e4f396539cf551755eebc6188c5c2b82.ppt
- Количество слайдов: 33
 UDATE ON REVASCAT: (Randomized Trial Of Revascularization With Solitaire FR® Device Versus Best Medical Therapy In The Treatment Of Acute Stroke Due To Anterior Circulation Large Vessel Occlusion Presenting Within 8 Hours Of Symptom Onset) Tudor G. Jovin, M. D. Associate Professor of Neurology and Neurosurgery Director, UPMC Stroke Institute Director, UPMC Center for Neuroendovascular Therapy University of Pittsburgh Medical Center
UDATE ON REVASCAT: (Randomized Trial Of Revascularization With Solitaire FR® Device Versus Best Medical Therapy In The Treatment Of Acute Stroke Due To Anterior Circulation Large Vessel Occlusion Presenting Within 8 Hours Of Symptom Onset) Tudor G. Jovin, M. D. Associate Professor of Neurology and Neurosurgery Director, UPMC Stroke Institute Director, UPMC Center for Neuroendovascular Therapy University of Pittsburgh Medical Center
 DISCLOSURES Consultant/Advisory Board: Ownership Interest: Silk Road Medical – modest Consultant: Air Liquide- modest Consultant/Advisory Board: Covidien/Medtronic: unpaid Consultant: Stryker Neurovascular unpaid PI: REVASCAT (Fundacio Ictus Malaltia Vascular), unpaid PI: DAWN (Stryker Neurovascular), unpaid
DISCLOSURES Consultant/Advisory Board: Ownership Interest: Silk Road Medical – modest Consultant: Air Liquide- modest Consultant/Advisory Board: Covidien/Medtronic: unpaid Consultant: Stryker Neurovascular unpaid PI: REVASCAT (Fundacio Ictus Malaltia Vascular), unpaid PI: DAWN (Stryker Neurovascular), unpaid
 Disclosures • Study funded with an unrestricted grant by Covidien • Sponsor: Fundació ICTUS (Non-profit foundation) • Covidien was not involved in the study design, conduct, writing or review of the protocol or manuscript AAN, 2015 Washington DC 3
Disclosures • Study funded with an unrestricted grant by Covidien • Sponsor: Fundació ICTUS (Non-profit foundation) • Covidien was not involved in the study design, conduct, writing or review of the protocol or manuscript AAN, 2015 Washington DC 3
 Study objective To determine efficacy and safety of neurovascular thrombectomy with Solitaire in conjunction with medical therapy versus medical therapy alone, among eligible acute ischemic stroke patients treatable within 8 hours of symptom onset; To assess the proportion of REVASCAT eligible patients treated outside the study.
Study objective To determine efficacy and safety of neurovascular thrombectomy with Solitaire in conjunction with medical therapy versus medical therapy alone, among eligible acute ischemic stroke patients treatable within 8 hours of symptom onset; To assess the proportion of REVASCAT eligible patients treated outside the study.
 Study design • Sequential, randomized and open trial with external blinded-endpoint evaluation • Clinical sites: four designated Comprehensive Stroke Centers in Catalonia, Spain • Randomization 1: 1 ratio of thrombectomy with the stentriever Solitaire FR® plus medical therapy (including IV t. PA when eligible) versus medical therapy alone • Randomization was done under a minimization process using : üAge (≤ 70 or >70 years) üBaseline NIHSS (6 -16, or 17 or more) üRandomization window (≤ 4. 5 or >4. 5 hours) üVessel occlusion site (Intracranial ICA or M 1) üInvestigational center Molina C et al. Int J Stroke 2014
