Адгезионные молекулы.ppt
- Количество слайдов: 31
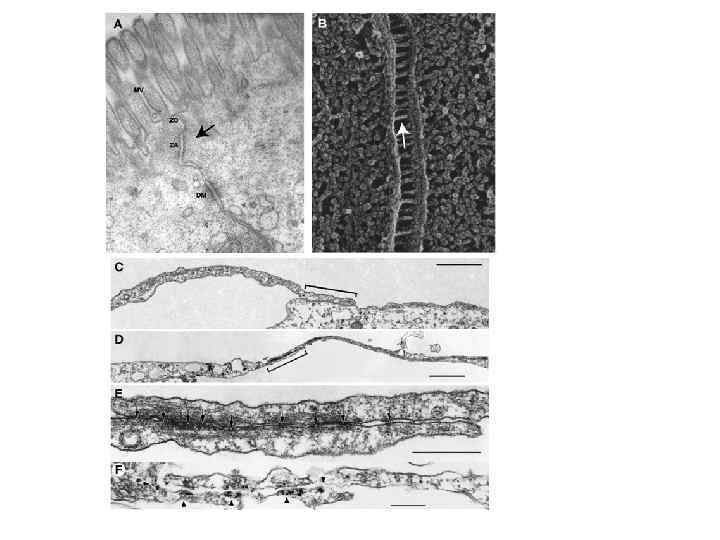
 Ultrastructural features of adherens junctions. A. Transmission electron micrograph of the zonula adherens (ZA) described by Farquhar and Palade [3] as part of a tripartite junction complex, where the ZA (black arrow) is located between the zonula occludens (ZO) and the desmosome (DM) just beneath the apical, microvillar (MV) domain. B. Quick-freeze, deep-etch image of the adherens junction between intestinal epithelial cells. Note the presence of rod-like bridging structures extending between adjacent cells at the attachment zone (white arrow) C–F. Electron micrographs of ultrathin sections from mesenchymal cell contacts. C. “Tentacle-like” process reveals contact without electron dense plaque structure (bracket). D and E. Different contact region at low (D) and higher magnification (E) reveals closely spaced adherens junctions characterized by dense, cytoplasmic plaques (arrows). F. Immunogold labeling shows β-catenin enrichment at these junctions (arrowheads).
Ultrastructural features of adherens junctions. A. Transmission electron micrograph of the zonula adherens (ZA) described by Farquhar and Palade [3] as part of a tripartite junction complex, where the ZA (black arrow) is located between the zonula occludens (ZO) and the desmosome (DM) just beneath the apical, microvillar (MV) domain. B. Quick-freeze, deep-etch image of the adherens junction between intestinal epithelial cells. Note the presence of rod-like bridging structures extending between adjacent cells at the attachment zone (white arrow) C–F. Electron micrographs of ultrathin sections from mesenchymal cell contacts. C. “Tentacle-like” process reveals contact without electron dense plaque structure (bracket). D and E. Different contact region at low (D) and higher magnification (E) reveals closely spaced adherens junctions characterized by dense, cytoplasmic plaques (arrows). F. Immunogold labeling shows β-catenin enrichment at these junctions (arrowheads).
