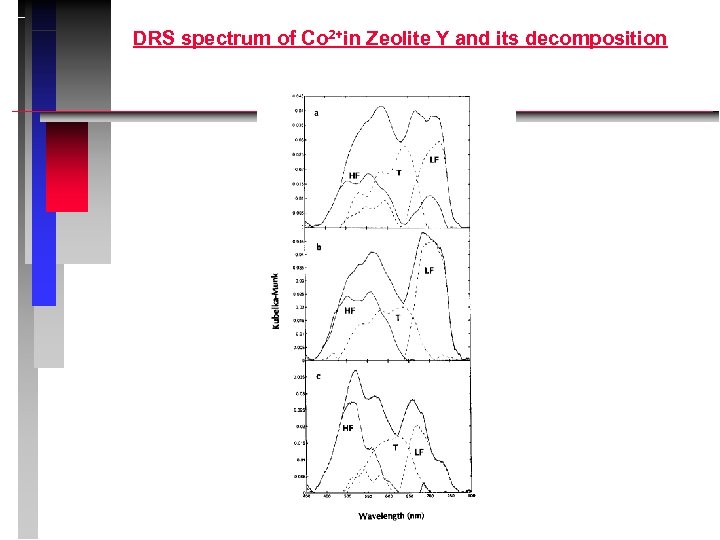
DRS spectrum of Co 2+in Zeolite Y and its decomposition
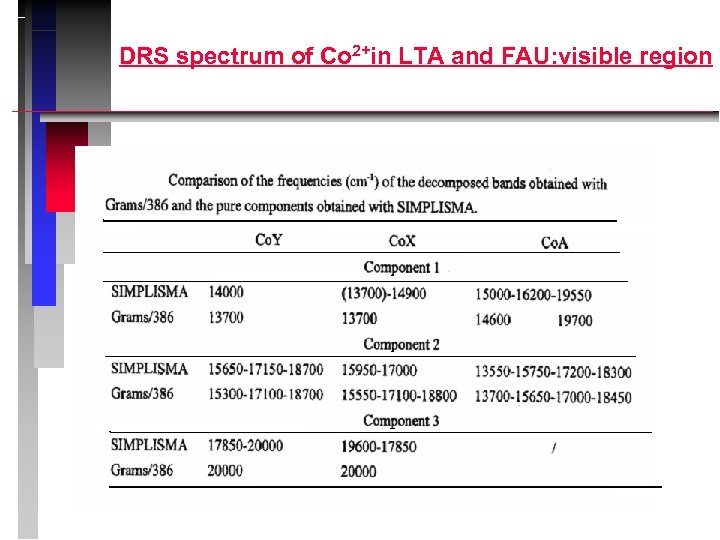
DRS spectrum of Co 2+in LTA and FAU: visible region
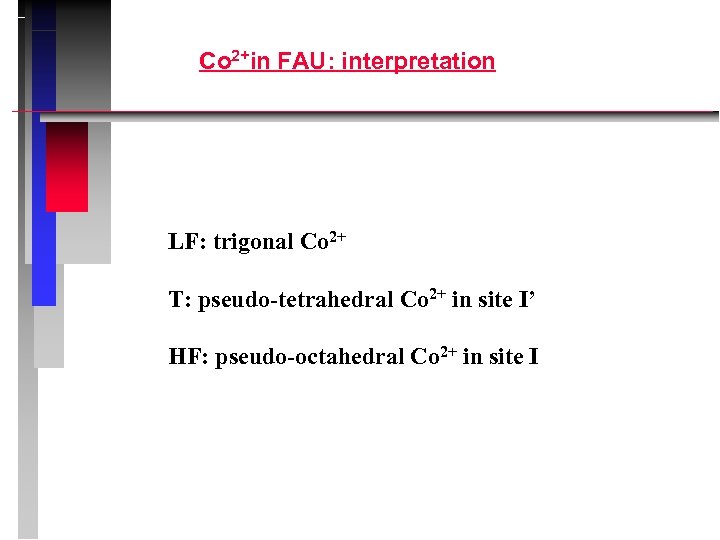
Co 2+in FAU: interpretation LF: trigonal Co 2+ T: pseudo-tetrahedral Co 2+ in site I’ HF: pseudo-octahedral Co 2+ in site I
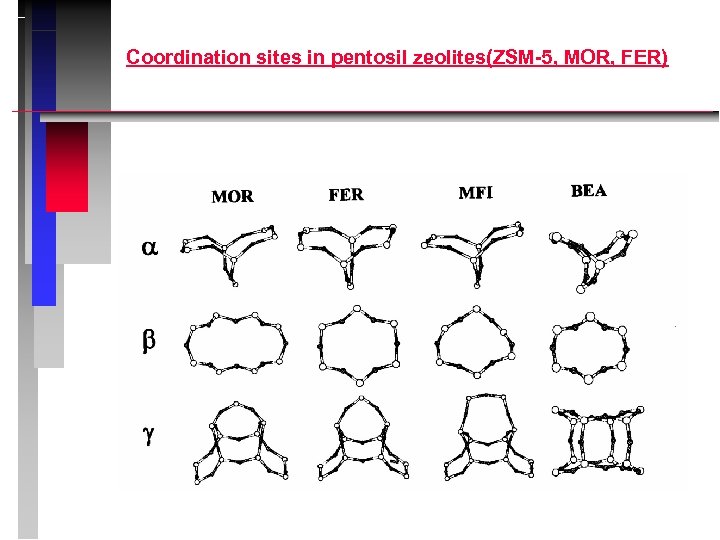
Coordination sites in pentosil zeolites(ZSM-5, MOR, FER)
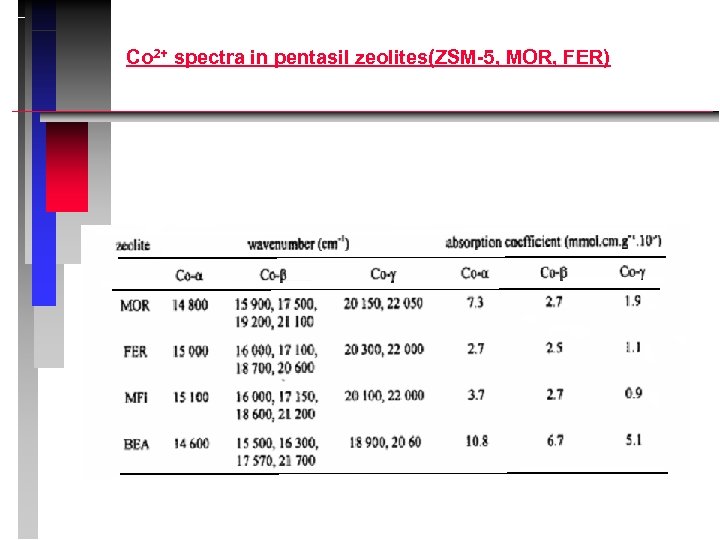
Co 2+ spectra in pentasil zeolites(ZSM-5, MOR, FER)
CONCLUSIONS 1. Significant technical advancement DRS in situ single crystal EPR wide range of resonance frequencies in situ pulse 2. Coordination of transition metal ions maximize coordination number site distortion number of Al tetrahedra 3. In situ UV-VIS: catalyst activation: chromate Cr 3+ active site: bis(µ-oxo)dicopper isomorphous substitution: Co 2+ EPR: isomorphous substitution of Fe 3+

CONCLUSIONS 4. Pulse EPR: - interaction TMI – Al in lattice - coordination chemistry of Cr(histidine)x in supercages - In situ techniques and pulse EPR give nice results in well-chosen problems. - Specialists are necessary; these are not routine measurements

Thanks to Collaborators: (D. Packet, S. De Tavernier, M. Uytterhoeven, B. Weckhuysen, A. Verberckmoes, M. Groothaert, H. Leeman) Collaborations: K. Pierloot and A. Ceulemans K. Klier Financial support: Concerted Research Action Fund for Scientific Research
8ec8bbe4a3443ef2528cb7ada4f07fc8.ppt