Study design • Sequential, randomized and open trial with external blinded-endpoint evaluation • Clinical sites: four designated Comprehensive Stroke Centers in Catalonia, Spain • Randomization 1: 1 ratio of thrombectomy with the stentriever Solitaire FR® plus medical therapy (including IV t. PA when eligible) versus medical therapy alone • Randomization was done under a minimization process using : üAge (≤ 70 or >70 years) üBaseline NIHSS (6 -16, or 17 or more) üRandomization window (≤ 4. 5 or >4. 5 hours) üVessel occlusion site (Intracranial ICA or M 1) üInvestigational center Molina C et al. Int J Stroke 2014
 Elegibility criteria • • Acute ischemic stroke ineligible for IV thrombolysis or where patient had received IV thrombolytic therapy without recanalization after 30 min from t. PA proven by CTA or MRA No pre-stroke functional disability (m. RS ≤ 1) Baseline NIHSS ≥ 6 points Age ≥ 18 and 80* Intracranial internal carotid (distal ICA or T occlusions) proximal MCA (M 1) occlusion or tandem occlusions (proximal ICA + M 1) as evidenced by CTA or MRA Patient treatable (groin puncture) within 8 hours of symptom onset and < 90 min from imaging Small ischemic core: Brain CT ASPECTS ≥ 7 or MR DWI ASPECTS ≥ 6 Informed consent * Age was amended up to 85 year in mid 2014 when ASPECTS 9 or 10 Molina C et al. Int J Stroke 2014
Elegibility criteria • • Acute ischemic stroke ineligible for IV thrombolysis or where patient had received IV thrombolytic therapy without recanalization after 30 min from t. PA proven by CTA or MRA No pre-stroke functional disability (m. RS ≤ 1) Baseline NIHSS ≥ 6 points Age ≥ 18 and 80* Intracranial internal carotid (distal ICA or T occlusions) proximal MCA (M 1) occlusion or tandem occlusions (proximal ICA + M 1) as evidenced by CTA or MRA Patient treatable (groin puncture) within 8 hours of symptom onset and < 90 min from imaging Small ischemic core: Brain CT ASPECTS ≥ 7 or MR DWI ASPECTS ≥ 6 Informed consent * Age was amended up to 85 year in mid 2014 when ASPECTS 9 or 10 Molina C et al. Int J Stroke 2014
 Primary efficacy endpoint Distribution of the modified Rankin Scale scores at 90 days as evaluated by two separate certified assessors who were masked to treatment: • Primary analysis carried out based on central video adjudication or in case this was missing on local blinded neurologist adjudication (31%) Molina C et al. Int J Stroke 2014
Primary efficacy endpoint Distribution of the modified Rankin Scale scores at 90 days as evaluated by two separate certified assessors who were masked to treatment: • Primary analysis carried out based on central video adjudication or in case this was missing on local blinded neurologist adjudication (31%) Molina C et al. Int J Stroke 2014