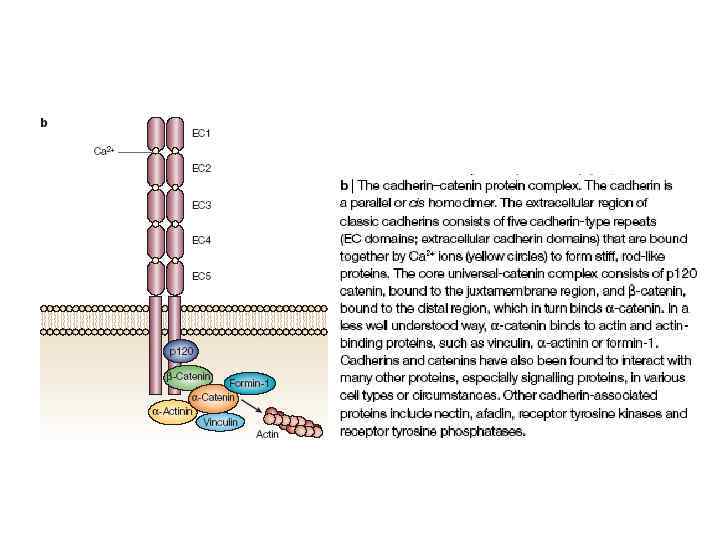
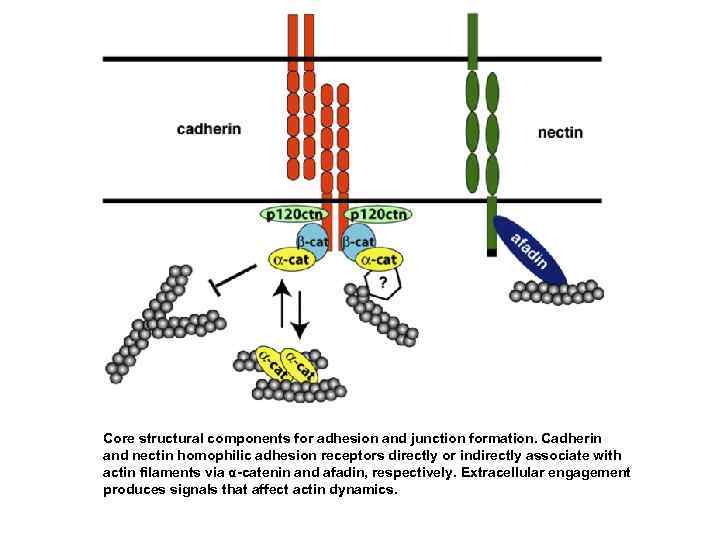 Core structural components for adhesion and junction formation. Cadherin and nectin homophilic adhesion receptors directly or indirectly associate with actin filaments via α-catenin and afadin, respectively. Extracellular engagement produces signals that affect actin dynamics.
Core structural components for adhesion and junction formation. Cadherin and nectin homophilic adhesion receptors directly or indirectly associate with actin filaments via α-catenin and afadin, respectively. Extracellular engagement produces signals that affect actin dynamics.
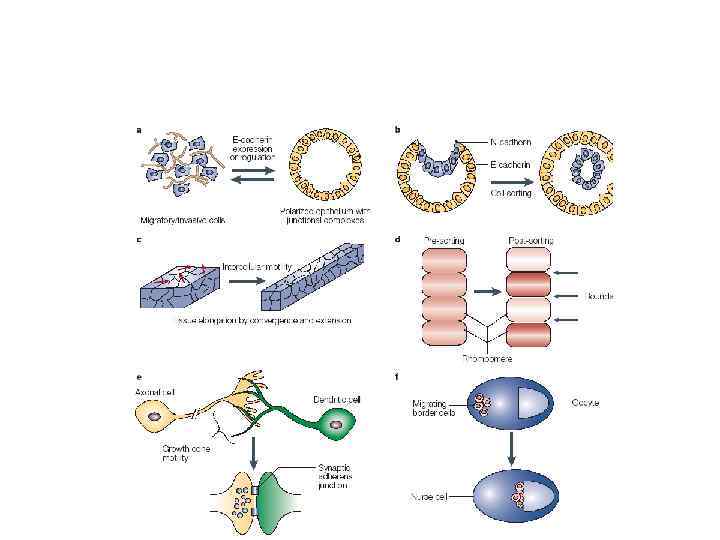
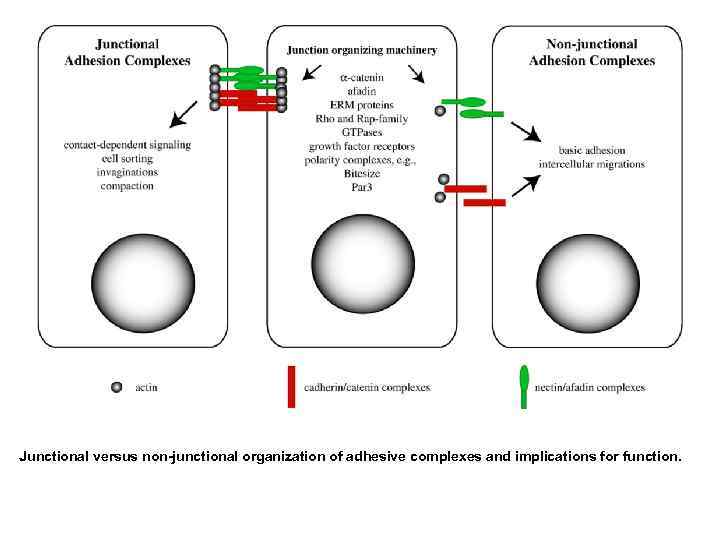 Junctional versus non-junctional organization of adhesive complexes and implications for function.
