a2aca8940c33de255a0c689a6f7e02bf.ppt
- Количество слайдов: 25
 U. S. EPA Design for the Environment Program NEWMOA November 18, 2010 Clive Davies
U. S. EPA Design for the Environment Program NEWMOA November 18, 2010 Clive Davies
 Contents • Df. E Background • Safer Product Labeling Program – Enhancements Now Being Implemented – Enhancements Being Considered, including ingredient communication • Action Plans and Chemical Alternatives Assessments – BPA in Thermal Paper – Deca. BDE 2
Contents • Df. E Background • Safer Product Labeling Program – Enhancements Now Being Implemented – Enhancements Being Considered, including ingredient communication • Action Plans and Chemical Alternatives Assessments – BPA in Thermal Paper – Deca. BDE 2
 What Df. E is About • Goals • Safer Products • Safer chemical ingredients is baseline • Life cycle impacts are considered • Protecting Consumers – Especially Children • Central Elements • OPPT technical tools and expertise • Multi-stakeholder participation • Results • Industry partners reduced more than 500 million pounds of chemicals of concern last year 3
What Df. E is About • Goals • Safer Products • Safer chemical ingredients is baseline • Life cycle impacts are considered • Protecting Consumers – Especially Children • Central Elements • OPPT technical tools and expertise • Multi-stakeholder participation • Results • Industry partners reduced more than 500 million pounds of chemicals of concern last year 3
 I. Safer Product Labeling 1) Review every ingredient by functional use class • • To promote green chemistry To understand toxicity • • • Lists Literature Analogous chemicals – SAR 2) Review formulation as a whole • • • Synergistic effects p. H Performance testing 3) Partnership Agreement • • Audits Logo Use 4
I. Safer Product Labeling 1) Review every ingredient by functional use class • • To promote green chemistry To understand toxicity • • • Lists Literature Analogous chemicals – SAR 2) Review formulation as a whole • • • Synergistic effects p. H Performance testing 3) Partnership Agreement • • Audits Logo Use 4
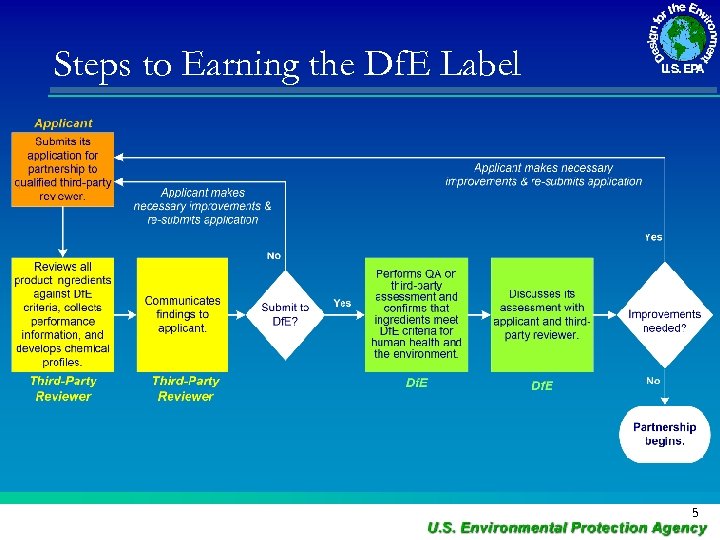 Steps to Earning the Df. E Label 5
Steps to Earning the Df. E Label 5
 Component-Class Criteria • Master Criteria – Sets the Standard for Green Chemistry for all ingredients – Based on New Chemicals Program, OPP/OPPT Harmonized Guidelines, and Globally Harmonized System criteria • Tailored Criteria Differentiate Highly Functional Alternatives for key ingredient classes – Surfactants – Solvents – Chelants – Builders – Fragrances • Future Criteria would enhance transparency and promote Green Chemistry – Disinfectant Actives & Preservatives – Colorants – Polymers 6
Component-Class Criteria • Master Criteria – Sets the Standard for Green Chemistry for all ingredients – Based on New Chemicals Program, OPP/OPPT Harmonized Guidelines, and Globally Harmonized System criteria • Tailored Criteria Differentiate Highly Functional Alternatives for key ingredient classes – Surfactants – Solvents – Chelants – Builders – Fragrances • Future Criteria would enhance transparency and promote Green Chemistry – Disinfectant Actives & Preservatives – Colorants – Polymers 6
 Hazard Endpoints for Safer Chemicals • Every chemical is assessed against criteria – Authoritative government lists of chemicals of concern – Data from studies – Modeling to fill data gaps • Based on internationally agreed toxicological endpoints and thresholds – – – Acute mammalian toxicity Aquatic toxicity Bioaccumulation Biodegradation Carcinogenicity Eutrophication Genetic toxicity Neurotoxicity Repeated dose toxicity Reproductive and developmental toxicity Respiratory sensitization Skin sensitization 7