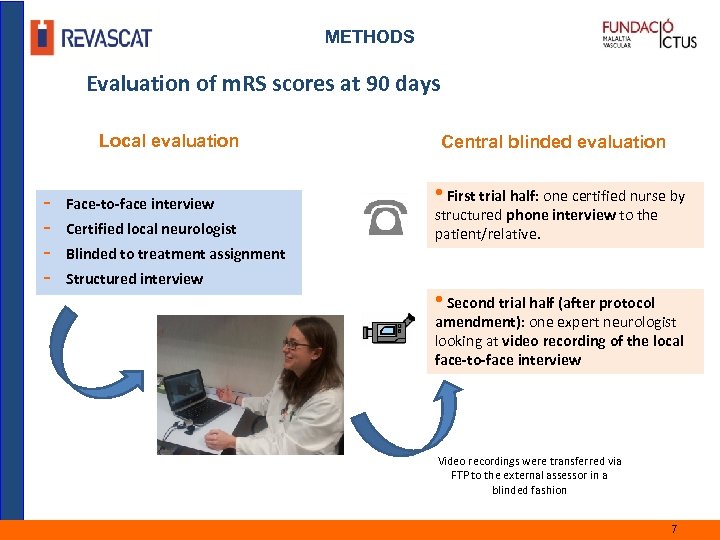 METHODS Evaluation of m. RS scores at 90 days Local evaluation - Face-to-face interview Certified local neurologist Blinded to treatment assignment Structured interview Central blinded evaluation • First trial half: one certified nurse by structured phone interview to the patient/relative. • Second trial half (after protocol amendment): one expert neurologist looking at video recording of the local face-to-face interview Video recordings were transferred via FTP to the external assessor in a blinded fashion 7
METHODS Evaluation of m. RS scores at 90 days Local evaluation - Face-to-face interview Certified local neurologist Blinded to treatment assignment Structured interview Central blinded evaluation • First trial half: one certified nurse by structured phone interview to the patient/relative. • Second trial half (after protocol amendment): one expert neurologist looking at video recording of the local face-to-face interview Video recordings were transferred via FTP to the external assessor in a blinded fashion 7
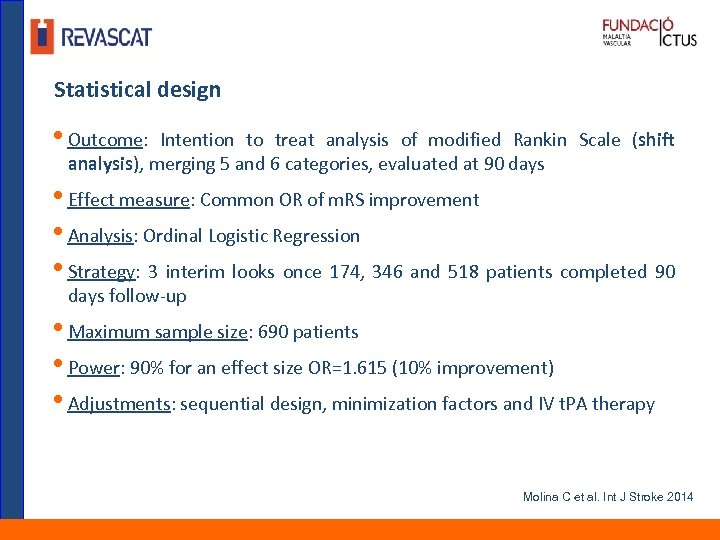 Statistical design • Outcome: Intention to treat analysis of modified Rankin Scale (shift analysis), merging 5 and 6 categories, evaluated at 90 days • Effect measure: Common OR of m. RS improvement • Analysis: Ordinal Logistic Regression • Strategy: 3 interim looks once 174, 346 and 518 patients completed 90 days follow-up • Maximum sample size: 690 patients • Power: 90% for an effect size OR=1. 615 (10% improvement) • Adjustments: sequential design, minimization factors and IV t. PA therapy Molina C et al. Int J Stroke 2014
Statistical design • Outcome: Intention to treat analysis of modified Rankin Scale (shift analysis), merging 5 and 6 categories, evaluated at 90 days • Effect measure: Common OR of m. RS improvement • Analysis: Ordinal Logistic Regression • Strategy: 3 interim looks once 174, 346 and 518 patients completed 90 days follow-up • Maximum sample size: 690 patients • Power: 90% for an effect size OR=1. 615 (10% improvement) • Adjustments: sequential design, minimization factors and IV t. PA therapy Molina C et al. Int J Stroke 2014
 Trial termination On December 12, 2014, following the first interim analysis (n=174), the steering committee decided to accept the DSMB’s recommendation to stop the trial due to loss of equipoise in the trial population. Consequently, adjustment for sequential design was not necessary. REVASCAT enrolled 206 patients from November 2012 through December 2014 at 2. 1 patients per center per month. 90 day follow-up ended in March 10 th 2015, and main primary and secondary outcomes were available in March 20 th. AAN, 2015 Washington DC 10
Trial termination On December 12, 2014, following the first interim analysis (n=174), the steering committee decided to accept the DSMB’s recommendation to stop the trial due to loss of equipoise in the trial population. Consequently, adjustment for sequential design was not necessary. REVASCAT enrolled 206 patients from November 2012 through December 2014 at 2. 1 patients per center per month. 90 day follow-up ended in March 10 th 2015, and main primary and secondary outcomes were available in March 20 th. AAN, 2015 Washington DC 10