Junctional versus non-junctional organization of adhesive complexes and implications for function.

 In adult tissues, cadherins control • orderly turnover of rapidly growing tissues such as the lining of the gut and the epidermis, • the plasticity and regulation of neuronal synapses, • the physiological regulation of epithelial and endothelial cell • junctions to allow controlled passage of solutes, water and lymphoid cells across the cell layer, • the maintenance of stable tissue organization to prevent the dissociation and spread of tumour cells
In adult tissues, cadherins control • orderly turnover of rapidly growing tissues such as the lining of the gut and the epidermis, • the plasticity and regulation of neuronal synapses, • the physiological regulation of epithelial and endothelial cell • junctions to allow controlled passage of solutes, water and lymphoid cells across the cell layer, • the maintenance of stable tissue organization to prevent the dissociation and spread of tumour cells
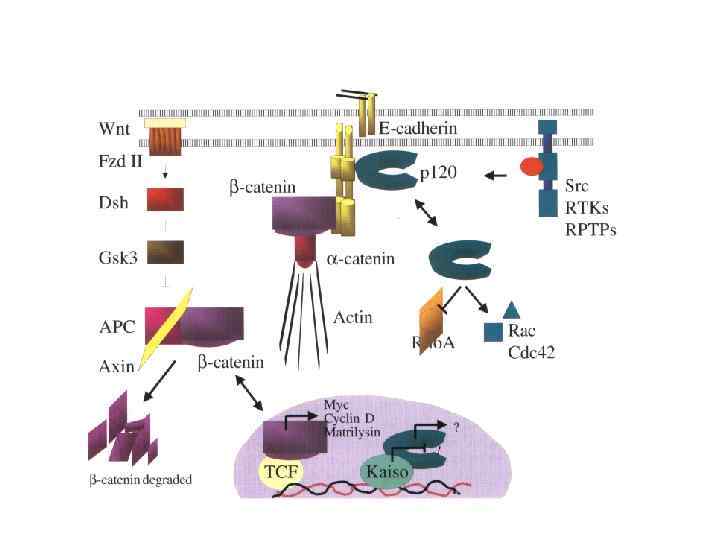
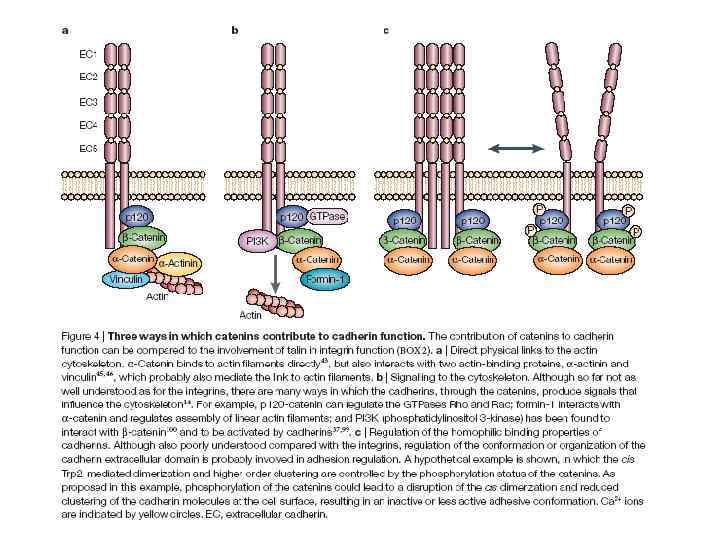
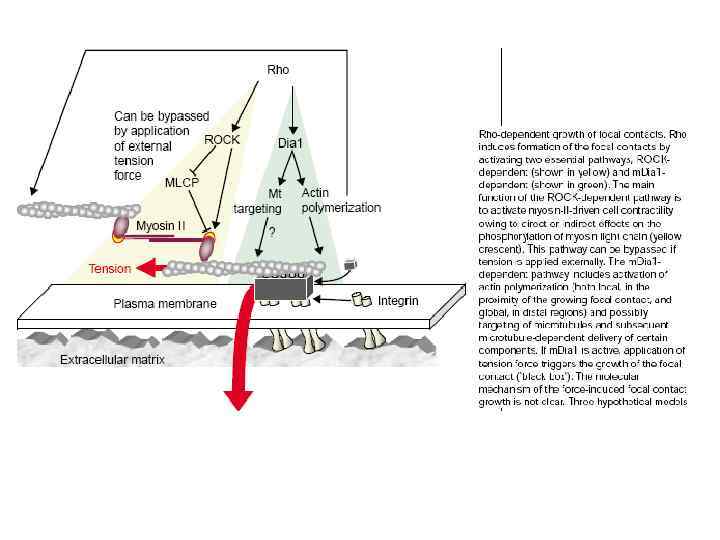
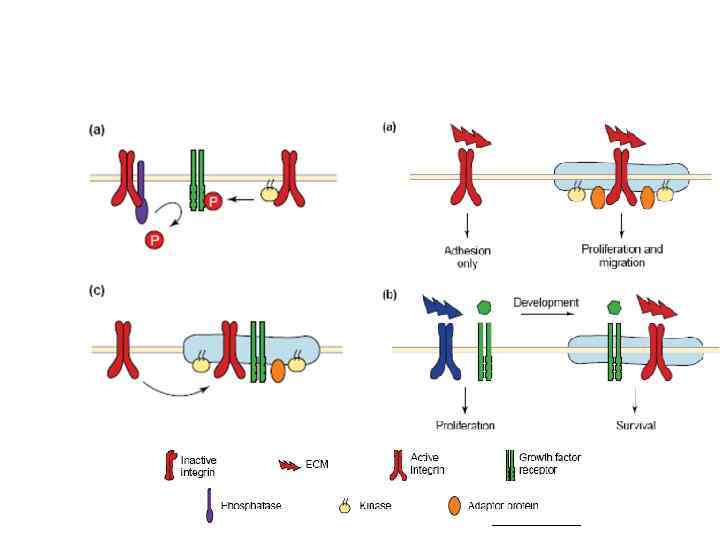
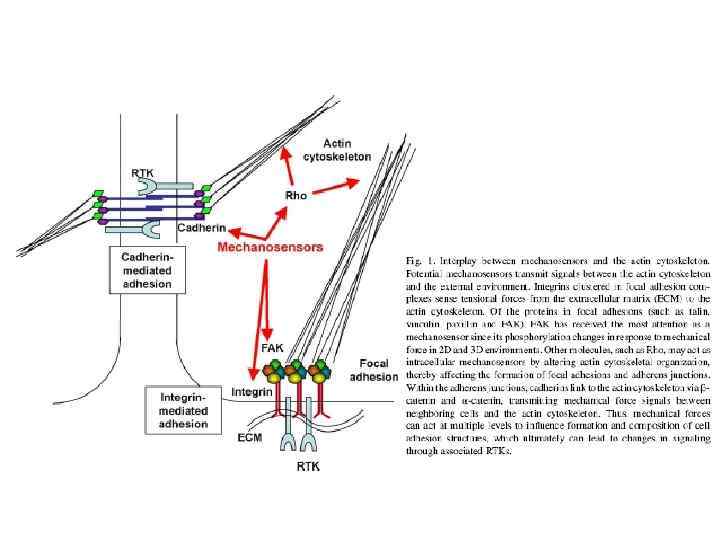
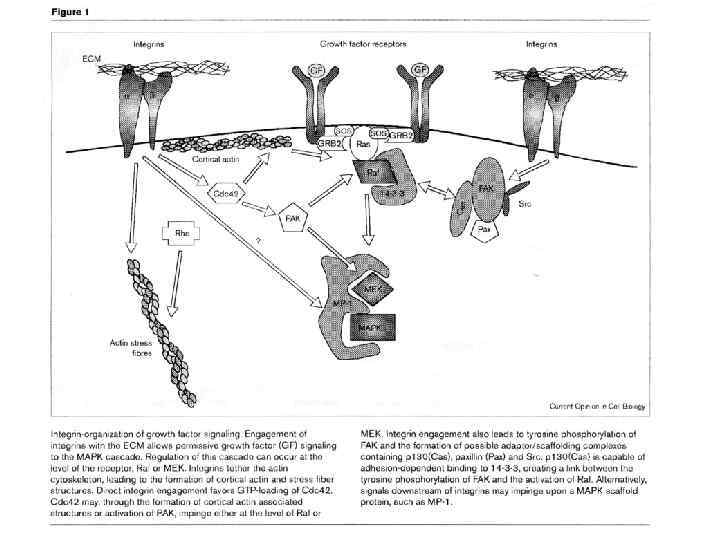
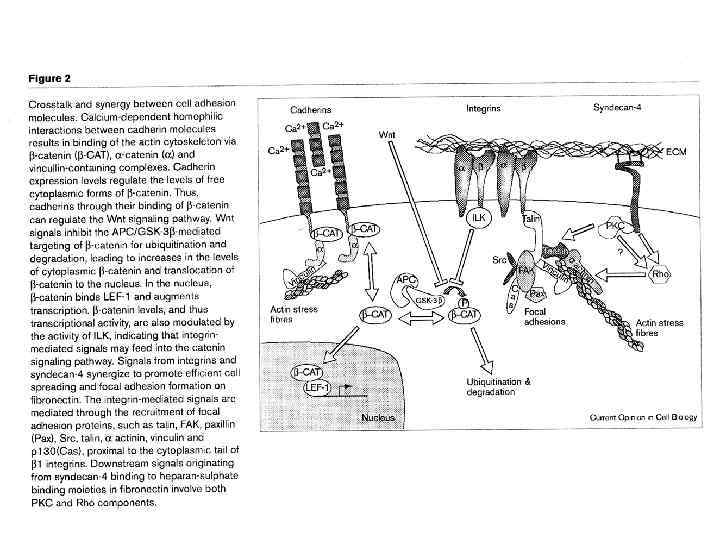
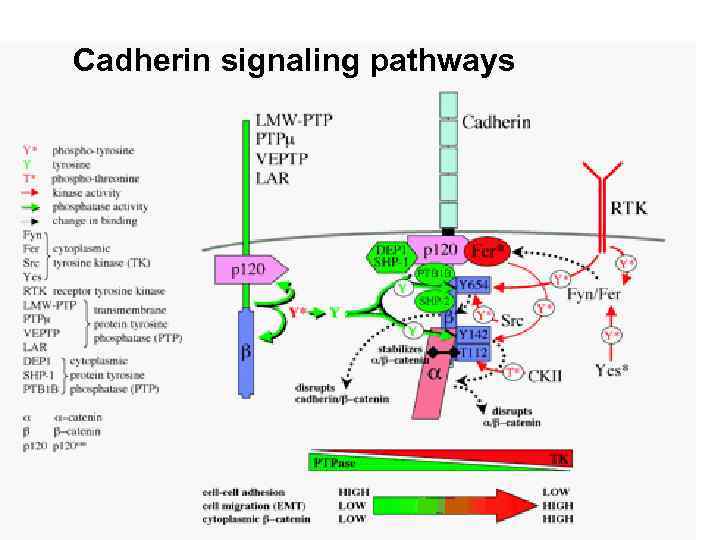 Cadherin signaling pathways
Cadherin signaling pathways
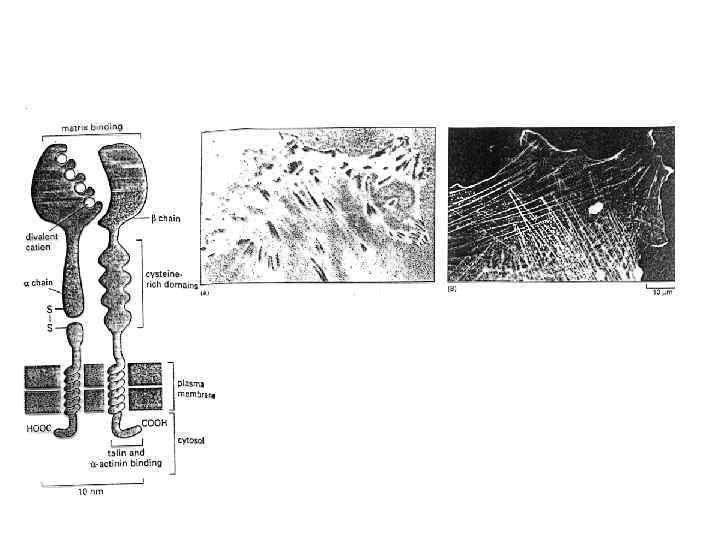
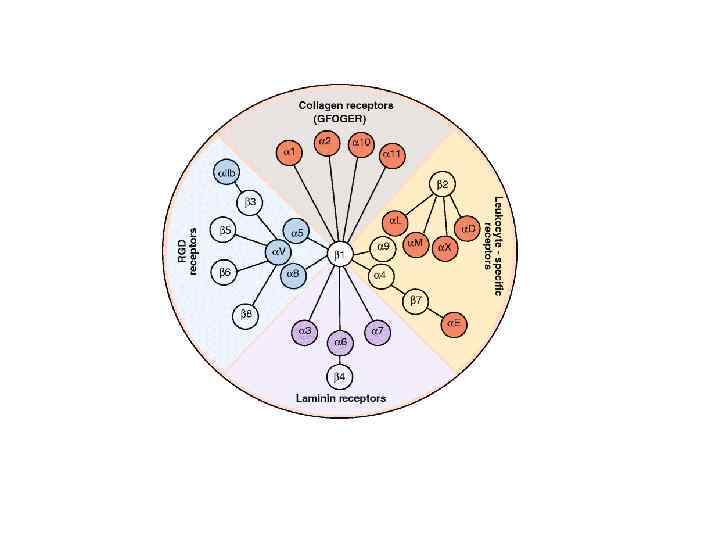
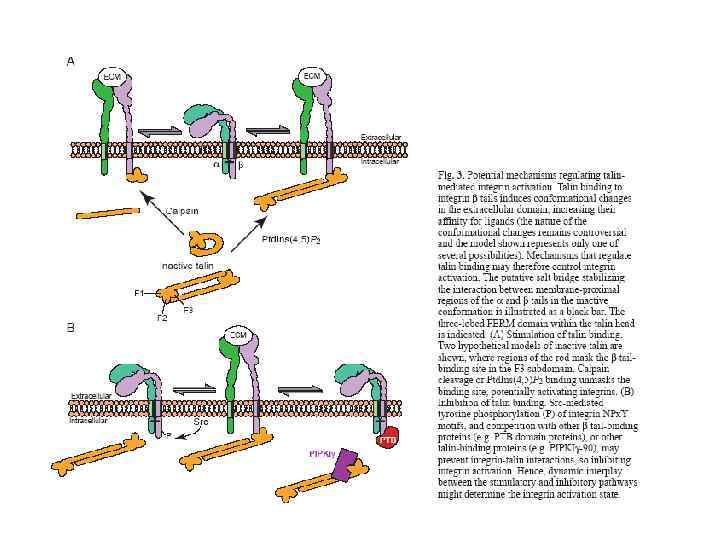
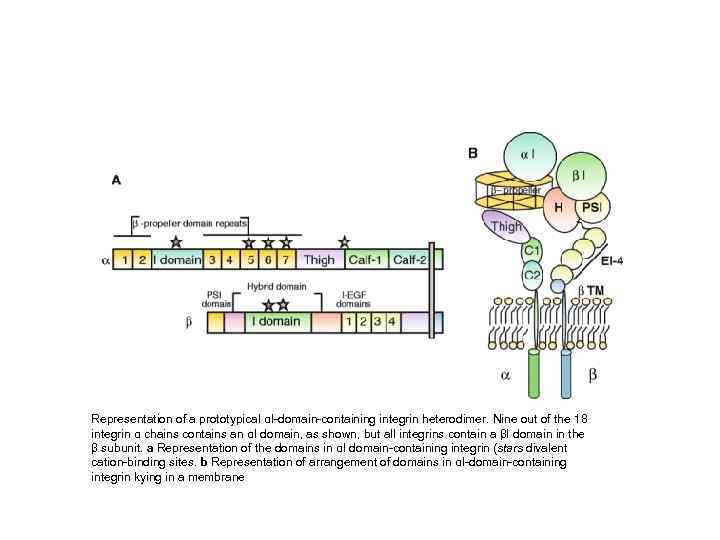 Representation of a prototypical αI-domain-containing integrin heterodimer. Nine out of the 18 integrin α chains contains an αI domain, as shown, but all