Hazard Endpoints for Safer Chemicals • Every chemical is assessed against criteria – Authoritative government lists of chemicals of concern – Data from studies – Modeling to fill data gaps • Based on internationally agreed toxicological endpoints and thresholds – – – Acute mammalian toxicity Aquatic toxicity Bioaccumulation Biodegradation Carcinogenicity Eutrophication Genetic toxicity Neurotoxicity Repeated dose toxicity Reproductive and developmental toxicity Respiratory sensitization Skin sensitization 7
 Life Cycle Considerations • Program focuses on “hotspots” in the lifecycle • Requirements of Df. E Standard: – Primary focus is on hazard for the chemical manufacturing, product manufacturing, use, and disposal phases (hazard reduction) – Packaging requirements reduce material use and make transportation phase more efficient (GHG reduction, resource conservation) – Performance requirements promote efficient product use (resource conservation) • Policy Goals: – Concentrates to reduce transportation needs. (GHG reduction) – Cold water detergents to reduce energy use. (GHG reduction) – Renewable raw materials and packaging (resource conservation) 8
Life Cycle Considerations • Program focuses on “hotspots” in the lifecycle • Requirements of Df. E Standard: – Primary focus is on hazard for the chemical manufacturing, product manufacturing, use, and disposal phases (hazard reduction) – Packaging requirements reduce material use and make transportation phase more efficient (GHG reduction, resource conservation) – Performance requirements promote efficient product use (resource conservation) • Policy Goals: – Concentrates to reduce transportation needs. (GHG reduction) – Cold water detergents to reduce energy use. (GHG reduction) – Renewable raw materials and packaging (resource conservation) 8
 Verification of Formulation • Partnership Agreement – Signed agreement between EPA and each manufacturer – 3 years in duration; will sunset unless renewed – Specifies • Chemicals in each formulation • Conditions of logo use • Audit procedures • Audits – Annual desk audits – Triennial on-site audit 9
Verification of Formulation • Partnership Agreement – Signed agreement between EPA and each manufacturer – 3 years in duration; will sunset unless renewed – Specifies • Chemicals in each formulation • Conditions of logo use • Audit procedures • Audits – Annual desk audits – Triennial on-site audit 9
 Enhancements Now Being Implemented • Stakeholder group helped document Df. E in the form of an ANSI Standard • Stakeholder group helped propose enhancements. – Audits – Continuous Delivery Systems for Consumer Products – Definitional Improvements 10
Enhancements Now Being Implemented • Stakeholder group helped document Df. E in the form of an ANSI Standard • Stakeholder group helped propose enhancements. – Audits – Continuous Delivery Systems for Consumer Products – Definitional Improvements 10
 Now Being Implemented – Audits • Annual Desk Audits – Verify contents of recognized products and labels – Ensure safer chemistry status (continuous improvement) – Review production volumes, use of logo, etc. • On-site Audit – Once during 3 -yr partnership period -- if more than one facility, two sites selected randomly will be audited – Confirms materials usage compared to Partnership Agreement (using batch tickets) – Ensures Good Manufacturing Practices (e. g. , non-contamination of labeled products) – Reviews overall partnership compliance (e. g. , documentation of enduser training, packaging) 11
Now Being Implemented – Audits • Annual Desk Audits – Verify contents of recognized products and labels – Ensure safer chemistry status (continuous improvement) – Review production volumes, use of logo, etc. • On-site Audit – Once during 3 -yr partnership period -- if more than one facility, two sites selected randomly will be audited – Confirms materials usage compared to Partnership Agreement (using batch tickets) – Ensures Good Manufacturing Practices (e. g. , non-contamination of labeled products) – Reviews overall partnership compliance (e. g. , documentation of enduser training, packaging) 11