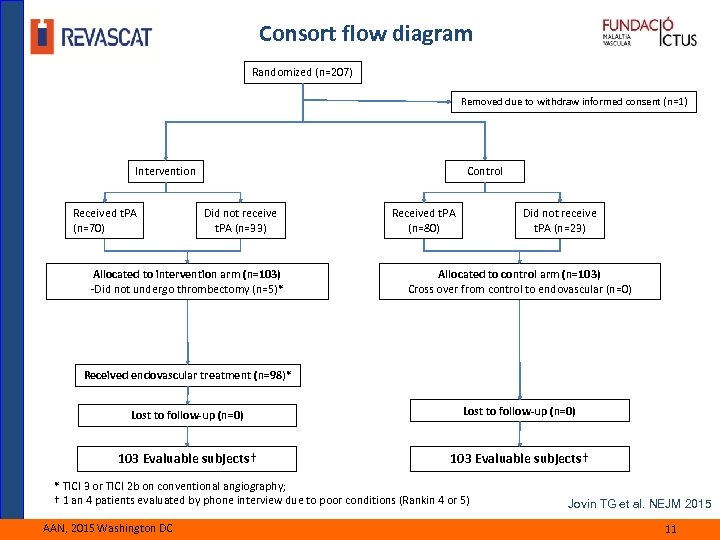 Consort flow diagram Randomized (n=207) Removed due to withdraw informed consent (n=1) Intervention Received t. PA (n=70) Control Did not receive t. PA (n=33) Allocated to intervention arm (n=103) -Did not undergo thrombectomy (n=5)* Received t. PA (n=80) Did not receive t. PA (n=23) Allocated to control arm (n=103) Cross over from control to endovascular (n=0) Received endovascular treatment (n=98)* Lost to follow-up (n=0) 103 Evaluable subjects† * TICI 3 or TICI 2 b on conventional angiography; † 1 an 4 patients evaluated by phone interview due to poor conditions (Rankin 4 or 5) AAN, 2015 Washington DC Jovin TG et al. NEJM 2015 11
Consort flow diagram Randomized (n=207) Removed due to withdraw informed consent (n=1) Intervention Received t. PA (n=70) Control Did not receive t. PA (n=33) Allocated to intervention arm (n=103) -Did not undergo thrombectomy (n=5)* Received t. PA (n=80) Did not receive t. PA (n=23) Allocated to control arm (n=103) Cross over from control to endovascular (n=0) Received endovascular treatment (n=98)* Lost to follow-up (n=0) 103 Evaluable subjects† * TICI 3 or TICI 2 b on conventional angiography; † 1 an 4 patients evaluated by phone interview due to poor conditions (Rankin 4 or 5) AAN, 2015 Washington DC Jovin TG et al. NEJM 2015 11
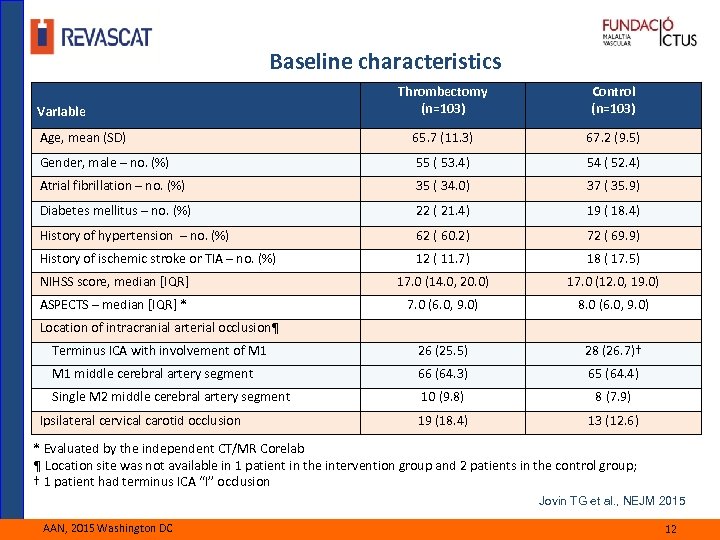 Baseline characteristics Thrombectomy (n=103) Control (n=103) Age, mean (SD) 65. 7 (11. 3) 67. 2 (9. 5) Gender, male – no. (%) 55 ( 53. 4) 54 ( 52. 4) Atrial fibrillation – no. (%) 35 ( 34. 0) 37 ( 35. 9) Diabetes mellitus – no. (%) 22 ( 21. 4) 19 ( 18. 4) History of hypertension – no. (%) 62 ( 60. 2) 72 ( 69. 9) History of ischemic stroke or TIA – no. (%) 12 ( 11. 7) 18 ( 17. 5) NIHSS score, median [IQR] 17. 0 (14. 0, 20. 0) 17. 0 (12. 0, 19. 0) ASPECTS – median [IQR] * 7. 0 (6. 0, 9. 0) 8. 0 (6. 0, 9. 0) Terminus ICA with involvement of M 1 26 (25. 5) 28 (26. 7)† M 1 middle cerebral artery segment 66 (64. 3) 65 (64. 4) Single M 2 middle cerebral artery segment 10 (9. 8) 8 (7. 9) Ipsilateral cervical carotid occlusion 19 (18. 4) 13 (12. 6) Variable Location of intracranial arterial occlusion¶ * Evaluated by the independent CT/MR Corelab ¶ Location site was not available in 1 patient in the intervention group and 2 patients in the control group; † 1 patient had terminus ICA “I” occlusion Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 12
Baseline characteristics Thrombectomy (n=103) Control (n=103) Age, mean (SD) 65. 7 (11. 3) 67. 2 (9. 5) Gender, male – no. (%) 55 ( 53. 4) 54 ( 52. 4) Atrial fibrillation – no. (%) 35 ( 34. 0) 37 ( 35. 9) Diabetes mellitus – no. (%) 22 ( 21. 4) 19 ( 18. 4) History of hypertension – no. (%) 62 ( 60. 2) 72 ( 69. 9) History of ischemic stroke or TIA – no. (%) 12 ( 11. 7) 18 ( 17. 5) NIHSS score, median [IQR] 17. 0 (14. 0, 20. 0) 17. 0 (12. 0, 19. 0) ASPECTS – median [IQR] * 7. 0 (6. 0, 9. 0) 8. 0 (6. 0, 9. 0) Terminus ICA with involvement of M 1 26 (25. 5) 28 (26. 7)† M 1 middle cerebral artery segment 66 (64. 3) 65 (64. 4) Single M 2 middle cerebral artery segment 10 (9. 8) 8 (7. 9) Ipsilateral cervical carotid occlusion 19 (18. 4) 13 (12. 6) Variable Location of intracranial arterial occlusion¶ * Evaluated by the independent CT/MR Corelab ¶ Location site was not available in 1 patient in the intervention group and 2 patients in the control group; † 1 patient had terminus ICA “I” occlusion Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 12