integrins contain a βI domain in the β subunit. a Representation of the domains in αI domain-containing integrin (stars divalent cation-binding sites. b Representation of arrangement of domains in αI-domain-containing integrin kying in a membrane
Representation of a prototypical αI-domain-containing integrin heterodimer. Nine out of the 18 integrin α chains contains an αI domain, as shown, but all integrins contain a βI domain in the β subunit. a Representation of the domains in αI domain-containing integrin (stars divalent cation-binding sites. b Representation of arrangement of domains in αI-domain-containing integrin kying in a membrane

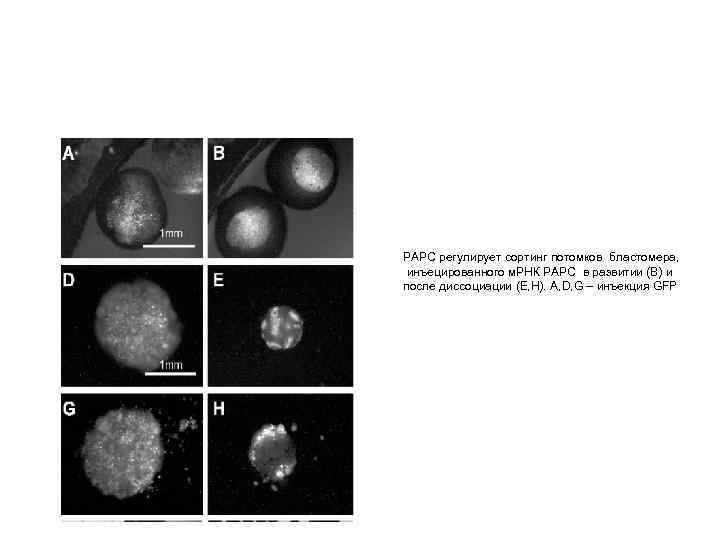 РАРС регулирует сортинг потомков бластомера, инъецированного м. РНК РАРС в развитии (В) и после диссоциации (Е, Н). A, D, G – инъекция GFP