 Enhancements Now Being Considered • New Areas – Ingredient Disclosure – Dermal Contact Products • Enhancements to Existing Criteria – Performance: Pass/fail thresholds. – Packaging: Sustainable Packaging Coalition measures; 25% compliance and continuous improvement. – VOC : Adopt CARB or OTC levels. – Flammability: Clarify limits (140 F). – Enzymes: Allow low-dust granulated enzymes in dry formulations, if adequate engineering controls present. 12
Enhancements Now Being Considered • New Areas – Ingredient Disclosure – Dermal Contact Products • Enhancements to Existing Criteria – Performance: Pass/fail thresholds. – Packaging: Sustainable Packaging Coalition measures; 25% compliance and continuous improvement. – VOC : Adopt CARB or OTC levels. – Flammability: Clarify limits (140 F). – Enzymes: Allow low-dust granulated enzymes in dry formulations, if adequate engineering controls present. 12
 Ingredient Disclosure • All must be listed • Non-confidential ingredients – On the bottle, or – An easily accessible location where ingredients can be found (e. g. , a place on the formulator’s website). • Confidential ingredients, dyes and preservatives -- use a chemical descriptive name. • Scent ingredients – Can be listed on the label as “Fragrance, ” – more detailed information must be provided elsewhere • E. g. , website list of the actual ingredients, or • reference to the IFRA list or a subset of chemicals on the IFRA list. 13
Ingredient Disclosure • All must be listed • Non-confidential ingredients – On the bottle, or – An easily accessible location where ingredients can be found (e. g. , a place on the formulator’s website). • Confidential ingredients, dyes and preservatives -- use a chemical descriptive name. • Scent ingredients – Can be listed on the label as “Fragrance, ” – more detailed information must be provided elsewhere • E. g. , website list of the actual ingredients, or • reference to the IFRA list or a subset of chemicals on the IFRA list. 13
 Packaging • Partners will be required to adopt sustainable packaging measures as a condition of partnership and show continuous improvement over time – At partnership initiation, must achieve at least 25% level in one of six sustainability measures (developed by Sustainable Packaging Coalition) • Report on packaging status will occur at partnership renewal • Materials must not contain heavy metals or other ingredients of concern (e. g. , BPA or phthalates) 14
Packaging • Partners will be required to adopt sustainable packaging measures as a condition of partnership and show continuous improvement over time – At partnership initiation, must achieve at least 25% level in one of six sustainability measures (developed by Sustainable Packaging Coalition) • Report on packaging status will occur at partnership renewal • Materials must not contain heavy metals or other ingredients of concern (e. g. , BPA or phthalates) 14
 II. EPA Chemical Action Plans • Chemicals for which action plans have been published: – – – – Benzidine dyes Bisphenol A (BPA) Hexabromocyclododecane (HBCD) Nonylphenol and nonylphenol ethoxylates (NP/NPE) Phthalates Perfluorinated chemicals (PFCs) Penta, octa, and decabromodiphenyl ethers (PBDEs) Short-chain chlorinated paraffins • Chemicals in the action plan development process: – Diisocyantes – Siloxanes See: http: //www. epa. gov/opptintr/existingchemicals/pubs/ecactionpln. html 15
II. EPA Chemical Action Plans • Chemicals for which action plans have been published: – – – – Benzidine dyes Bisphenol A (BPA) Hexabromocyclododecane (HBCD) Nonylphenol and nonylphenol ethoxylates (NP/NPE) Phthalates Perfluorinated chemicals (PFCs) Penta, octa, and decabromodiphenyl ethers (PBDEs) Short-chain chlorinated paraffins • Chemicals in the action plan development process: – Diisocyantes – Siloxanes See: http: //www. epa. gov/opptintr/existingchemicals/pubs/ecactionpln. html 15