![Workflow Times Time from onset to IV t-PA infusion, min – median [IQR] Time Workflow Times Time from onset to IV t-PA infusion, min – median [IQR] Time](https://present5.com/presentation/e4f396539cf551755eebc6188c5c2b82/image-13.jpg) Workflow Times Time from onset to IV t-PA infusion, min – median [IQR] Time from onset to imaging, min – median [IQR] Time from onset to randomization, min – median [IQR] Time from hospital arrival to groin puncture, min – median [IQR] Time from onset to reperfusion, min – median [IQR] Thrombectomy (n=103) Control (n=103) N=70 117. 5 (90. 0, 150. 0) N=80 105. 0 (86. 0, 137. 5) 192 (129, 272) 183 (132, 263) 223 (170, 312) 226 (168, 308) 109 [85, 163] NA 355 (269, 430) NA Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 13
Workflow Times Time from onset to IV t-PA infusion, min – median [IQR] Time from onset to imaging, min – median [IQR] Time from onset to randomization, min – median [IQR] Time from hospital arrival to groin puncture, min – median [IQR] Time from onset to reperfusion, min – median [IQR] Thrombectomy (n=103) Control (n=103) N=70 117. 5 (90. 0, 150. 0) N=80 105. 0 (86. 0, 137. 5) 192 (129, 272) 183 (132, 263) 223 (170, 312) 226 (168, 308) 109 [85, 163] NA 355 (269, 430) NA Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 13
 SAFETY RESULTS AAN, 2015 Washington DC 14
SAFETY RESULTS AAN, 2015 Washington DC 14
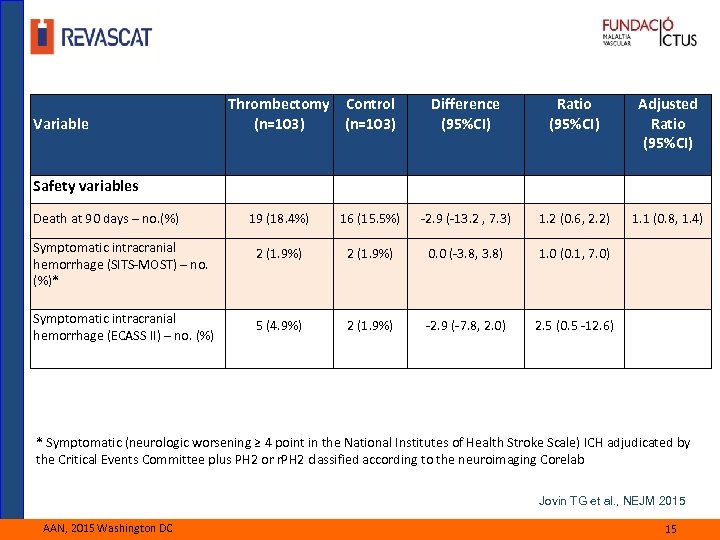 Variable Thrombectomy Control (n=103) Difference (95%CI) Ratio (95%CI) Adjusted Ratio (95%CI) 1. 1 (0. 8, 1. 4) Safety variables Death at 90 days – no. (%) 19 (18. 4%) 16 (15. 5%) -2. 9 (-13. 2 , 7. 3) 1. 2 (0. 6, 2. 2) Symptomatic intracranial hemorrhage (SITS-MOST) – no. (%)* 2 (1. 9%) 0. 0 (-3. 8, 3. 8) 1. 0 (0. 1, 7. 0) Symptomatic intracranial hemorrhage (ECASS II) – no. (%) 5 (4. 9%) 2 (1. 9%) -2. 9 (-7. 8, 2. 0) 2. 5 (0. 5 -12. 6) * Symptomatic (neurologic worsening ≥ 4 point in the National Institutes of Health Stroke Scale) ICH adjudicated by the Critical Events Committee plus PH 2 or r. PH 2 classified according to the neuroimaging Corelab Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 15
Variable Thrombectomy Control (n=103) Difference (95%CI) Ratio (95%CI) Adjusted Ratio (95%CI) 1. 1 (0. 8, 1. 4) Safety variables Death at 90 days – no. (%) 19 (18. 4%) 16 (15. 5%) -2. 9 (-13. 2 , 7. 3) 1. 2 (0. 6, 2. 2) Symptomatic intracranial hemorrhage (SITS-MOST) – no. (%)* 2 (1. 9%) 0. 0 (-3. 8, 3. 8) 1. 0 (0. 1, 7. 0) Symptomatic intracranial hemorrhage (ECASS II) – no. (%) 5 (4. 9%) 2 (1. 9%) -2. 9 (-7. 8, 2. 0) 2. 5 (0. 5 -12. 6) * Symptomatic (neurologic worsening ≥ 4 point in the National Institutes of Health Stroke Scale) ICH adjudicated by the Critical Events Committee plus PH 2 or r. PH 2 classified according to the neuroimaging Corelab Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 15