РАРС регулирует сортинг потомков бластомера, инъецированного м. РНК РАРС в развитии (В) и после диссоциации (Е, Н). A, D, G – инъекция GFP
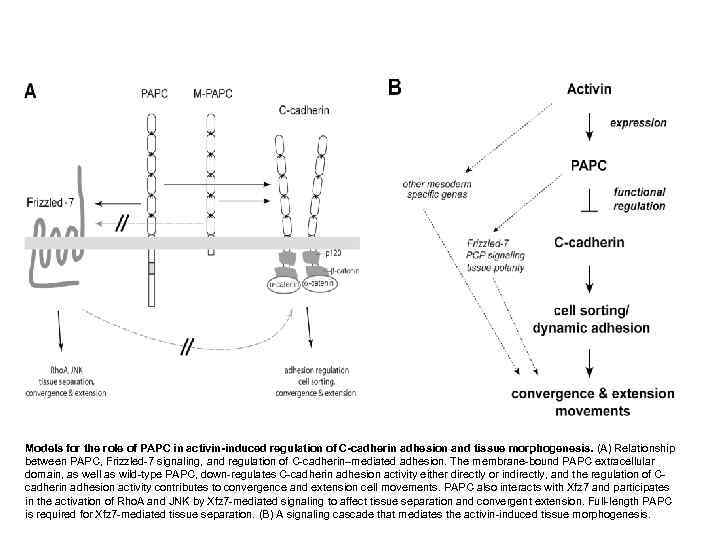 Models for the role of PAPC in activin-induced regulation of C-cadherin adhesion and tissue morphogenesis. (A) Relationship between PAPC, Frizzled-7 signaling, and regulation of C-cadherin–mediated adhesion. The membrane-bound PAPC extracellular domain, as well as wild-type PAPC, down-regulates C-cadherin adhesion activity either directly or indirectly, and the regulation of C- cadherin adhesion activity contributes to convergence and extension cell movements. PAPC also interacts with Xfz 7 and participates in the activation of Rho. A and JNK by Xfz 7 -mediated signaling to affect tissue separation and convergent extension. Full-length PAPC is required for Xfz 7 -mediated tissue separation. (B) A signaling cascade that mediates the activin-induced tissue morphogenesis.
Models for the role of PAPC in activin-induced regulation of C-cadherin adhesion and tissue morphogenesis. (A) Relationship between PAPC, Frizzled-7 signaling, and regulation of C-cadherin–mediated adhesion. The membrane-bound PAPC extracellular domain, as well as wild-type PAPC, down-regulates C-cadherin adhesion activity either directly or indirectly, and the regulation of C- cadherin adhesion activity contributes to convergence and extension cell movements. PAPC also interacts with Xfz 7 and participates in the activation of Rho. A and JNK by Xfz 7 -mediated signaling to affect tissue separation and convergent extension. Full-length PAPC is required for Xfz 7 -mediated tissue separation. (B) A signaling cascade that mediates the activin-induced tissue morphogenesis.