 EPA Chemical Action Plans • Of these action plan chemicals, Df. E plans to conduct chemical alternatives assessments for the following: – – – Bisphenol A (BPA) Decabromodiphenyl ether (deca. BDE) Hexabromocyclododecane (HBCD) Nonylphenol and nonylphenol ethoxylates (NP/NPE) Phthalates Others 16
EPA Chemical Action Plans • Of these action plan chemicals, Df. E plans to conduct chemical alternatives assessments for the following: – – – Bisphenol A (BPA) Decabromodiphenyl ether (deca. BDE) Hexabromocyclododecane (HBCD) Nonylphenol and nonylphenol ethoxylates (NP/NPE) Phthalates Others 16
 What are Alternatives Assessments? • Evaluates the impacts of a chemical and its alternatives. High, Moderate, or Low ratings are given for: • Human health effects • Environmental fate and effects • The goal is inform substitution to safer chemicals, and avoid simply switching to chemicals that are poorly understood. • Alternatives to the chemical of concern must provide the same function. • Life-cycle thinking ensures that other impacts are not overlooked. 17
What are Alternatives Assessments? • Evaluates the impacts of a chemical and its alternatives. High, Moderate, or Low ratings are given for: • Human health effects • Environmental fate and effects • The goal is inform substitution to safer chemicals, and avoid simply switching to chemicals that are poorly understood. • Alternatives to the chemical of concern must provide the same function. • Life-cycle thinking ensures that other impacts are not overlooked. 17
 Hazard Endpoints in the Assessment Criteria Human Health Toxicity • Acute mammalian toxicity • Carcinogenicity • Mutagenicity/ Genotoxicity • Reproductive and Developmental Toxicity • Neurotoxicity • Repeated Dose Toxicity • Respiratory and Skin Sensitization • Eye and Skin Irritation/Corrosivity Environmental Fate & Effects • Aquatic toxicity • Environmental persistence • Bioaccumulation 18
Hazard Endpoints in the Assessment Criteria Human Health Toxicity • Acute mammalian toxicity • Carcinogenicity • Mutagenicity/ Genotoxicity • Reproductive and Developmental Toxicity • Neurotoxicity • Repeated Dose Toxicity • Respiratory and Skin Sensitization • Eye and Skin Irritation/Corrosivity Environmental Fate & Effects • Aquatic toxicity • Environmental persistence • Bioaccumulation 18
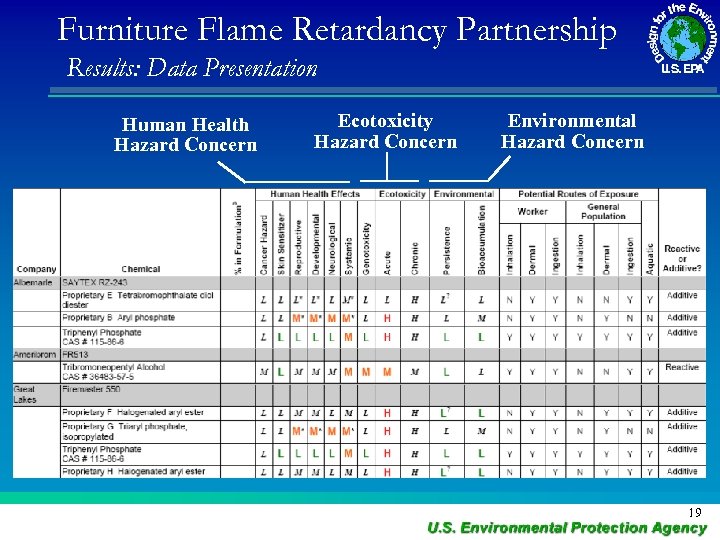 Furniture Flame Retardancy Partnership Results: Data Presentation Human Health Hazard Concern Ecotoxicity Hazard Concern Environmental Hazard Concern 19
Furniture Flame Retardancy Partnership Results: Data Presentation Human Health Hazard Concern Ecotoxicity Hazard Concern Environmental Hazard Concern 19
 Endocrine Activity for Alternatives Assessment • This criterion would evaluate endocrine activity rather than characterize hazard in terms of “endocrine disruption”. • Including this endpoint in the alternatives assessment will provide information that could inform decision-making. • In consultation with EPA toxicologists and risk assessors, EPA will provide: – Summary statement of available data – Qualitative assessment of the level of evidence supporting designation • Presence of equivocal or conflicting data • Limitations • Level of confidence in the assessment 20
Endocrine Activity for Alternatives Assessment • This criterion would evaluate endocrine activity rather than characterize hazard in terms of “endocrine disruption”. • Including this endpoint in the alternatives assessment will provide information that could inform decision-making. • In consultation with EPA toxicologists and risk assessors, EPA will provide: – Summary statement of available data – Qualitative assessment of the level of evidence supporting designation • Presence of equivocal or conflicting data • Limitations • Level of confidence in the assessment 20