 EFFICACY RESULTS (ITT Population) AAN, 2015 Washington DC 16
EFFICACY RESULTS (ITT Population) AAN, 2015 Washington DC 16
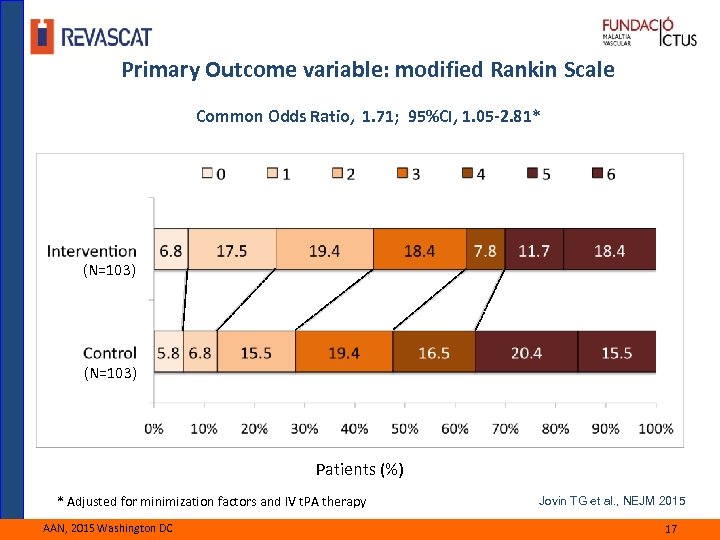 Primary Outcome variable: modified Rankin Scale Common Odds Ratio, 1. 71; 95%CI, 1. 05 -2. 81* (N=103) Patients (%) * Adjusted for minimization factors and IV t. PA therapy AAN, 2015 Washington DC Jovin TG et al. , NEJM 2015 17
Primary Outcome variable: modified Rankin Scale Common Odds Ratio, 1. 71; 95%CI, 1. 05 -2. 81* (N=103) Patients (%) * Adjusted for minimization factors and IV t. PA therapy AAN, 2015 Washington DC Jovin TG et al. , NEJM 2015 17
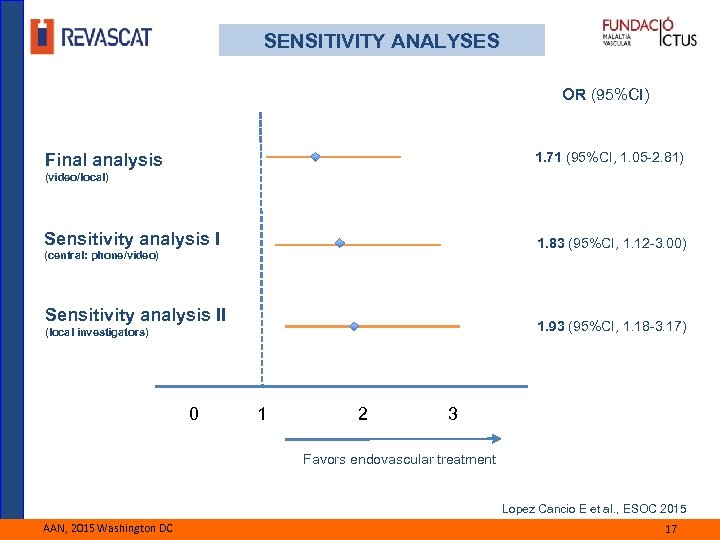 SENSITIVITY ANALYSES OR (95%CI) 1. 71 (95%CI, 1. 05 -2. 81) Final analysis (video/local) Sensitivity analysis I 1. 83 (95%CI, 1. 12 -3. 00) (central: phone/video) Sensitivity analysis II 1. 93 (95%CI, 1. 18 -3. 17) (local investigators) 0 1 2 3 Favors endovascular treatment Lopez Cancio E et al. , ESOC 2015 AAN, 2015 Washington DC 17
SENSITIVITY ANALYSES OR (95%CI) 1. 71 (95%CI, 1. 05 -2. 81) Final analysis (video/local) Sensitivity analysis I 1. 83 (95%CI, 1. 12 -3. 00) (central: phone/video) Sensitivity analysis II 1. 93 (95%CI, 1. 18 -3. 17) (local investigators) 0 1 2 3 Favors endovascular treatment Lopez Cancio E et al. , ESOC 2015 AAN, 2015 Washington DC 17
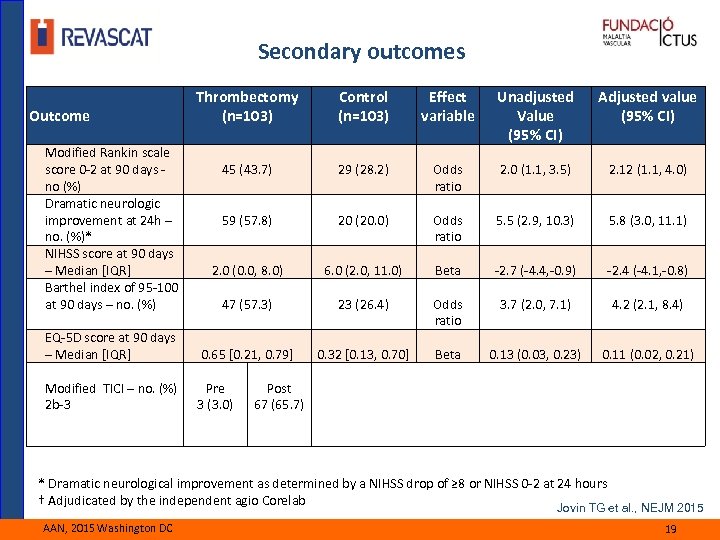 Secondary outcomes Thrombectomy (n=103) Control (n=103) Effect variable Unadjusted Value (95% CI) Adjusted value (95% CI) 45 (43. 7) 29 (28. 2) Odds ratio 2. 0 (1. 1, 3. 5) 2. 12 (1. 1, 4. 0) 59 (57. 8) 20 (20. 0) Odds ratio 5. 5 (2. 9, 10. 3) 5. 8 (3. 0, 11. 1) 2. 0 (0. 0, 8. 0) 6. 0 (2. 0, 11. 0) Beta -2. 7 (-4. 4, -0. 9) -2. 4 (-4. 1, -0. 8) 47 (57. 3) 23 (26. 4) 3. 7 (2. 0, 7. 1) 4. 2 (2. 1, 8. 4) EQ-5 D score at 90 days – Median [IQR] Odds ratio 0. 65 [0. 21, 0. 79] 0. 32 [0. 13, 0. 70] Beta 0. 13 (0. 03, 0. 23) 0. 11 (0. 02, 0. 21) Modified TICI – no. (%) 2 b-3 Pre 3 (3. 0) Outcome Modified Rankin scale score 0 -2 at 90 days - no (%) Dramatic neurologic improvement at 24 h – no. (%)* NIHSS score at 90 days – Median [IQR] Barthel index of 95 -100 at 90 days – no. (%) Post 67 (65. 7) * Dramatic neurological improvement as determined by a NIHSS drop of ≥ 8 or NIHSS 0 -2 at 24 hours † Adjudicated by the independent agio Corelab Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 19
Secondary outcomes Thrombectomy (n=103) Control (n=103) Effect variable Unadjusted Value (95% CI) Adjusted value (95% CI) 45 (43. 7) 29 (28. 2) Odds ratio 2. 0 (1. 1, 3. 5) 2. 12 (1. 1, 4. 0) 59 (57. 8) 20 (20. 0) Odds ratio 5. 5 (2. 9, 10. 3) 5. 8 (3. 0, 11. 1) 2. 0 (0. 0, 8. 0) 6. 0 (2. 0, 11. 0) Beta -2. 7 (-4. 4, -0. 9) -2. 4 (-4. 1, -0. 8) 47 (57. 3) 23 (26. 4) 3. 7 (2. 0, 7. 1) 4. 2 (2. 1, 8. 4) EQ-5 D score at 90 days – Median [IQR] Odds ratio 0. 65 [0. 21, 0. 79] 0. 32 [0. 13, 0. 70] Beta 0. 13 (0. 03, 0. 23) 0. 11 (0. 02, 0. 21) Modified TICI – no. (%) 2 b-3 Pre 3 (3. 0) Outcome Modified Rankin scale score 0 -2 at 90 days - no (%) Dramatic neurologic improvement at 24 h – no. (%)* NIHSS score at 90 days – Median [IQR] Barthel index of 95 -100 at 90 days – no. (%) Post 67 (65. 7) * Dramatic neurological improvement as determined by a NIHSS drop of ≥ 8 or NIHSS 0 -2 at 24 hours † Adjudicated by the independent agio Corelab Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 19
![Infarct volume at 24 h by CT (n=189) or DWI (n=15) Median [IQR], m. Infarct volume at 24 h by CT (n=189) or DWI (n=15) Median [IQR], m.](https://present5.com/presentation/e4f396539cf551755eebc6188c5c2b82/image-20.jpg) Infarct volume at 24 h by CT (n=189) or DWI (n=15) Median [IQR], m. L 38. 6 [12, 87] 17. 2 [9, 58] † Wilcoxon-Mann Whitney, p=0. 030 Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 20
Infarct volume at 24 h by CT (n=189) or DWI (n=15) Median [IQR], m. L 38. 6 [12, 87] 17. 2 [9, 58] † Wilcoxon-Mann Whitney, p=0. 030 Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 20
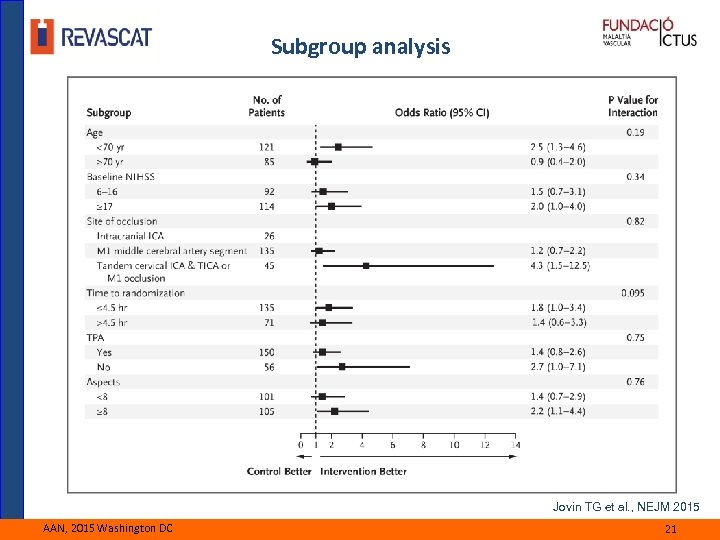 Subgroup analysis Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 21
Subgroup analysis Jovin TG et al. , NEJM 2015 AAN, 2015 Washington DC 21
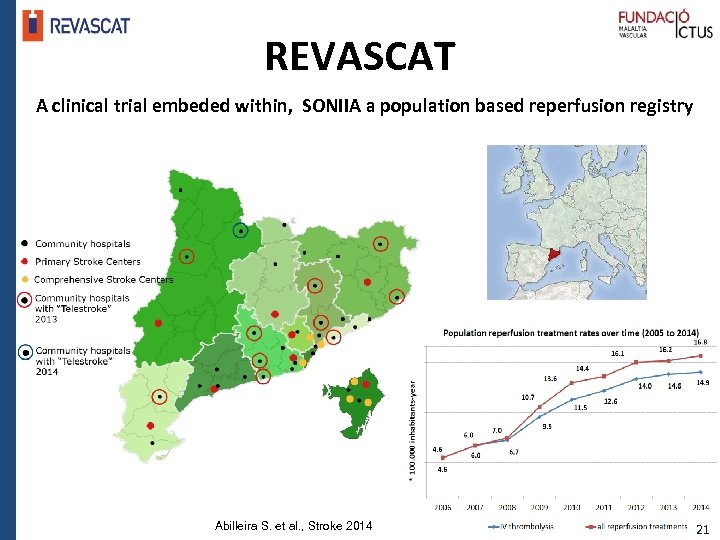 REVASCAT A clinical trial embeded within, SONIIA a population based reperfusion registry ESOC 2015 Glasgow Abilleira S. et al. , Stroke 2014 21 2/12
REVASCAT A clinical trial embeded within, SONIIA a population based reperfusion registry ESOC 2015 Glasgow Abilleira S. et al. , Stroke 2014 21 2/12
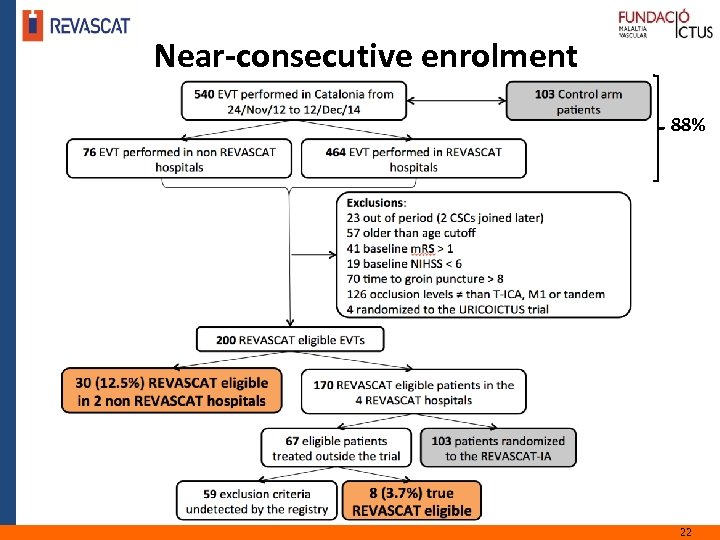 Near-consecutive enrolment 88% 22 5/12
Near-consecutive enrolment 88% 22 5/12
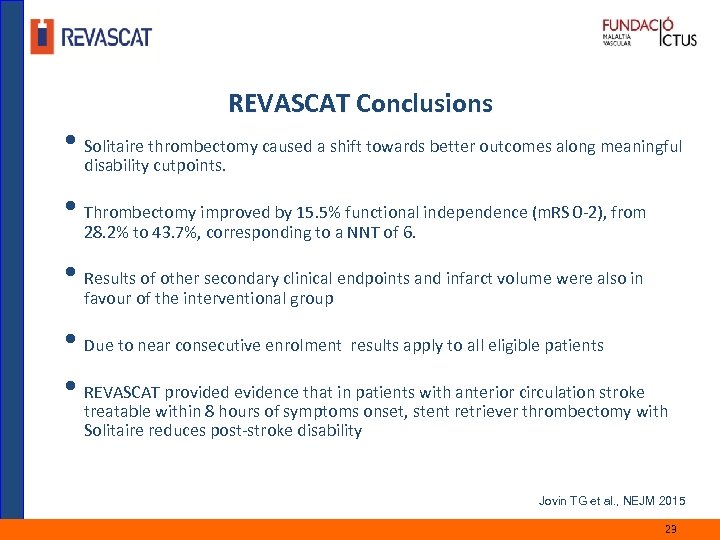 REVASCAT Conclusions • Solitaire thrombectomy caused a shift towards better outcomes along meaningful disability cutpoints. • Thrombectomy improved by 15. 5% functional independence (m. RS 0 -2), from 28. 2% to 43. 7%, corresponding to a NNT of 6. • Results of other secondary clinical endpoints and infarct volume were also in favour of the interventional group • Due to near consecutive enrolment results apply to all eligible patients • REVASCAT provided evidence that in patients with anterior circulation stroke treatable within 8 hours of symptoms onset, stent retriever thrombectomy with Solitaire reduces post-stroke disability Jovin TG et al. , NEJM 2015 23
REVASCAT Conclusions • Solitaire thrombectomy caused a shift towards better outcomes along meaningful disability cutpoints. • Thrombectomy improved by 15. 5% functional independence (m. RS 0 -2), from 28. 2% to 43. 7%, corresponding to a NNT of 6. • Results of other secondary clinical endpoints and infarct volume were also in favour of the interventional group • Due to near consecutive enrolment results apply to all eligible patients • REVASCAT provided evidence that in patients with anterior circulation stroke treatable within 8 hours of symptoms onset, stent retriever thrombectomy with Solitaire reduces post-stroke disability Jovin TG et al. , NEJM 2015 23