 BPA Alternatives in Thermal Paper Partnership • Bisphenol A (BPA) is a high production volume chemical that is considered to be a reproductive, developmental and systemic toxicant, as well as weakly estrogenic. Aquatic toxicity is also of concern. • BPA is used in thermal paper as a developer that reacts with other chemicals in the presence of heat to create color and may be an important source of exposure and release to the environment. • The alternatives assessment will evaluate alternatives to BPA. • Timing: July 2010 – October 2011. 21
BPA Alternatives in Thermal Paper Partnership • Bisphenol A (BPA) is a high production volume chemical that is considered to be a reproductive, developmental and systemic toxicant, as well as weakly estrogenic. Aquatic toxicity is also of concern. • BPA is used in thermal paper as a developer that reacts with other chemicals in the presence of heat to create color and may be an important source of exposure and release to the environment. • The alternatives assessment will evaluate alternatives to BPA. • Timing: July 2010 – October 2011. 21
 Stakeholders for the BPA Partnership • Thermal Paper Manufacturers • Thermal Paper Converters • Suppliers • POS OEM Manufacturers • Retailers • International • Green Chemistry Consultants • Chemical Manufacturers (Developers and Colorformers) • Trade Associations • Trade Unions • Government 22
Stakeholders for the BPA Partnership • Thermal Paper Manufacturers • Thermal Paper Converters • Suppliers • POS OEM Manufacturers • Retailers • International • Green Chemistry Consultants • Chemical Manufacturers (Developers and Colorformers) • Trade Associations • Trade Unions • Government 22
 Partnership on FR Alternatives to deca. BDE • Based on concerns for human health and the environment, decabromodiphenyl ether (deca. BDE) is being phased-out by manufacturers. • Deca. BDE is used as a flame retardant in a variety of materials that have applications in electronics, wire and cable, construction, automotive, aviation, and textile industries, and is used in plastic shipping pallets. • The alternatives assessment will evaluate alternatives to deca. BDE. • Timing: October 2010 to December 2011. 23
Partnership on FR Alternatives to deca. BDE • Based on concerns for human health and the environment, decabromodiphenyl ether (deca. BDE) is being phased-out by manufacturers. • Deca. BDE is used as a flame retardant in a variety of materials that have applications in electronics, wire and cable, construction, automotive, aviation, and textile industries, and is used in plastic shipping pallets. • The alternatives assessment will evaluate alternatives to deca. BDE. • Timing: October 2010 to December 2011. 23
 Stakeholders for deca. BDE Partnership • • Academics Consultants NGOs Flame Retardant Manufacturers • Compounders and Resin • Manufacturers • Automotive Industry • • • Electronics Industry Shipping Pallet Industry Textile Industry Recyclers U. S. Federal Government State and Local Governments • International 24
Stakeholders for deca. BDE Partnership • • Academics Consultants NGOs Flame Retardant Manufacturers • Compounders and Resin • Manufacturers • Automotive Industry • • • Electronics Industry Shipping Pallet Industry Textile Industry Recyclers U. S. Federal Government State and Local Governments • International 24
 Thank you! For more information: Clive Davies davies. clive@epa. gov 202 -564 -3821 For more information: Df. E: epa. gov/dfe facebook. com/epadfe Action Plans: epa. gov/opptintr/existingchemicals/pubs/ecactionpln. html 25
Thank you! For more information: Clive Davies davies. clive@epa. gov 202 -564 -3821 For more information: Df. E: epa. gov/dfe facebook. com/epadfe Action Plans: epa. gov/opptintr/existingchemicals/pubs/ecactionpln. html 25