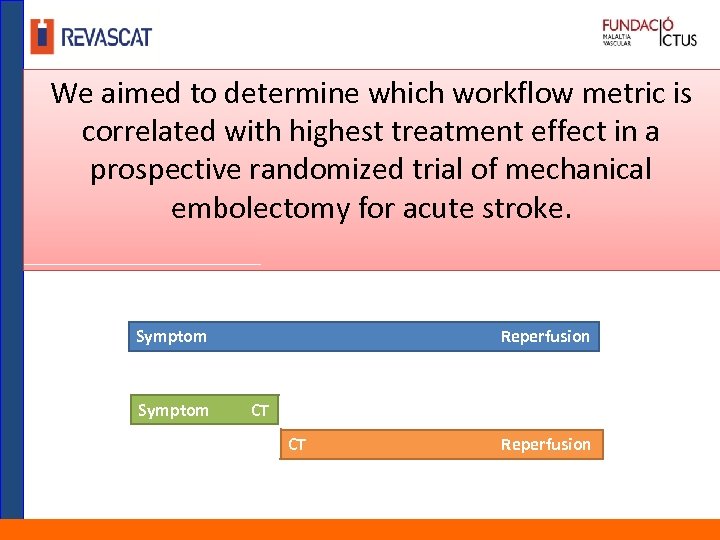 We aimed to determine which workflow metric is correlated with highest treatment effect in a prospective randomized trial of mechanical embolectomy for acute stroke. Symptom Reperfusion CT CT Reperfusion
We aimed to determine which workflow metric is correlated with highest treatment effect in a prospective randomized trial of mechanical embolectomy for acute stroke. Symptom Reperfusion CT CT Reperfusion
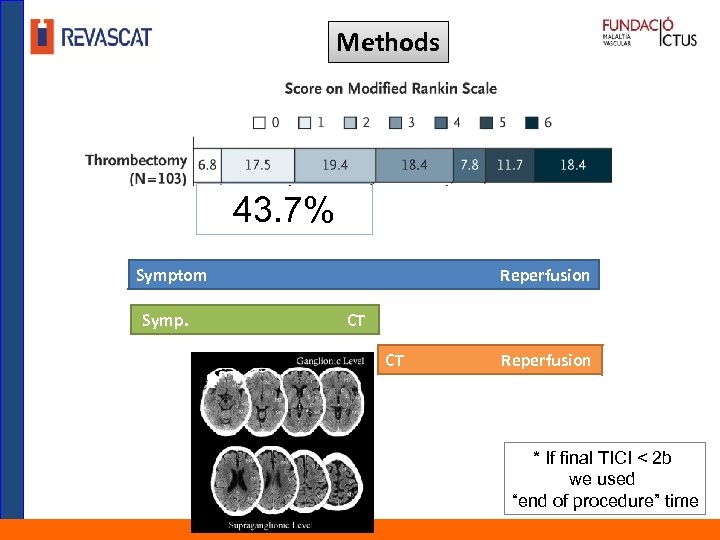 Methods 43. 7% Symptom Symp. Reperfusion CT CT Reperfusion * If final TICI < 2 b we used “end of procedure” time
Methods 43. 7% Symptom Symp. Reperfusion CT CT Reperfusion * If final TICI < 2 b we used “end of procedure” time
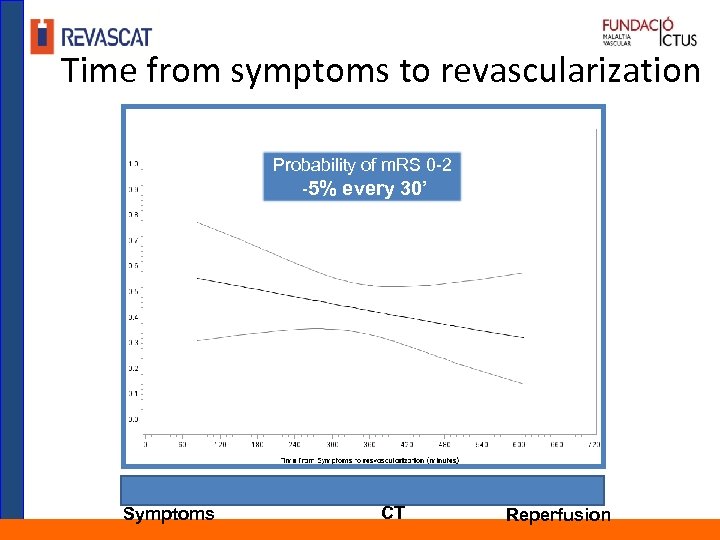 Time from symptoms to revascularization Probability of m. RS 0 -2 -5% every 30’ Symptoms CT Reperfusion
Time from symptoms to revascularization Probability of m. RS 0 -2 -5% every 30’ Symptoms CT Reperfusion
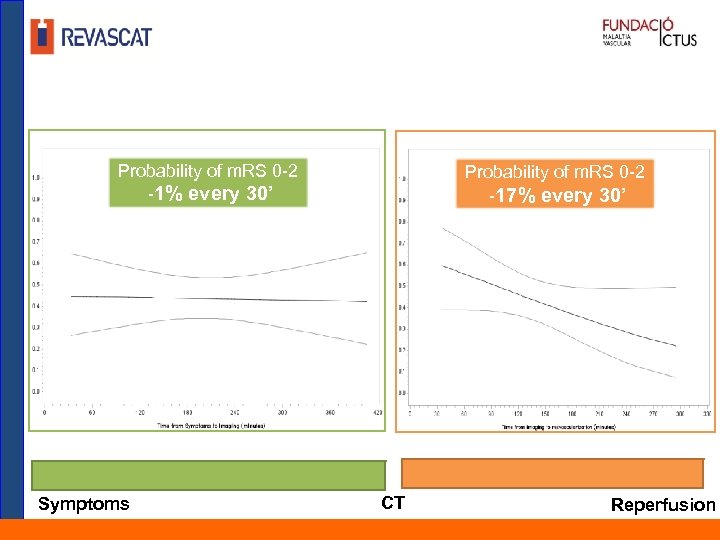 Probability of m. RS 0 -2 -1% every 30’ Symptoms Probability of m. RS 0 -2 -17% every 30’ CT Reperfusion
Probability of m. RS 0 -2 -1% every 30’ Symptoms Probability of m. RS 0 -2 -17% every 30’ CT Reperfusion
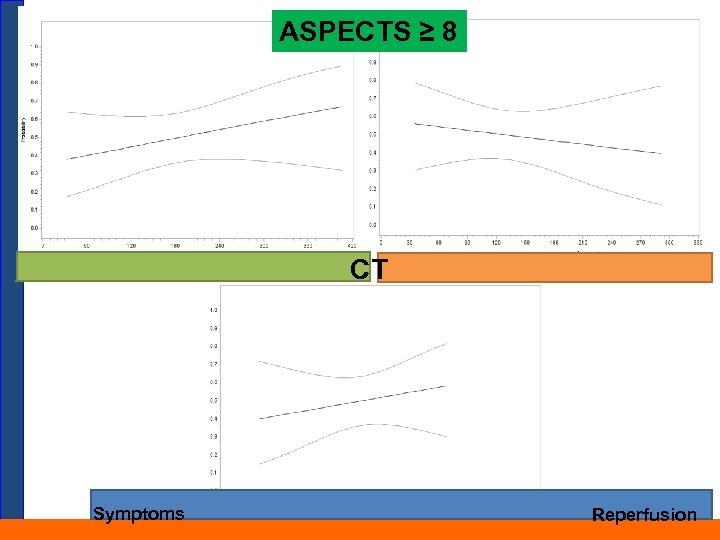 ASPECTS ≥ 8 CT Symptoms Reperfusion
ASPECTS ≥ 8 CT Symptoms Reperfusion
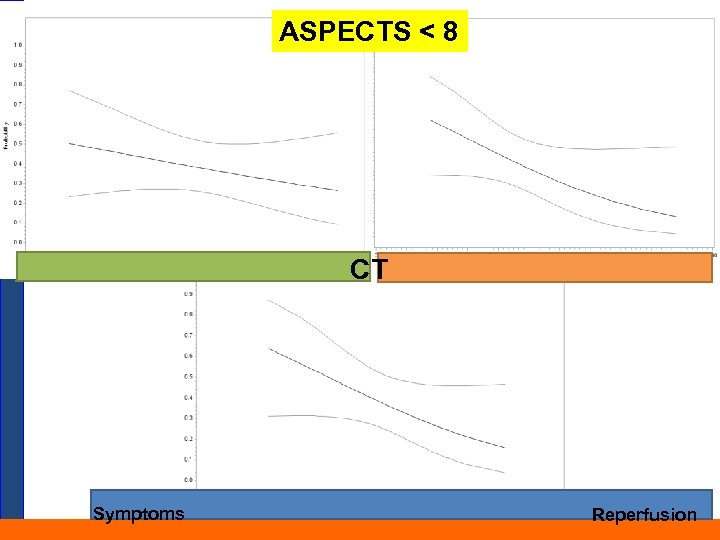 Figure 1. Time from symptoms to revascularization (minutes) (unadjusted) – Aspects < 8 ASPECTS < 8 CT Symptoms Reperfusion
Figure 1. Time from symptoms to revascularization (minutes) (unadjusted) – Aspects < 8 ASPECTS < 8 CT Symptoms Reperfusion
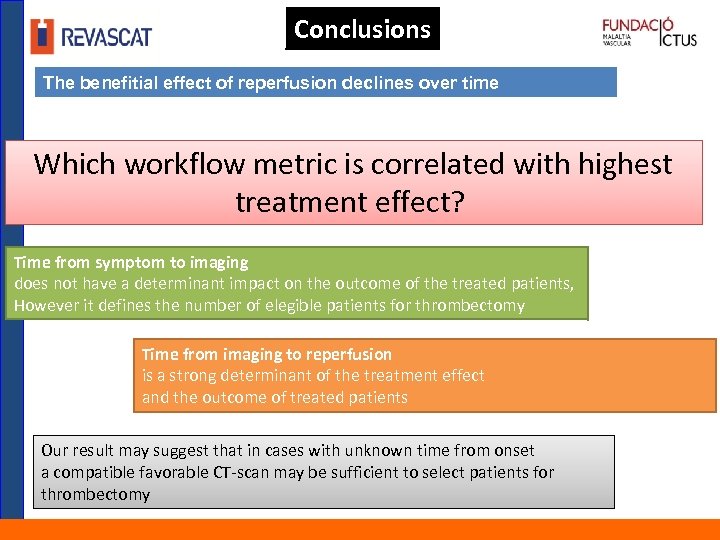 Conclusions The benefitial effect of reperfusion declines over time Which workflow metric is correlated with highest treatment effect? Time from symptom to imaging does not have a determinant impact on the outcome of the treated patients, However it defines the number of elegible patients for thrombectomy Time from imaging to reperfusion is a strong determinant of the treatment effect and the outcome of treated patients Our result may suggest that in cases with unknown time from onset a compatible favorable CT-scan may be sufficient to select patients for thrombectomy
Conclusions The benefitial effect of reperfusion declines over time Which workflow metric is correlated with highest treatment effect? Time from symptom to imaging does not have a determinant impact on the outcome of the treated patients, However it defines the number of elegible patients for thrombectomy Time from imaging to reperfusion is a strong determinant of the treatment effect and the outcome of treated patients Our result may suggest that in cases with unknown time from onset a compatible favorable CT-scan may be sufficient to select patients for thrombectomy
 Thank you! AAN, 2015 Washington DC ESOC 2015 Glasgow 25
Thank you! AAN, 2015 Washington DC ESOC 2015 Glasgow 25
 Investigators SONIIA Registry Miquel Gallofré Sonia Abilleira Other Comprehensive Stroke Centers Hospital del Mar Hospital Parc Taulí 24
Investigators SONIIA Registry Miquel Gallofré Sonia Abilleira Other Comprehensive Stroke Centers Hospital del Mar Hospital Parc Taulí 24


